OSMOSIS AND WATER POTENTIAL Osmosis Osmosis is the










- Slides: 25

OSMOSIS AND WATER POTENTIAL

Osmosis ■ Osmosis is the net movement of water molecules across a partially permeable membrane. ■ Water molecules move randomly with a certain amount of kinetic energy.

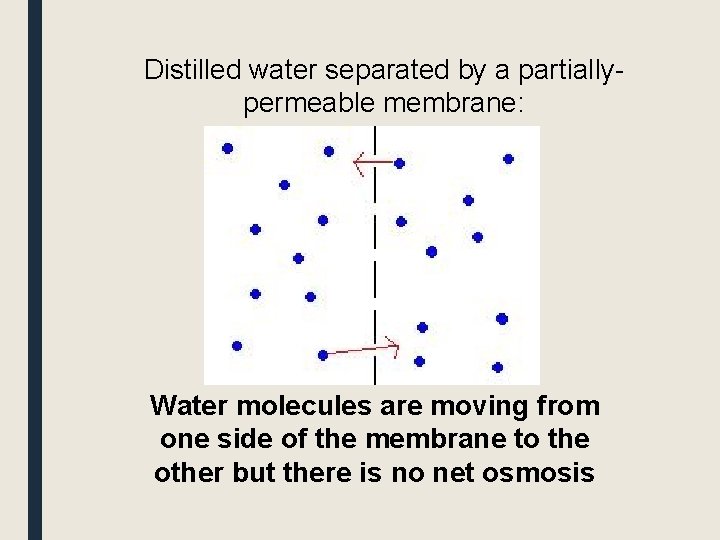
Distilled water separated by a partiallypermeable membrane: Water molecules are moving from one side of the membrane to the other but there is no net osmosis

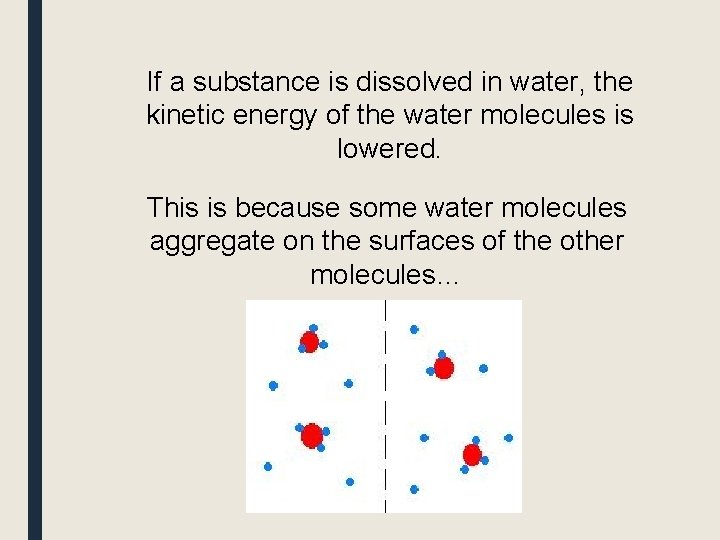
If a substance is dissolved in water, the kinetic energy of the water molecules is lowered. This is because some water molecules aggregate on the surfaces of the other molecules…

