Osmosis diffusion of water across a semipermeable membrane
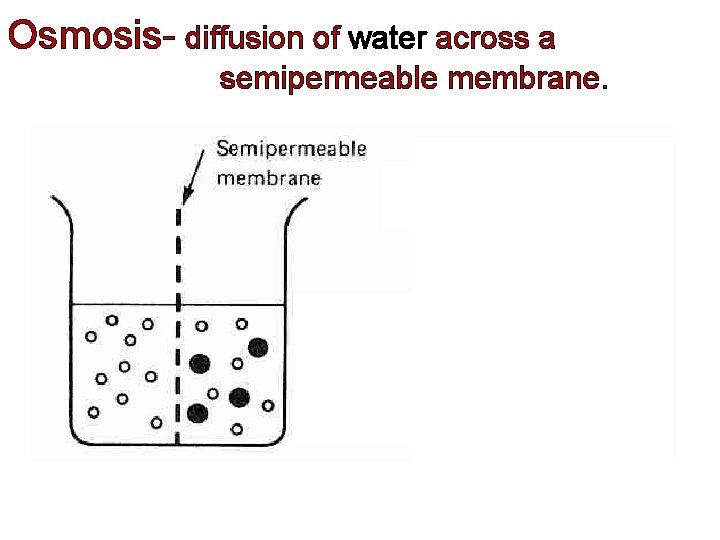
Osmosis- diffusion of water across a semipermeable membrane.
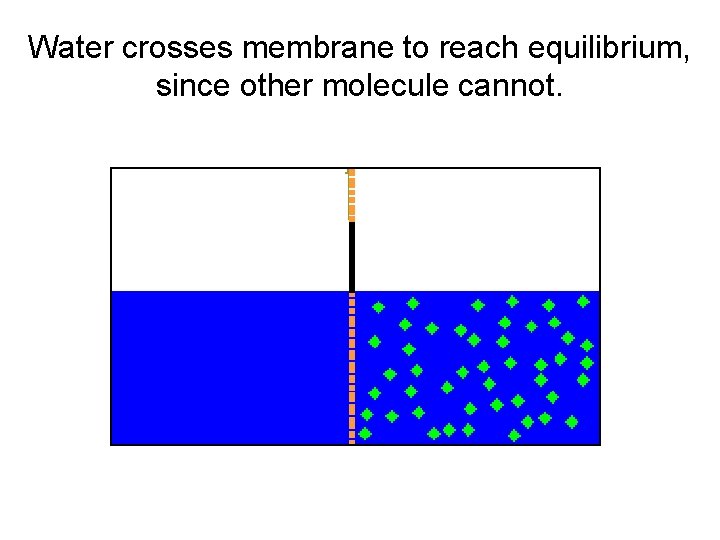
Water crosses membrane to reach equilibrium, since other molecule cannot.

Water crosses membrane to reach equilibrium, since other molecule cannot.
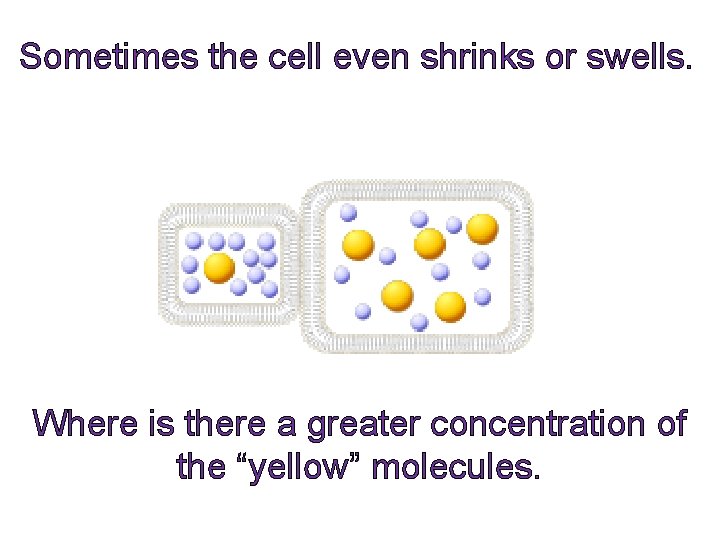
Sometimes the cell even shrinks or swells. Where is there a greater concentration of the “yellow” molecules.
