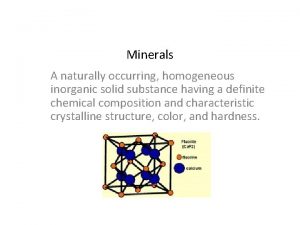
Orthosilicates n n Isolated tetrahedron Common examples Olivine






















- Slides: 41

Orthosilicates n n Isolated tetrahedron Common examples Olivine, garnet, and zircon n Al 2 Si. O 5 polymorphs, staurolite, topaz, titanite n Oxygen coordinate with other anions n

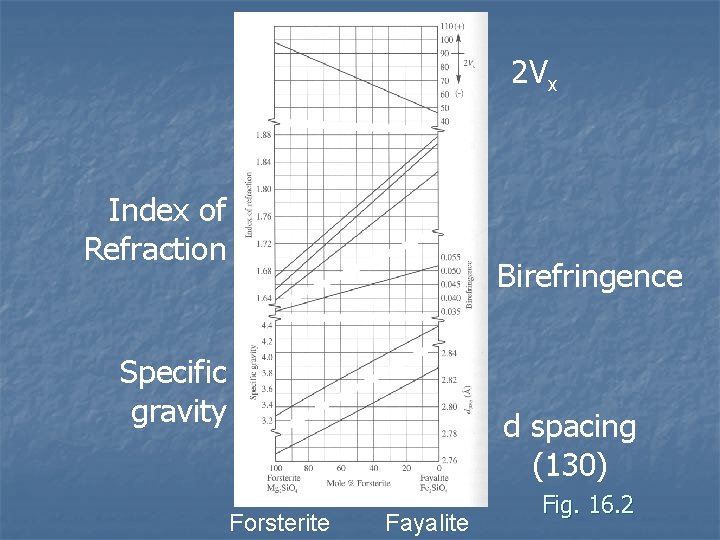
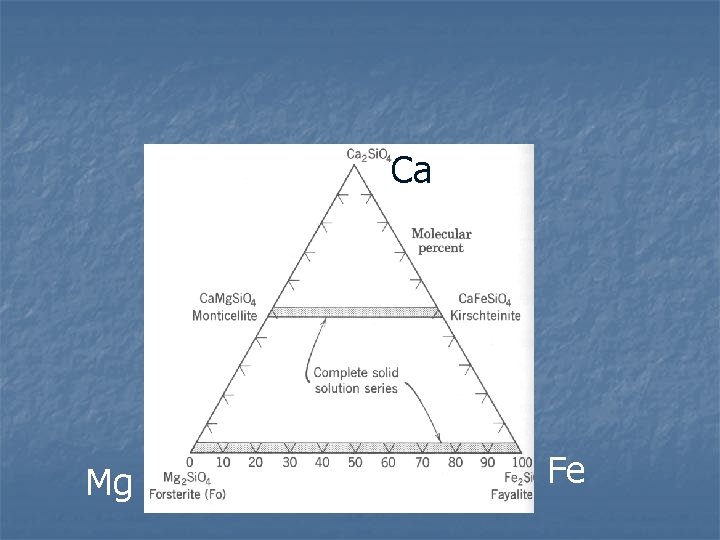
Olivine Composition n Complete solid solution between forsterite (Mg) and fayalite (Fe) Mn end members as well – rare n Ca can be around 50% of cations, still has Fe. Mg solid solution n n Fe and Mg contents cause variations in physical properties Can be used to identify composition Zoning can be common