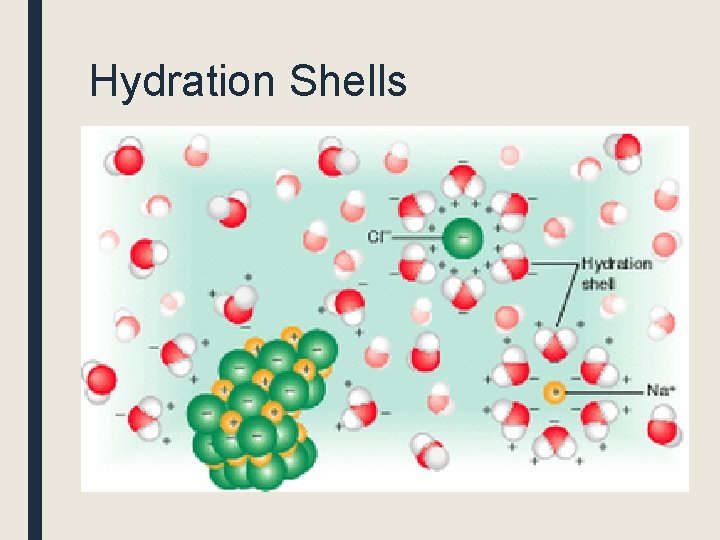
Hydration Shells

For osmosis we talk about the potential water molecules have to move – the water POTENTIAL. Distilled water has the highest potential (zero). When water has another substance dissolved in it, the water molecules have less potential to move. The osmotic potential is NEGATIVE.

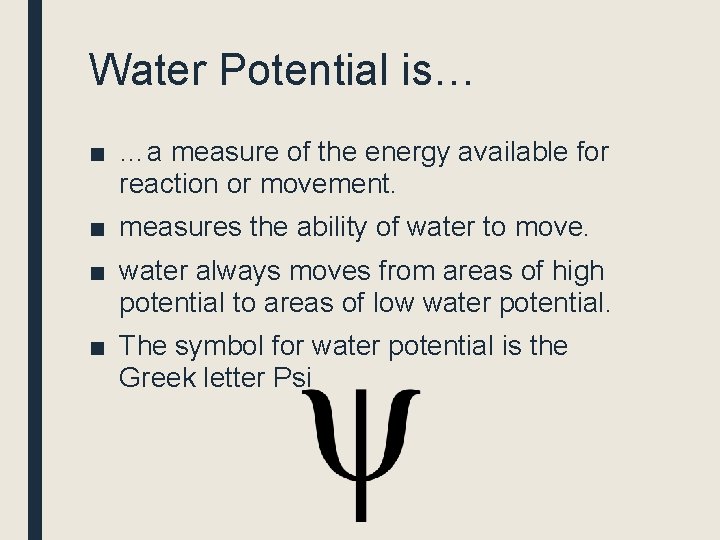
Water Potential is… ■ …a measure of the energy available for reaction or movement. ■ measures the ability of water to move. ■ water always moves from areas of high potential to areas of low water potential. ■ The symbol for water potential is the Greek letter Psi

Water Potential ■ Two components: – Osmotic potential (due to solutes) – Pressure potential (due to turgor pressure).

Calculating WP ■ water potential is just the sum of the pressure and osmotic components. ■ Water Potential = Osmotic Potential + Pressure Potential ■ Pure water always flows to the lower potential, so, WP must be negative (lower than zero) for any water containing solutes.

Some Basic Principals ■ Water always moves from high water potential to low water potential. ■ Distilled water in an open beaker has a water potential of 0(zero). ■ The addition of solute decreases water potential. ■ The addition of pressure increases water potential. ■ In cells, water moves by osmosis to areas where water potential is lower. – A hypertonic solution has lower water potential. – A hypotonic solution has higher water potential.

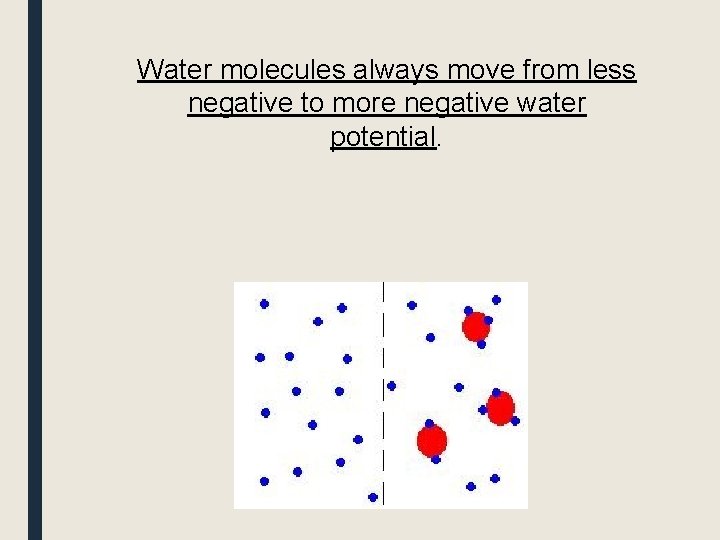
Water molecules always move from less negative to more negative water potential.

