OSMOSIS and DIFFUSION Molecules are always moving Molecules

![Diffusion concentrated, high energy molecules [High] [Low] diffuse, low energy molecules Diffusion concentrated, high energy molecules [High] [Low] diffuse, low energy molecules](https://slidetodoc.com/presentation_image_h2/130ed681ad4d0955c460fe05be83cfee/image-6.jpg)





- Slides: 33

OSMOSIS and DIFFUSION

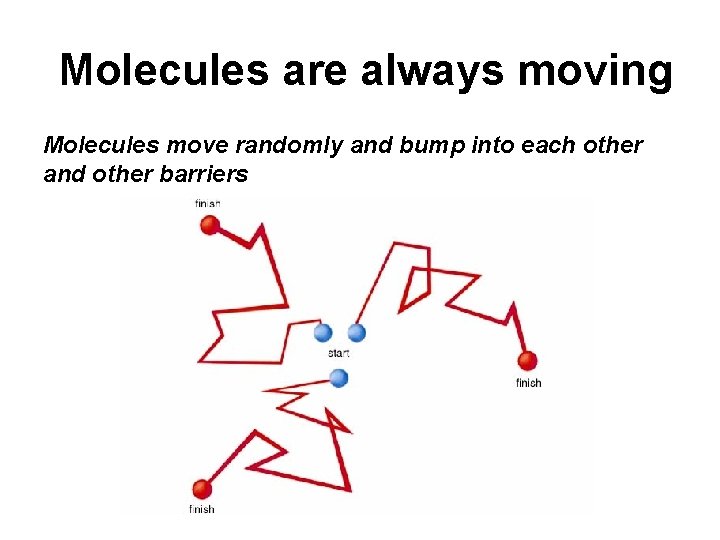
Molecules are always moving Molecules move randomly and bump into each other and other barriers

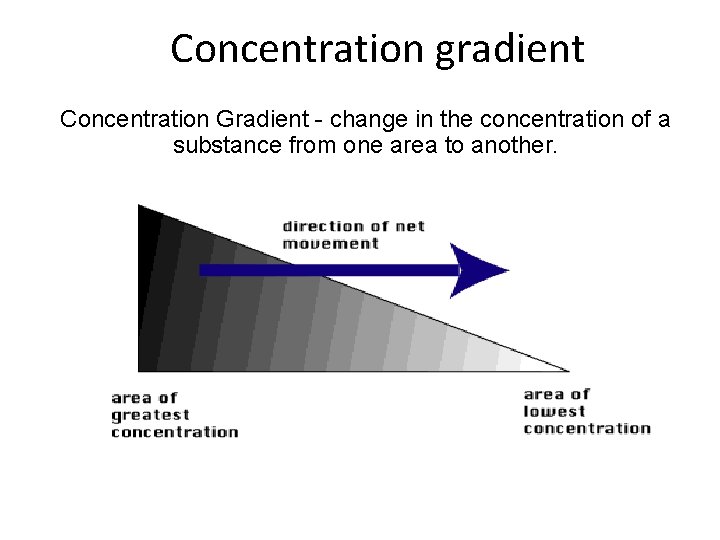
Concentration gradient Concentration Gradient - change in the concentration of a substance from one area to another.

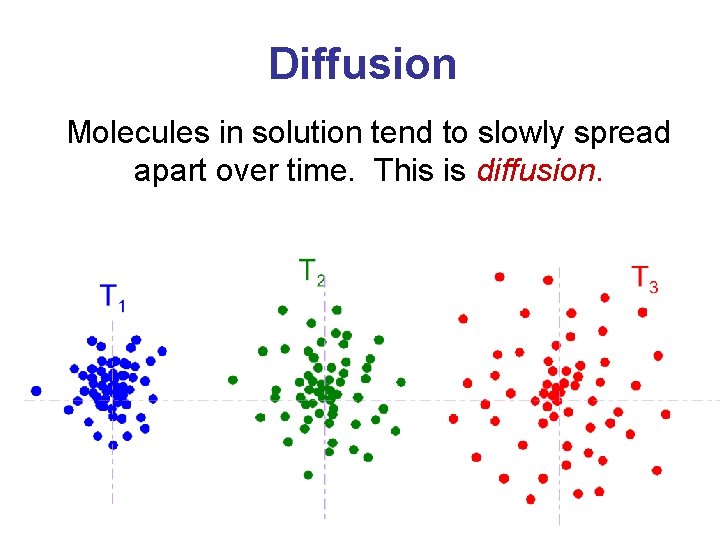
Diffusion Molecules in solution tend to slowly spread apart over time. This is diffusion.