2 Vx Index of Refraction Birefringence Specific gravity d spacing (130) Forsterite Fayalite Fig. 16. 2

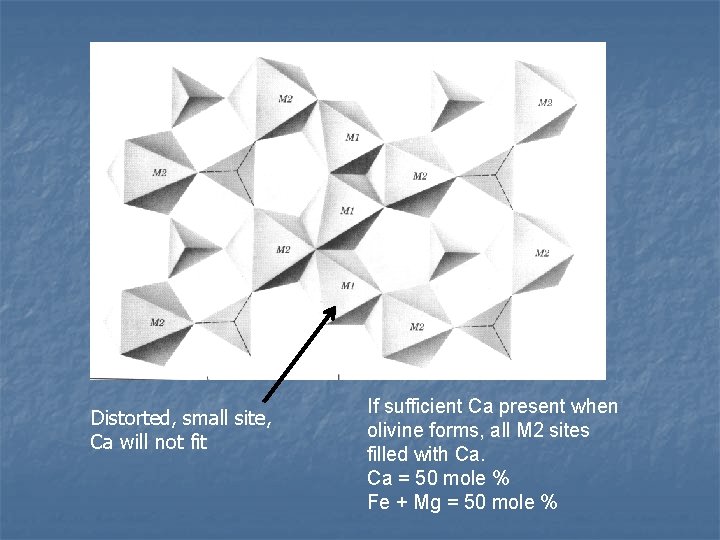
Structure and composition n Two distinct sites for cations: M 1 = distorted, so smaller than M 2 = regular octahedron n n Controls distribution of cations M 2 only site for Ca, 1. 12 Å, also may hold Fe and Mg n M 1 and M 2 n n both Fe = 0. 78 Å and Mg = 0. 72 Å

Distorted, small site, Ca will not fit If sufficient Ca present when olivine forms, all M 2 sites filled with Ca. Ca = 50 mole % Fe + Mg = 50 mole %

Ca Mg Fe

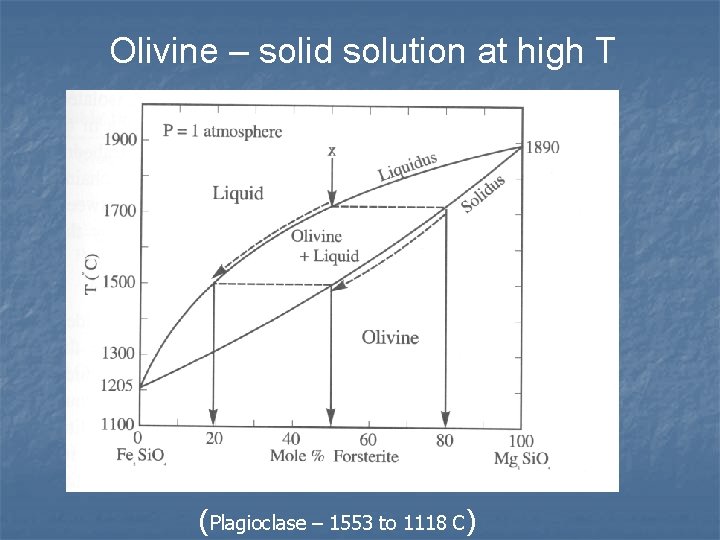
Olivine – solid solution at high T (Plagioclase – 1553 to 1118 C)

Inosilicates (chain) n n Common Fe/Mg – bearing silicates Two common groups Pyroxenes: single chains n Amphiboles: double chains n n n Pyroxenes are common in MORB Amphiboles more common on continents because of weathering

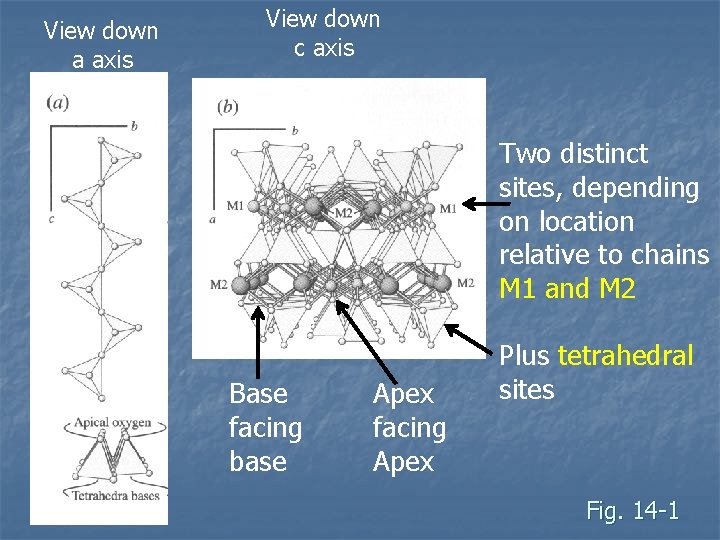
Pyroxene group n n n General formula: XYZ 2 O 6 Z/O ratio = 1/3 Z cations usually Si, occasionally Al Single chain extend along c axis Chains are stacked along a axis, alternating: Base faces base n Apex faces apex n