An animal cell with water potential – 50 is placed in a solution…

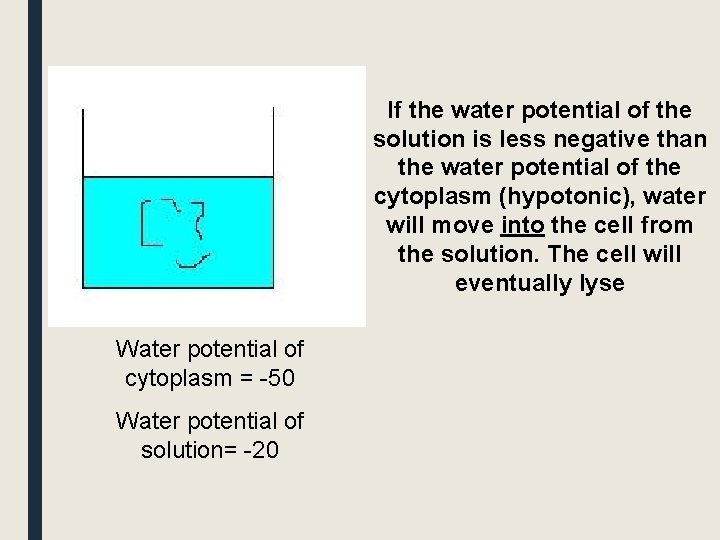
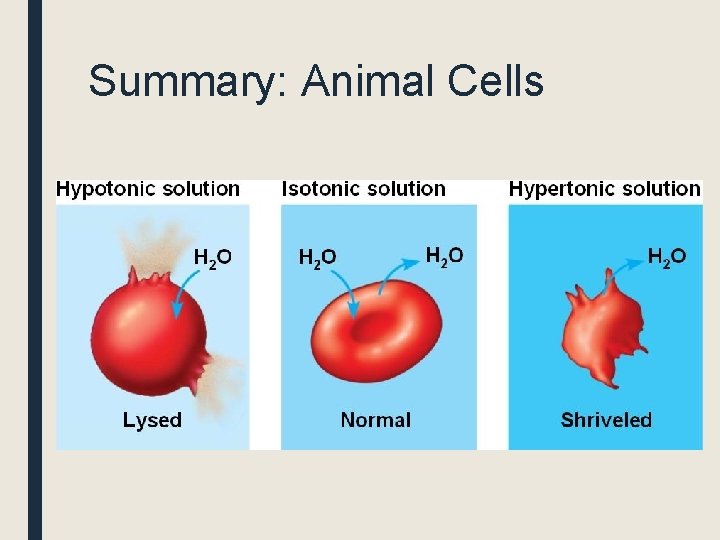
If the water potential of the solution is less negative than the water potential of the cytoplasm (hypotonic), water will move into the cell from the solution. The cell will eventually lyse Water potential of cytoplasm = -50 Water potential of solution= -20