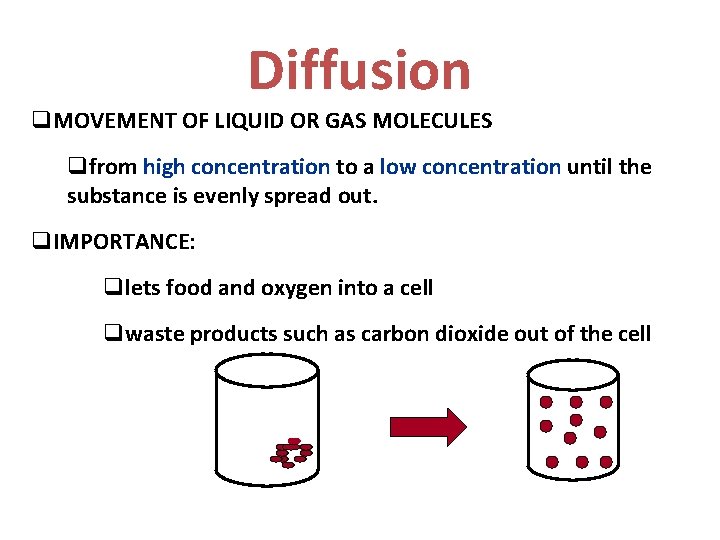
Diffusion q. MOVEMENT OF LIQUID OR GAS MOLECULES qfrom high concentration to a low concentration until the substance is evenly spread out. q. IMPORTANCE: qlets food and oxygen into a cell qwaste products such as carbon dioxide out of the cell
![Diffusion concentrated high energy molecules High Low diffuse low energy molecules Diffusion concentrated, high energy molecules [High] [Low] diffuse, low energy molecules](https://slidetodoc.com/presentation_image_h2/130ed681ad4d0955c460fe05be83cfee/image-6.jpg)
Diffusion concentrated, high energy molecules [High] [Low] diffuse, low energy molecules

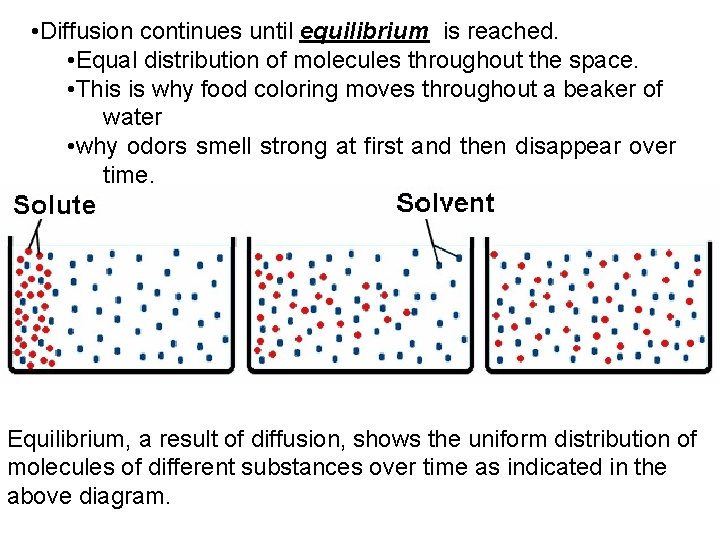
• Diffusion continues until equilibrium is reached. • Equal distribution of molecules throughout the space. • This is why food coloring moves throughout a beaker of water • why odors smell strong at first and then disappear over time. Equilibrium, a result of diffusion, shows the uniform distribution of molecules of different substances over time as indicated in the above diagram.

Definition of Osmosis • Osmosis is the movement of water molecules, • from a region of high water concentration LEARN THIS !!!! • to a region of low water concentration • ACROSS A SELECTIVELY PERMEABLE MEMBRANE.

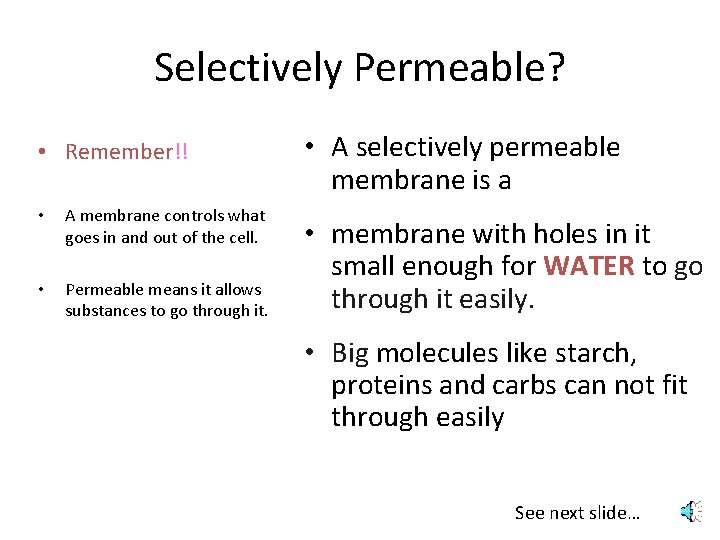
Selectively Permeable? • Remember!! • A membrane controls what goes in and out of the cell. • Permeable means it allows substances to go through it. • A selectively permeable membrane is a • membrane with holes in it small enough for WATER to go through it easily. • Big molecules like starch, proteins and carbs can not fit through easily See next slide…