View down a axis View down c axis Two distinct sites, depending on location relative to chains M 1 and M 2 Base facing base Apex facing Apex Plus tetrahedral sites Fig. 14 -1

XYZ 2 O 6 n n Z/O ratio 1/3 X cations in M 2 sites Between bases of tetrahedrons n Distorted 6 - and 8 - fold coordination n Depends on stacking and the size of the cations n n Y cations in M 1 sites n 6 -fold coordination between apical oxygen

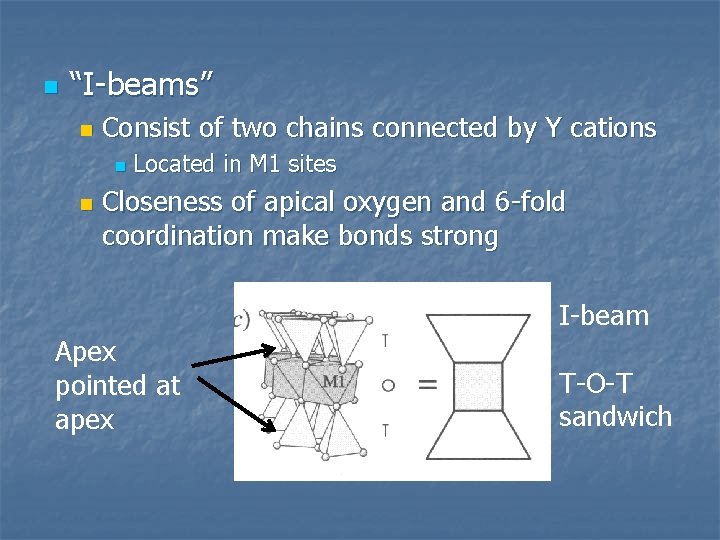
n “I-beams” n Consist of two chains connected by Y cations n n Located in M 1 sites Closeness of apical oxygen and 6 -fold coordination make bonds strong I-beam Apex pointed at apex T-O-T sandwich

n I-beams held together by X cations in M 2 site Coordination number depends on how chains line up n 6 -fold coordination gives orthorhombic symmetry – Orthopyroxenes or OPX n 8 -fold coordination gives monoclinic symmetry – Clinopyroxenes or CPX n

n Crystal shapes Blocky prisms, nearly square n Elongate along c axis n n Cleavage controlled by I-beams Cleavage typically between 87º and 93º n Only when viewed down the c axis n Mineral grain must be cut parallel to (001) n

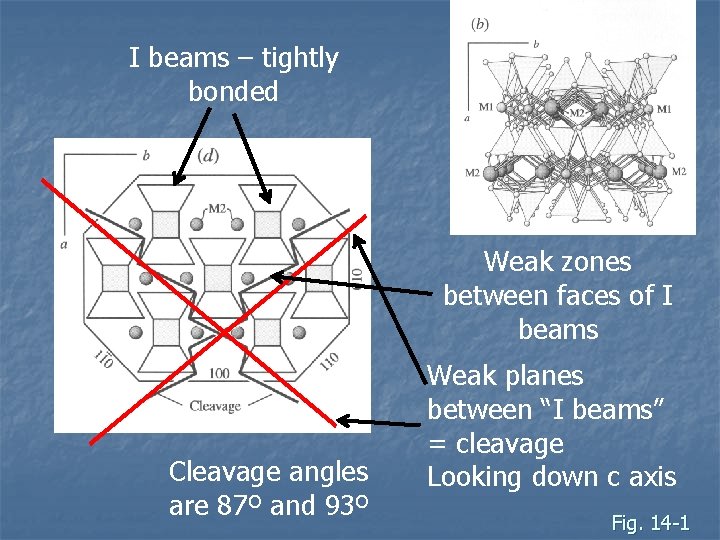
I beams – tightly bonded Weak zones between faces of I beams Cleavage angles are 87º and 93º Weak planes between “I beams” = cleavage Looking down c axis Fig. 14 -1