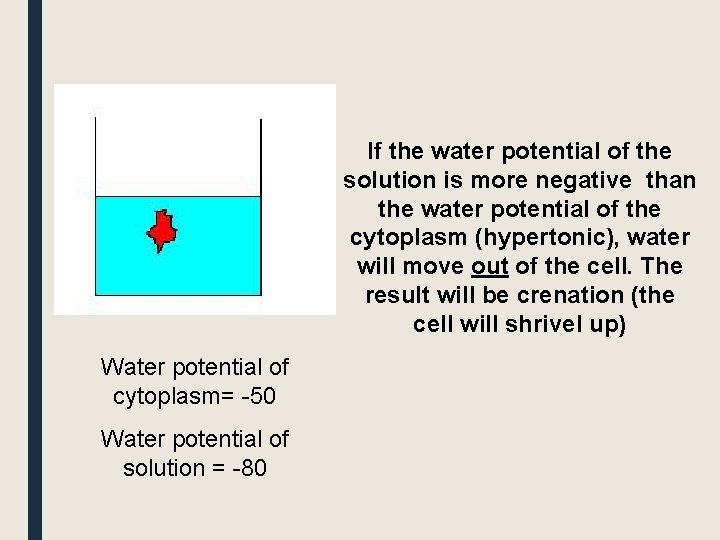
If the water potential of the solution is more negative than the water potential of the cytoplasm (hypertonic), water will move out of the cell. The result will be crenation (the cell will shrivel up) Water potential of cytoplasm= -50 Water potential of solution = -80

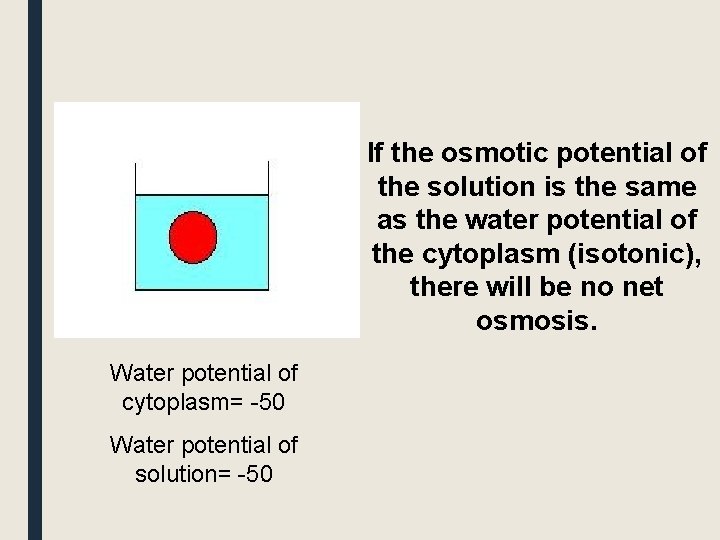
If the osmotic potential of the solution is the same as the water potential of the cytoplasm (isotonic), there will be no net osmosis. Water potential of cytoplasm= -50 Water potential of solution= -50

Summary: Animal Cells

In animal cells, the water potential is equal to the osmotic potential of the cytoplasm, but this is different in plant cells… Plant cells have a cell wall, which exerts an inward pressure when the cell is turgid. This is known as the pressure potential. The water potential of an plant cell is equal to the osmotic potential of the cytoplasm plus the cell wall pressure: W. P. = O. P. + P. P.

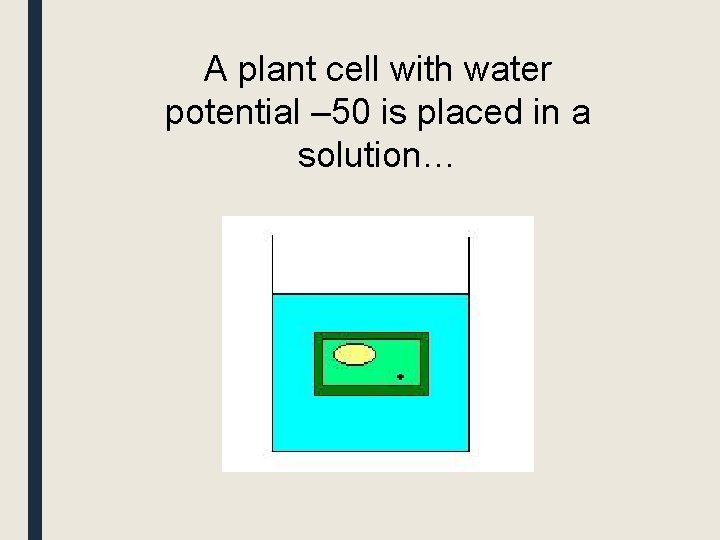
A plant cell with water potential – 50 is placed in a solution…