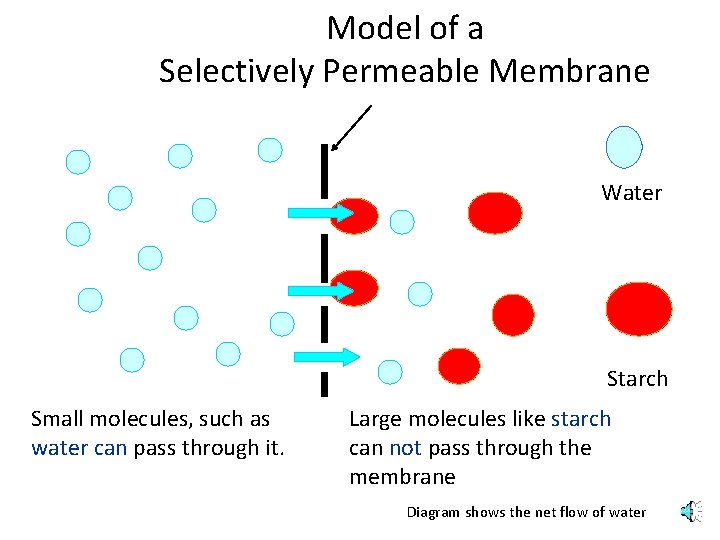
Model of a Selectively Permeable Membrane Water Starch Small molecules, such as water can pass through it. Large molecules like starch can not pass through the membrane Diagram shows the net flow of water

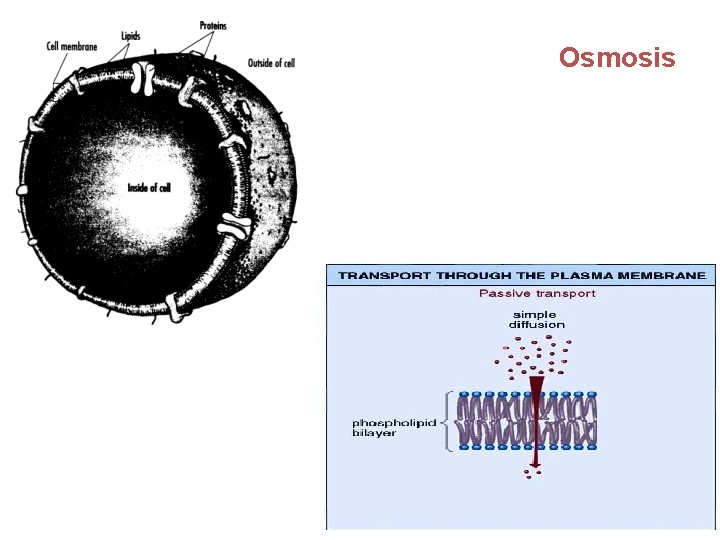
Osmosis

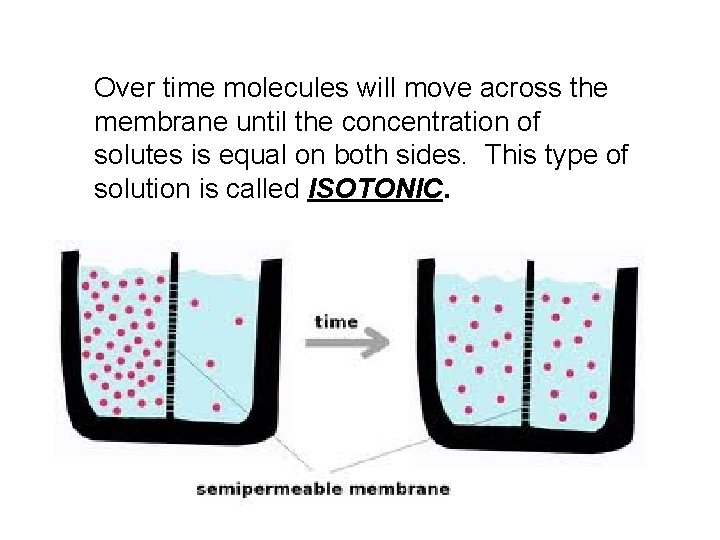
Over time molecules will move across the membrane until the concentration of solutes is equal on both sides. This type of solution is called ISOTONIC.

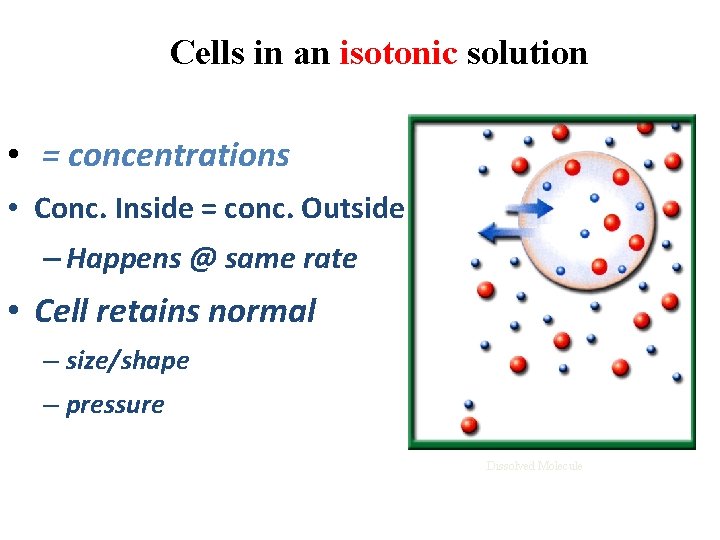
Cells in an isotonic solution • = concentrations • Conc. Inside = conc. Outside – Happens @ same rate H 2 O • Cell retains normal – size/shape – pressure Water Molecule Dissolved Molecule