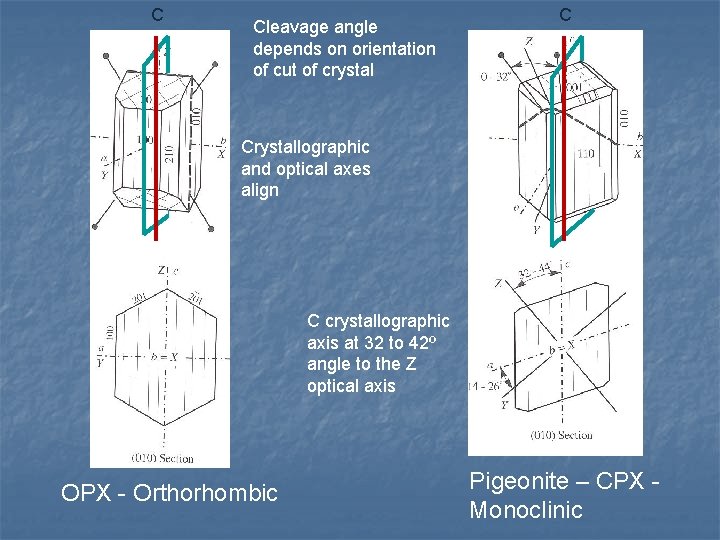
C Cleavage angle depends on orientation of cut of crystal C Crystallographic and optical axes align C crystallographic axis at 32 to 42º angle to the Z optical axis OPX - Orthorhombic Pigeonite – CPX Monoclinic

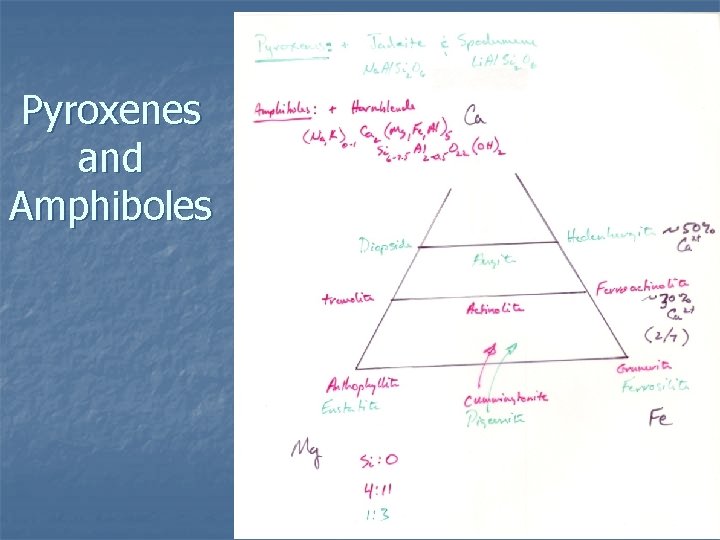
Classification n Based on two linked things Composition: which cations occurs in M 2 sites (facing bases of tetrahedron) n Symmetry: determined by composition n n Most plot on ternary diagram with apices: Wollastonite, Wo (Ca 2+) n Enstatite, En (Mg 2+) n Ferrosilite, Fe (Fe 2+) n

n Three major groups Orthopyroxenes (opx) – orthorhombic n Ca-poor clinopyroxenes (cpx) – monoclinic n Ca-rich clinopyroxenes (cpx) – monoclinic n n The amount of Ca in the mineral controls the crystal system, symmetry, and extinction angle

n Orthopyroxenes: Fe and Mg, but little Ca Both M 1 and M 2 are octahedral n Larger Fe ion more concentrated in M 2 site n n These minerals are the enstatite – ferrosilite solid solution series