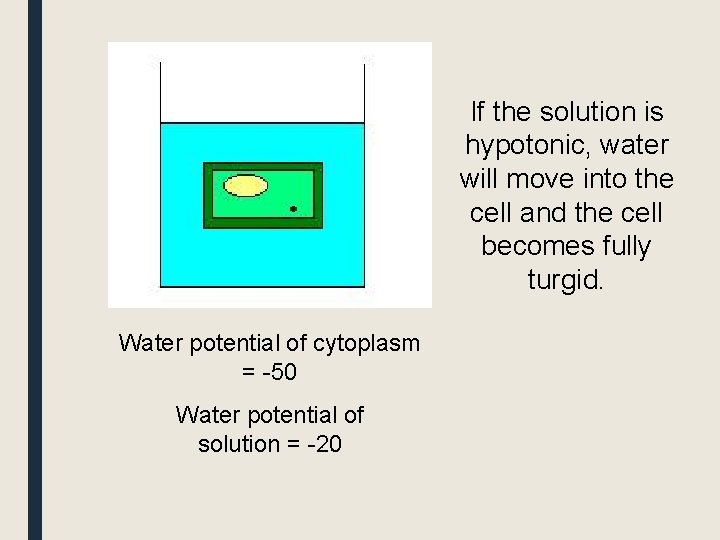
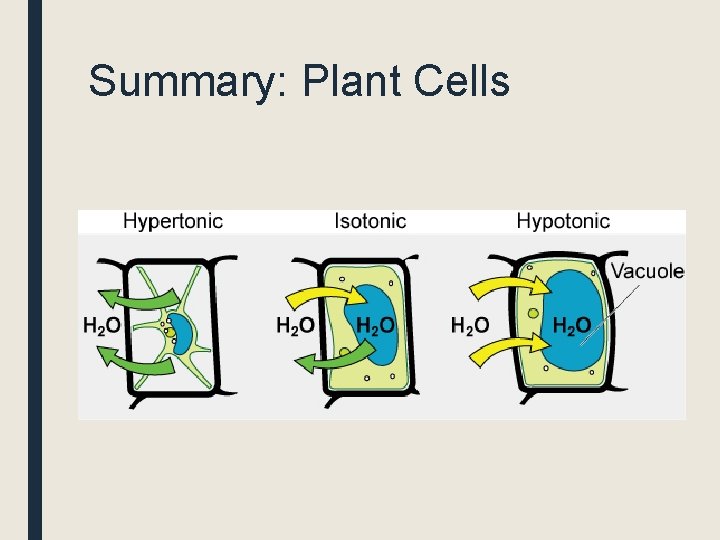
If the solution is hypotonic, water will move into the cell and the cell becomes fully turgid. Water potential of cytoplasm = -50 Water potential of solution = -20

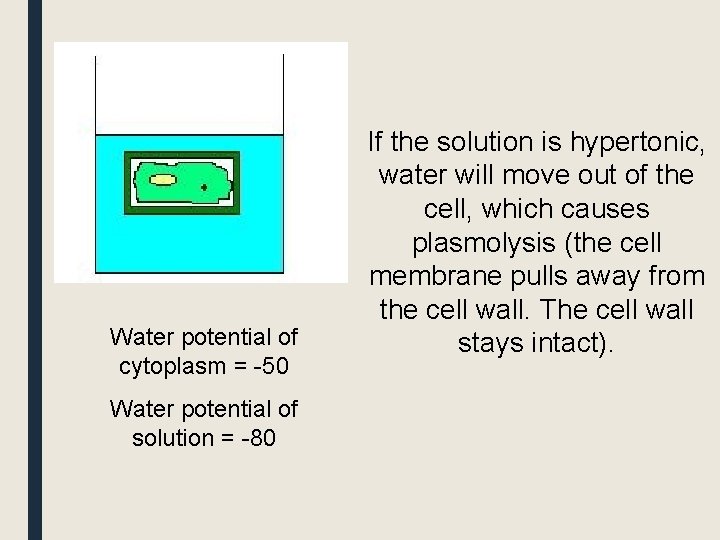
Water potential of cytoplasm = -50 Water potential of solution = -80 If the solution is hypertonic, water will move out of the cell, which causes plasmolysis (the cell membrane pulls away from the cell wall. The cell wall stays intact).