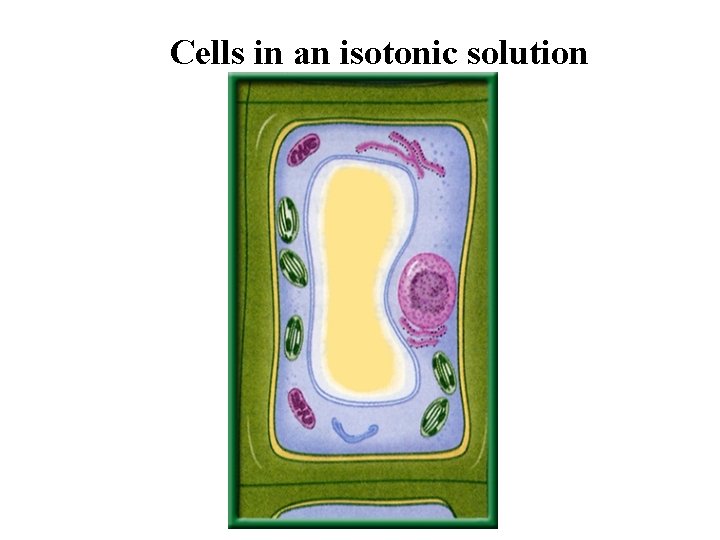
Cells in an isotonic solution

PASSIVE TRANSPORT qoccurs without expenditure of energy q. NO ENERGY IS USED BY THE CELL qmove using their own kinetic energy q. Particles go DOWN OR WITH their concentration gradient. q. Diffusion and osmosis are PT qallows cells to get water, oxygen and other small molecules that they need qallows the cell to get rid of waste such as carbon dioxide.

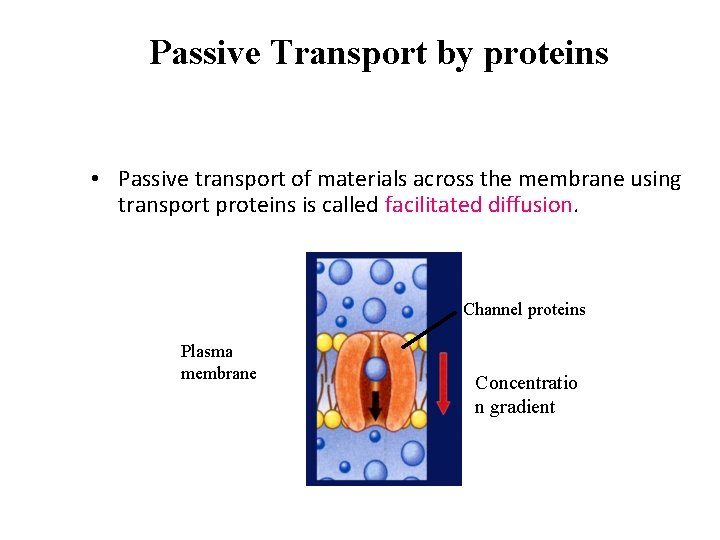
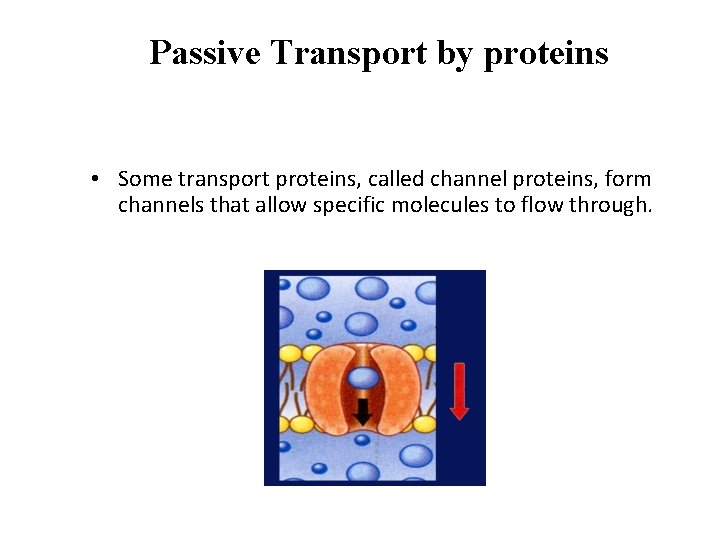
Passive Transport by proteins • Passive transport of materials across the membrane using transport proteins is called facilitated diffusion. Channel proteins Plasma membrane Concentratio n gradient

Passive Transport by proteins • Some transport proteins, called channel proteins, form channels that allow specific molecules to flow through.