n Low-Ca clinopyroxene: more Ca, but no solid solution with Hi-Ca clinopyroxene Mineral species is Pigeonite n Ca restricted to M 2 sites, these still mostly Fe and Mg n M 1 sites all Mg and Fe n

n Ca- clinopyroxene Diopside Mg(+Ca) to Hedenbergite Fe (+Ca) n M 2 site contains mostly Ca n M 1 site contains mostly Fe and Mg n n Most common specie is augite Al can substitute in M 1 site, and for Si in tetrahedral site n Na, Fe or Mg can substitute for Ca in M 2 site n

n n Other common pyroxenes Don’t fall neatly on Ca-Fe-Mg ternary diagram: Jadeite Na. Al. Si 2 O 6 n Spodumene Li. Al. Si 2 O 6 n

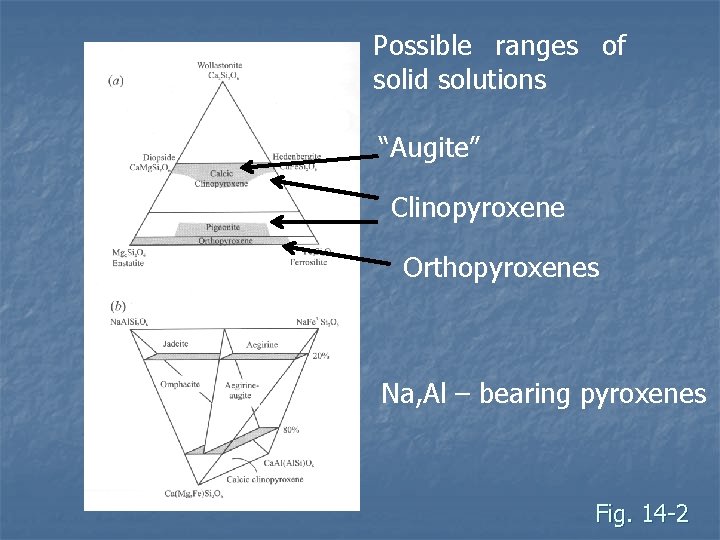
Possible ranges of solid solutions “Augite” Clinopyroxene Orthopyroxenes Na, Al – bearing pyroxenes Fig. 14 -2

Amphibole Group n n n Structure, composition, and classification similar to pyroxenes Primary difference is they are double chains Z/O ratio is 4/11

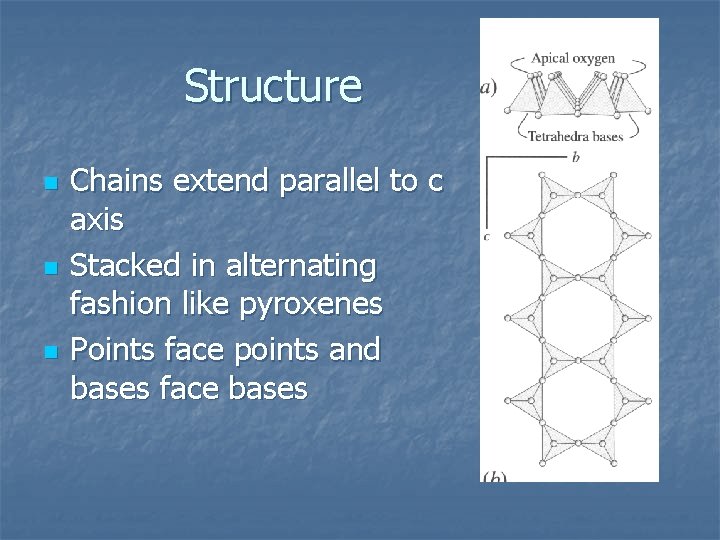
Structure n n n Chains extend parallel to c axis Stacked in alternating fashion like pyroxenes Points face points and bases face bases

