Osmosis Movement of Water across the Cell Membrane

Osmosis Movement of Water across the Cell Membrane

Let’s review diffusion! • Diffusion requires NO ENERGY • Molecules move from HIGH to LOW • Downhill!!!! Let’s see! • Osmosis: • Movement of WATER from an area of more water (H) to an area of less water (L) • Water moves OPPOSITE of other molecules!

How does osmosis work? • Across a SEMI-PERMEABLE membrane • Only certain molecules, like water, can move freely across the membrane • Water molecules move from HIGH to LOW • They want to BALANCE OUT a solution • Therefore, water moves OPPOSITE of other molecules
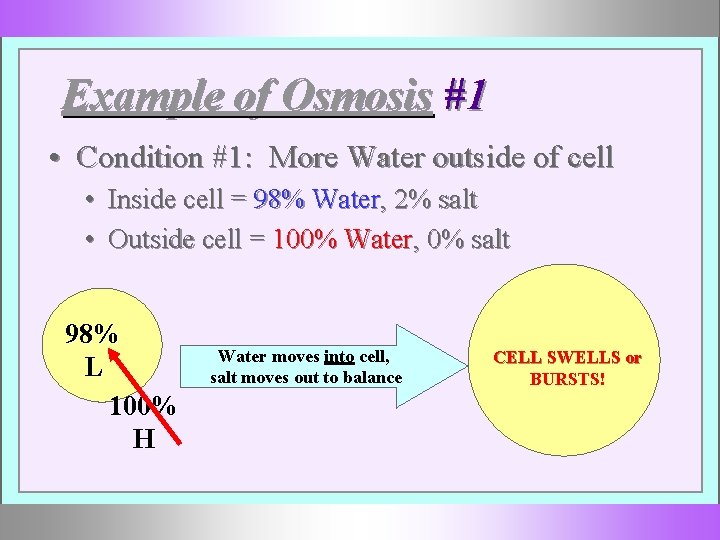
Example of Osmosis #1 • Condition #1: More Water outside of cell • Inside cell = 98% Water, 2% salt • Outside cell = 100% Water, 0% salt 98% L 100% H Water moves into cell, salt moves out to balance CELL SWELLS or BURSTS!
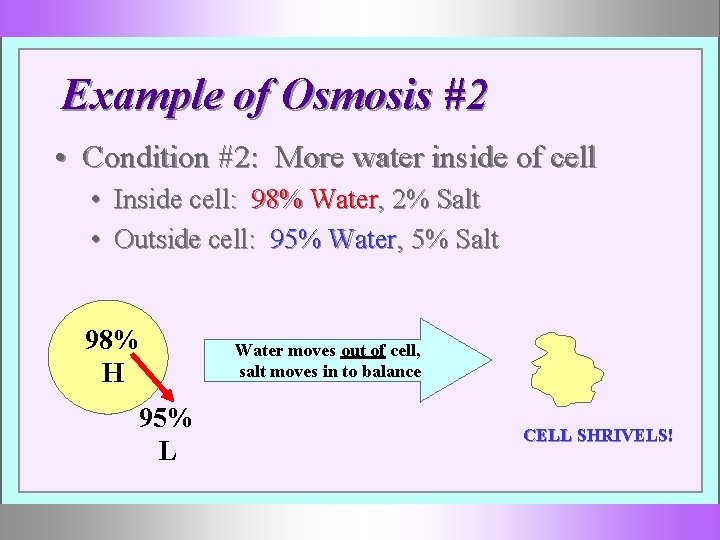
Example of Osmosis #2 • Condition #2: More water inside of cell • Inside cell: 98% Water, 2% Salt • Outside cell: 95% Water, 5% Salt 98% H 95% L Water moves out of cell, salt moves in to balance CELL SHRIVELS!

Osmotic Pressure • Cells behave differently when placed in different solutions! • This is due to OSMOSIS! • Most cells are ~ 98% water! • Three types of osmotic pressure: • • • Hypotonic Hypertonic Isotonic
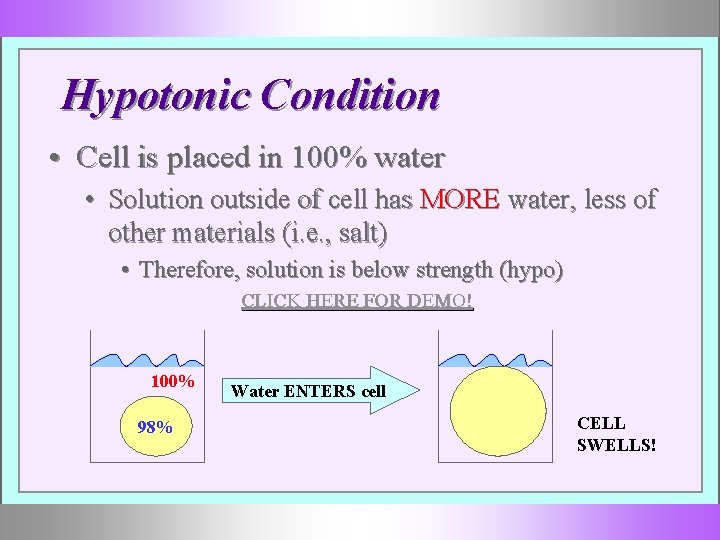
Hypotonic Condition • Cell is placed in 100% water • Solution outside of cell has MORE water, less of other materials (i. e. , salt) • Therefore, solution is below strength (hypo) CLICK HERE FOR DEMO! 100% 98% Water ENTERS cell CELL SWELLS!

Hypertonic Condition • Cell is placed in 95% water • Solution outside of cell has LESS water, more of other materials (i. e. , salt) • Therefore, solution is above strength (hyper) CLICK HERE FOR DEMO! 95% 98% Water LEAVES cell CELL SHRINKS!
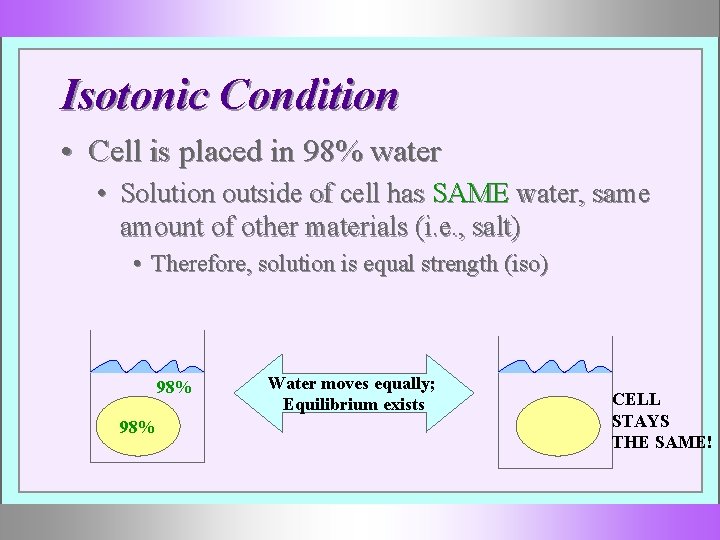
Isotonic Condition • Cell is placed in 98% water • Solution outside of cell has SAME water, same amount of other materials (i. e. , salt) • Therefore, solution is equal strength (iso) 98% Water moves equally; Equilibrium exists CELL STAYS THE SAME!

Review of Osmosis
- Slides: 10