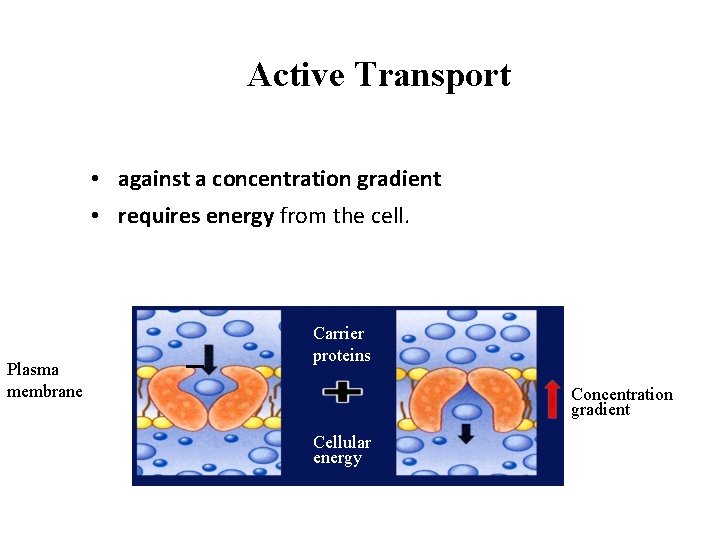
Active Transport • against a concentration gradient • requires energy from the cell. Cellular energy Plasma membrane Carrier proteins Concentration gradient Cellular energy

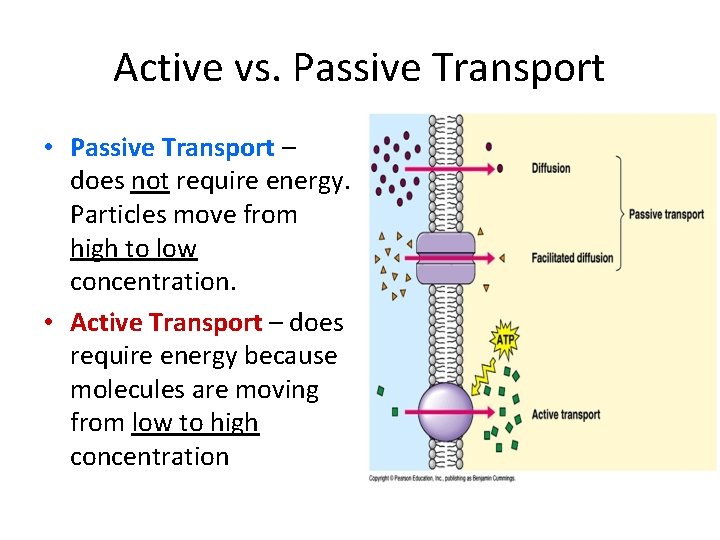
Active vs. Passive Transport • Passive Transport – does not require energy. Particles move from high to low concentration. • Active Transport – does require energy because molecules are moving from low to high concentration

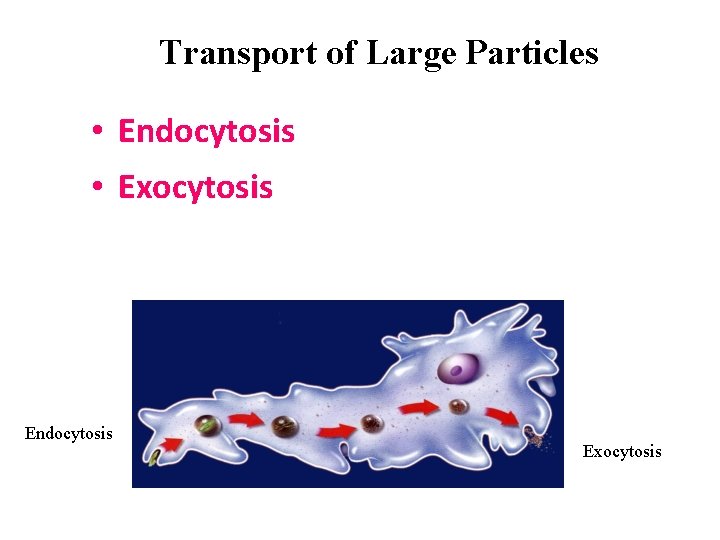
Transport of Large Particles • Endocytosis • Exocytosis Endocytosis Exocytosis