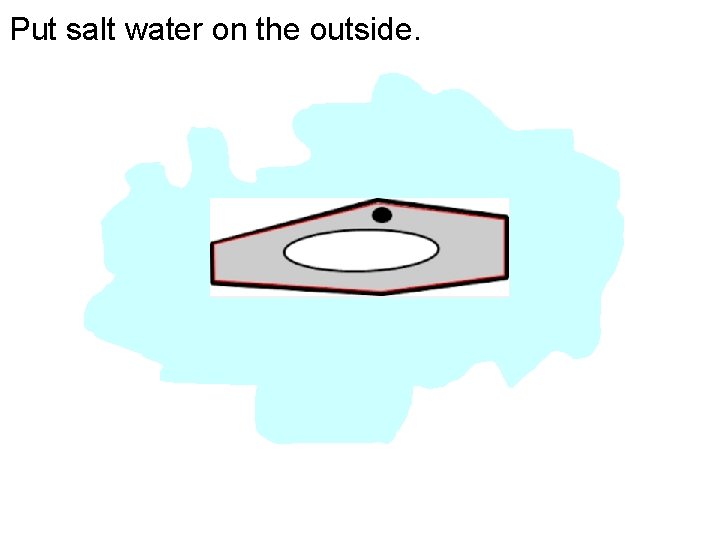
Put salt water on the outside.
Why?
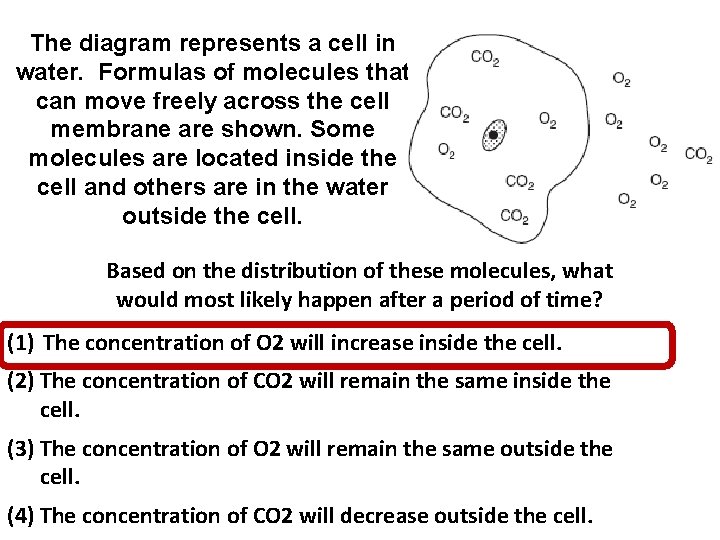
The diagram represents a cell in water. Formulas of molecules that can move freely across the cell membrane are shown. Some molecules are located inside the cell and others are in the water outside the cell. Based on the distribution of these molecules, what would most likely happen after a period of time? (1) The concentration of O 2 will increase inside the cell. (2) The concentration of CO 2 will remain the same inside the cell. (3) The concentration of O 2 will remain the same outside the cell. (4) The concentration of CO 2 will decrease outside the cell.
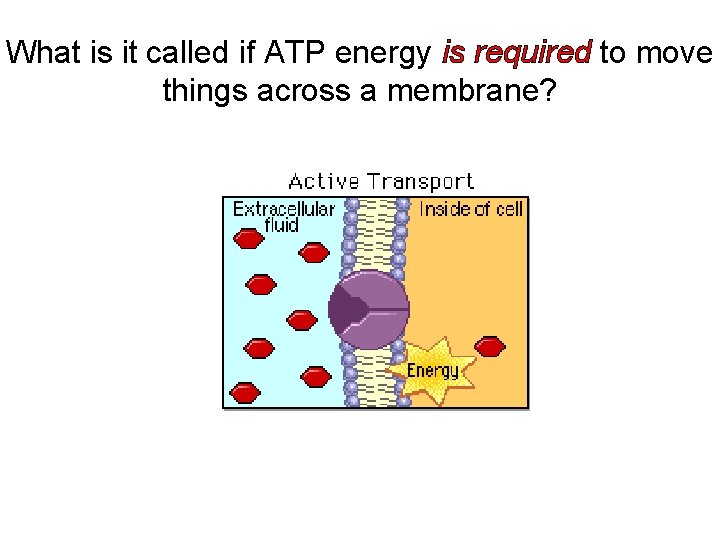
What is it called if ATP energy is required to move things across a membrane?