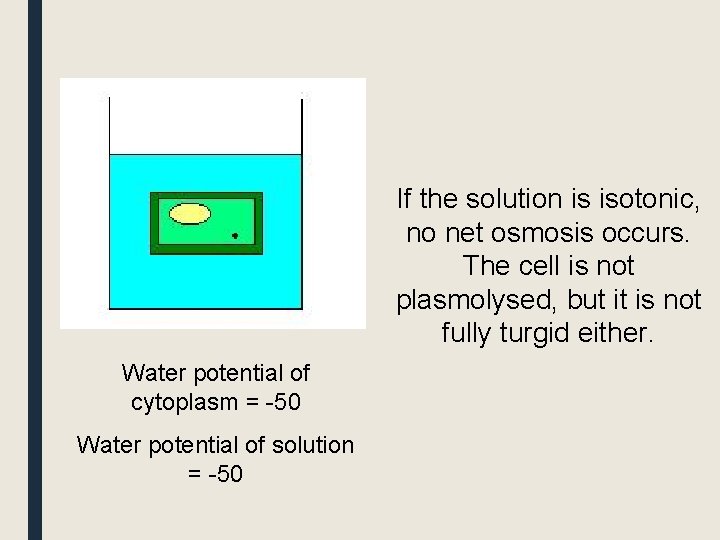
If the solution is isotonic, no net osmosis occurs. The cell is not plasmolysed, but it is not fully turgid either. Water potential of cytoplasm = -50 Water potential of solution = -50

Summary: Plant Cells

Some Review ■ The following are p. H values: cola-2; orange juice-3; beer-4; coffee-5; human blood-7. 4. Which of these liquids has the highest molar concentration of OH-? a. cola b. orange juice c. beer d. coffee e. human blood

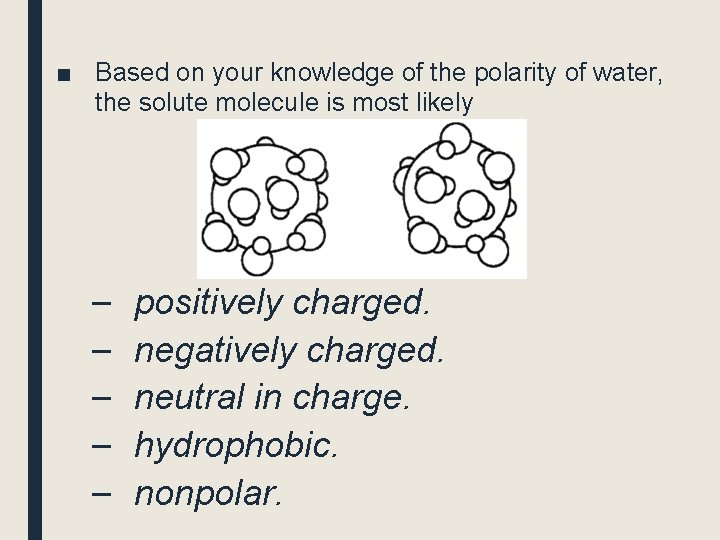
■ Based on your knowledge of the polarity of water, the solute molecule is most likely – – – positively charged. negatively charged. neutral in charge. hydrophobic. nonpolar.

■ If the p. H of a solution is increased from p. H 8 to p. H 9, it means that the – concentration of H+ is 10 times greater than what it was at p. H 8. – concentration of H+ is 100 times less than what it was at p. H 8. – concentration of OH- is 10 times greater than what it was at p. H 8. – concentration of OH- is 100 times less than what it was at p. H 8. – concentration of H+ is greater and the concentration of OH- is less than at p. H 8.