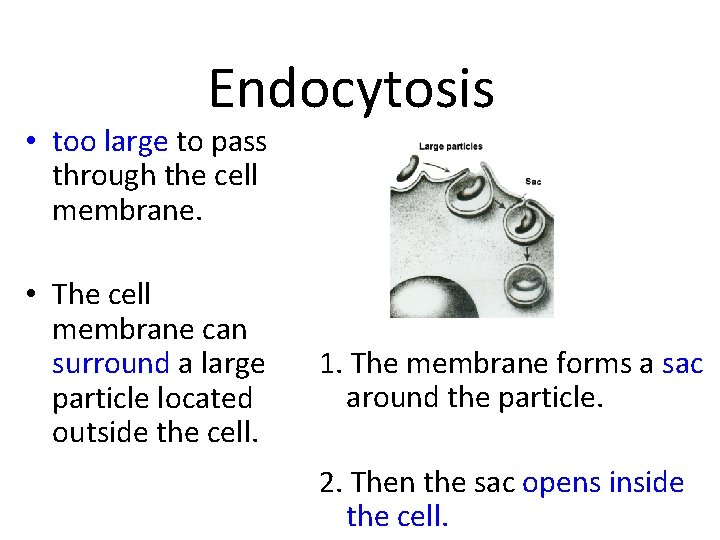
Endocytosis • too large to pass through the cell membrane. • The cell membrane can surround a large particle located outside the cell. 1. The membrane forms a sac around the particle. 2. Then the sac opens inside the cell.

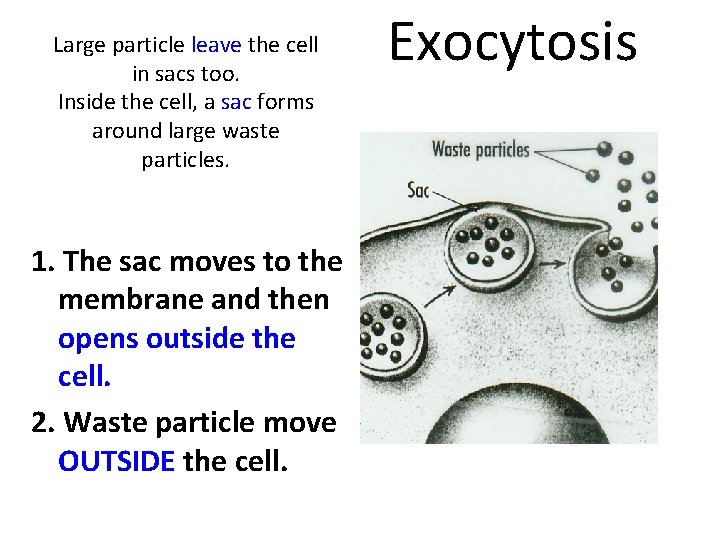
Large particle leave the cell in sacs too. Inside the cell, a sac forms around large waste particles. 1. The sac moves to the membrane and then opens outside the cell. 2. Waste particle move OUTSIDE the cell. Exocytosis

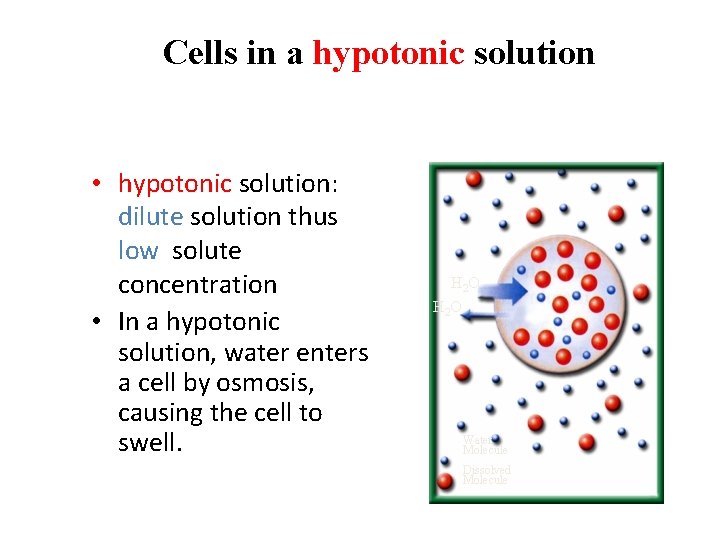
Cells in a hypotonic solution • hypotonic solution: dilute solution thus low solute concentration • In a hypotonic solution, water enters a cell by osmosis, causing the cell to swell. H 2 O Water Molecule Dissolved Molecule

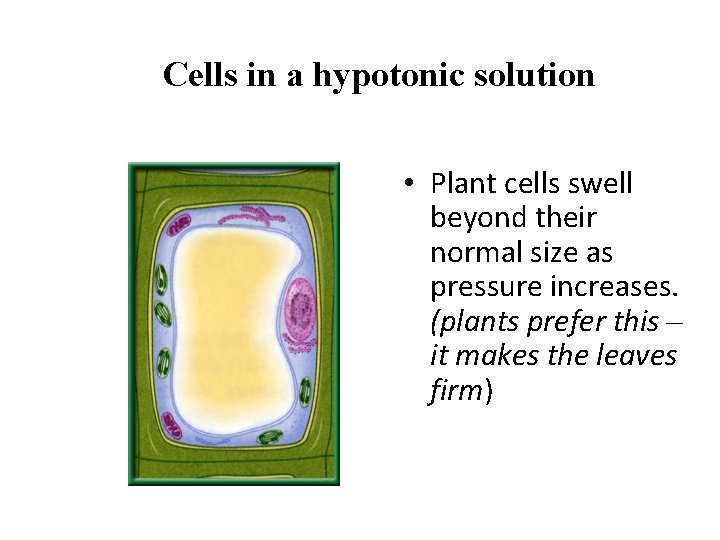
Cells in a hypotonic solution • Plant cells swell beyond their normal size as pressure increases. (plants prefer this – it makes the leaves firm)