Add fresh water Add salty water
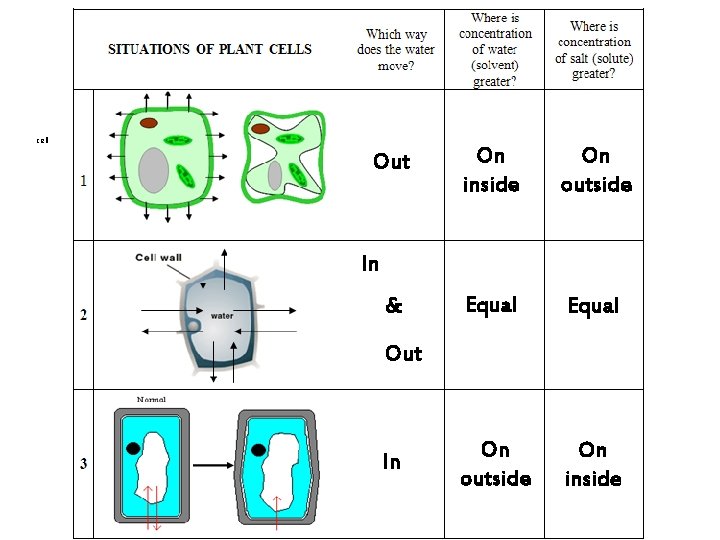
cell Out On inside On outside Equal On outside On inside In & Out In
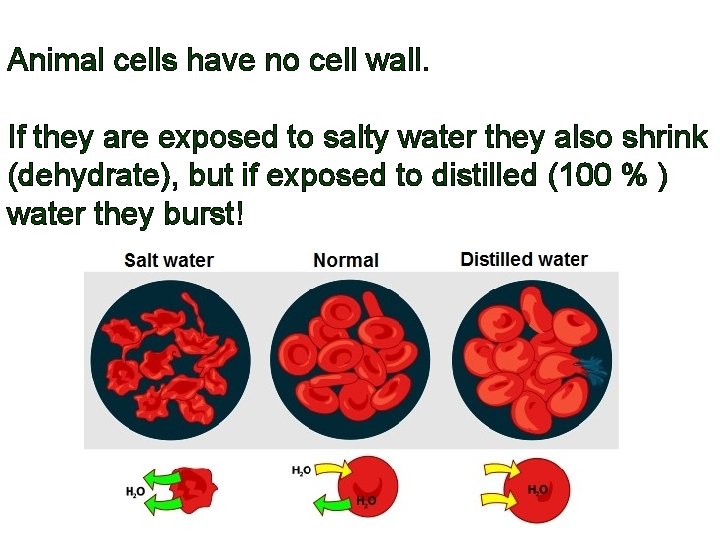
Animal cells have no cell wall. If they are exposed to salty water they also shrink (dehydrate), but if exposed to distilled (100 % ) water they burst!

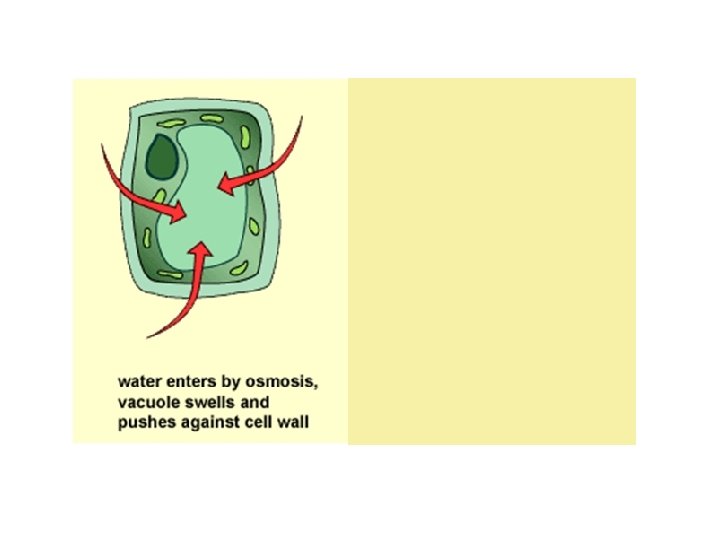
Why is the plant “sad”?

Why is the plant now “happy”?
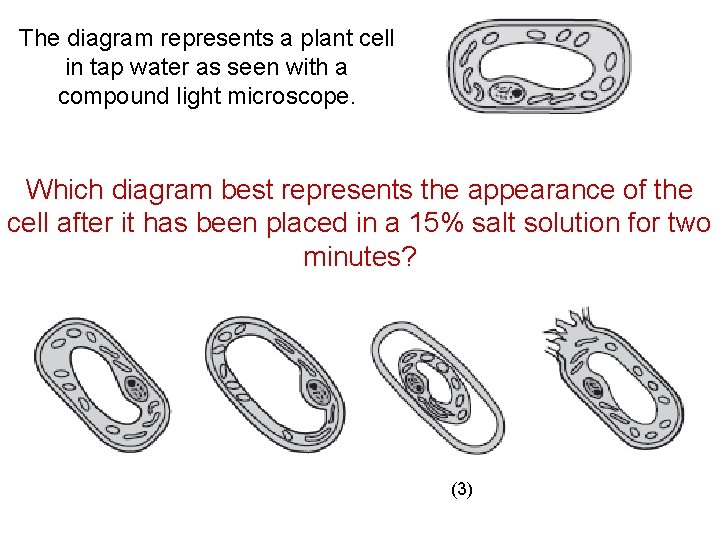
The diagram represents a plant cell in tap water as seen with a compound light microscope. Which diagram best represents the appearance of the cell after it has been placed in a 15% salt solution for two minutes? (1) (2) (3) (4)
- Slides: 15