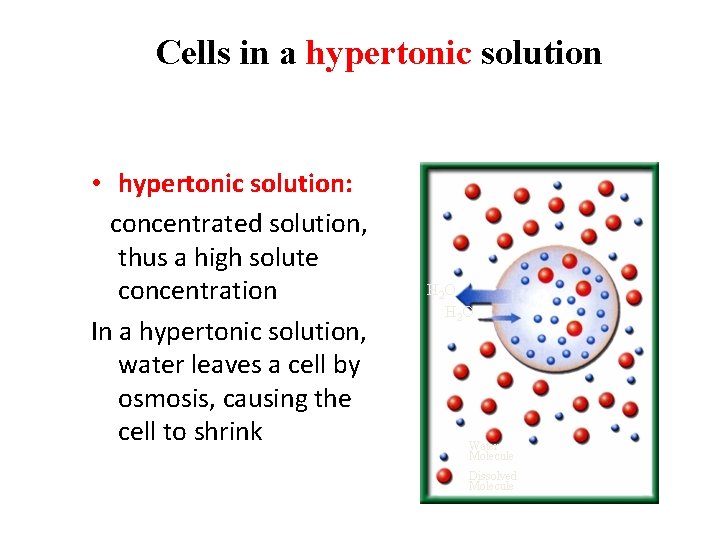
Cells in a hypertonic solution • hypertonic solution: concentrated solution, thus a high solute concentration In a hypertonic solution, water leaves a cell by osmosis, causing the cell to shrink H 2 O Water Molecule Dissolved Molecule

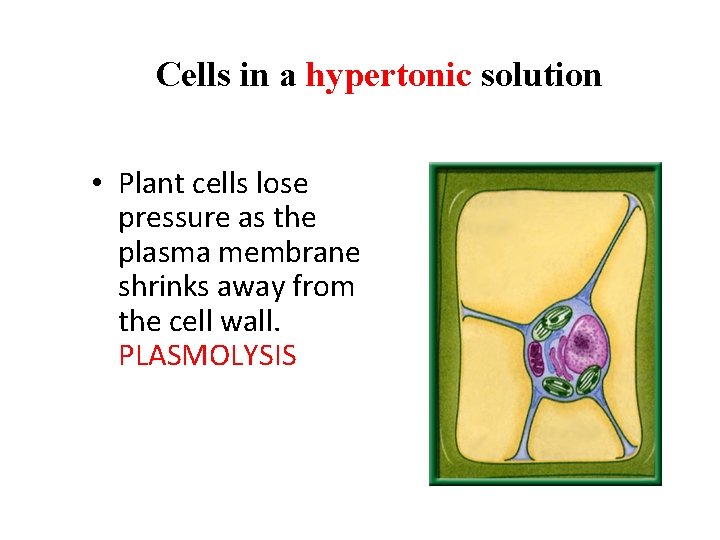
Cells in a hypertonic solution • Plant cells lose pressure as the plasma membrane shrinks away from the cell wall. PLASMOLYSIS

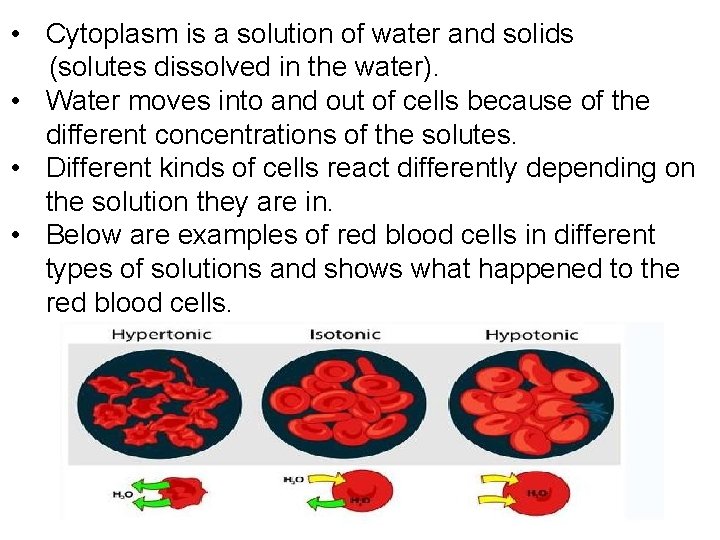
• Cytoplasm is a solution of water and solids (solutes dissolved in the water). • Water moves into and out of cells because of the different concentrations of the solutes. • Different kinds of cells react differently depending on the solution they are in. • Below are examples of red blood cells in different types of solutions and shows what happened to the red blood cells.

Animal Cells in different solutions • A red blood cell in pure water, water will move into the cell and the cell will BURST (there is no cell wall to prevent this happening) • In a strong salt/sugar solution water will move out and the cell will SHRINK (the cell in described as CRENATED)

PLANT CELLS Hypotonic Solution Turgor Pressure builds in the cell and causes osmosis to stop because of the rigid cell wall. Hypertonic Solution Plants will wilt when cells lose water through osmosis.

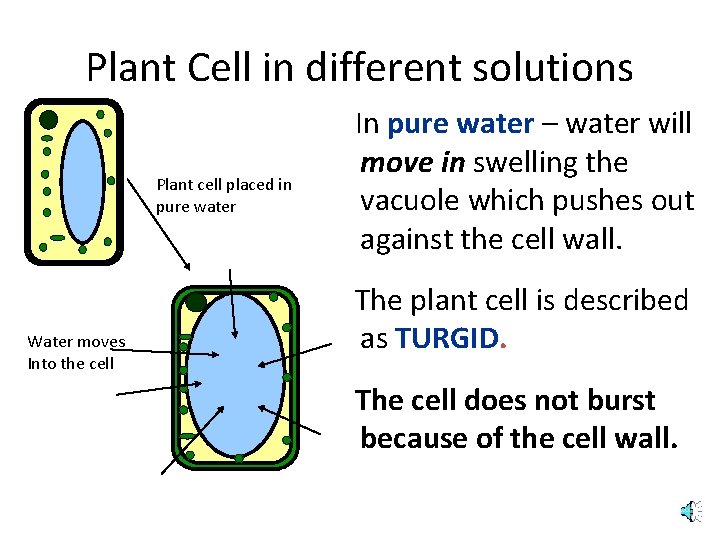
Plant Cell in different solutions Plant cell placed in pure water Water moves Into the cell In pure water – water will move in swelling the vacuole which pushes out against the cell wall. The plant cell is described as TURGID. The cell does not burst because of the cell wall.

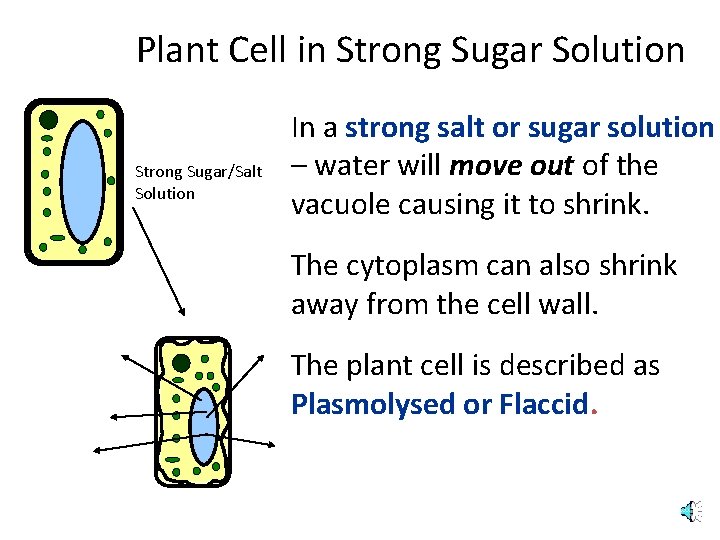
Plant Cell in Strong Sugar Solution Strong Sugar/Salt Solution In a strong salt or sugar solution – water will move out of the vacuole causing it to shrink. The cytoplasm can also shrink away from the cell wall. The plant cell is described as Plasmolysed or Flaccid.

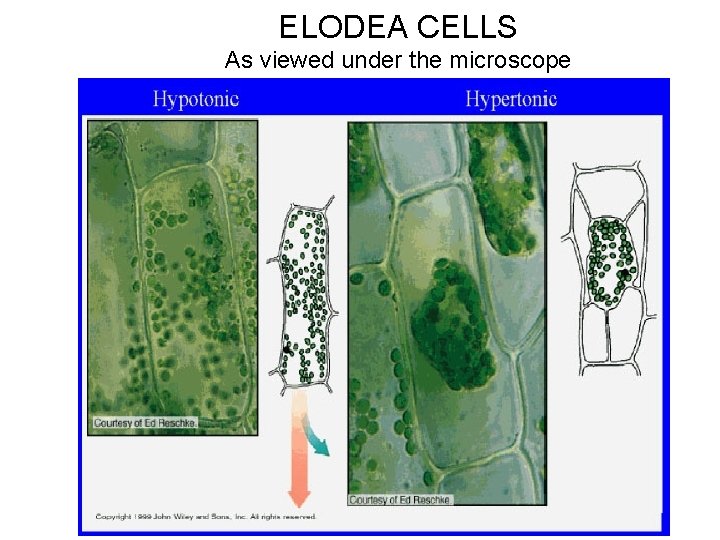
ELODEA CELLS As viewed under the microscope

THE END