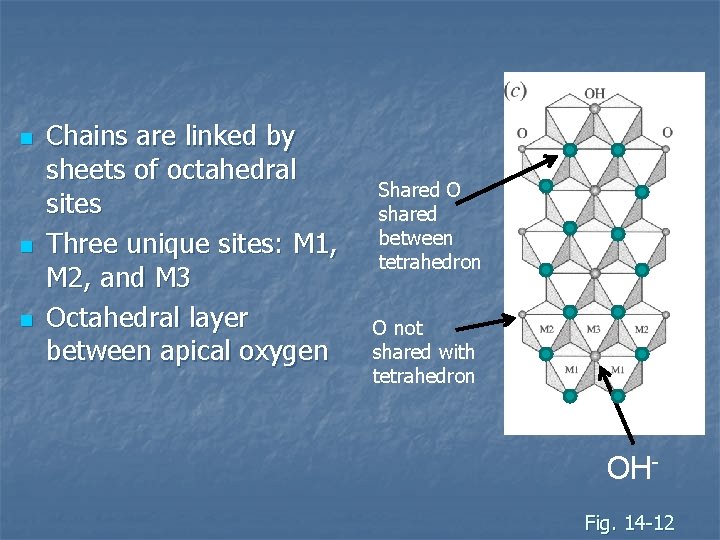
n n n Chains are linked by sheets of octahedral sites Three unique sites: M 1, M 2, and M 3 Octahedral layer between apical oxygen Shared O shared between tetrahedron O not shared with tetrahedron OHFig. 14 -12

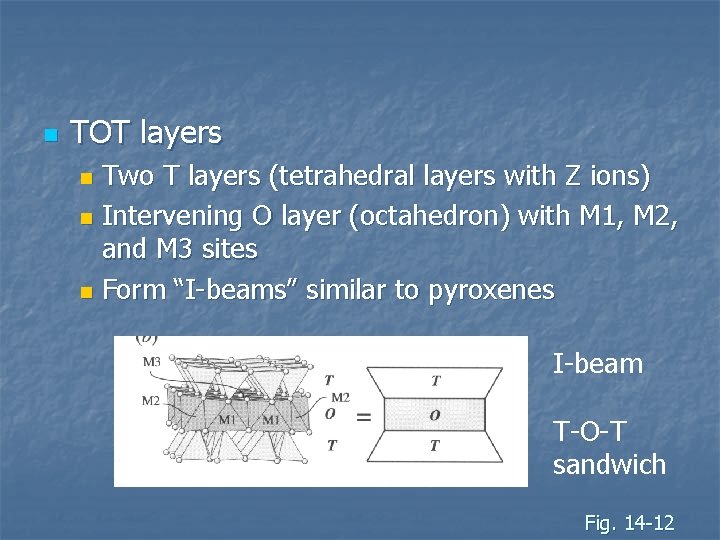
n TOT layers Two T layers (tetrahedral layers with Z ions) n Intervening O layer (octahedron) with M 1, M 2, and M 3 sites n Form “I-beams” similar to pyroxenes n I-beam T-O-T sandwich Fig. 14 -12

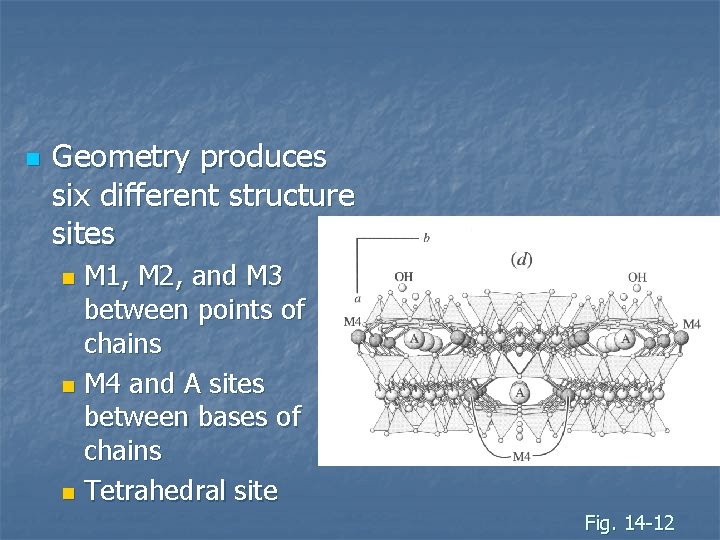
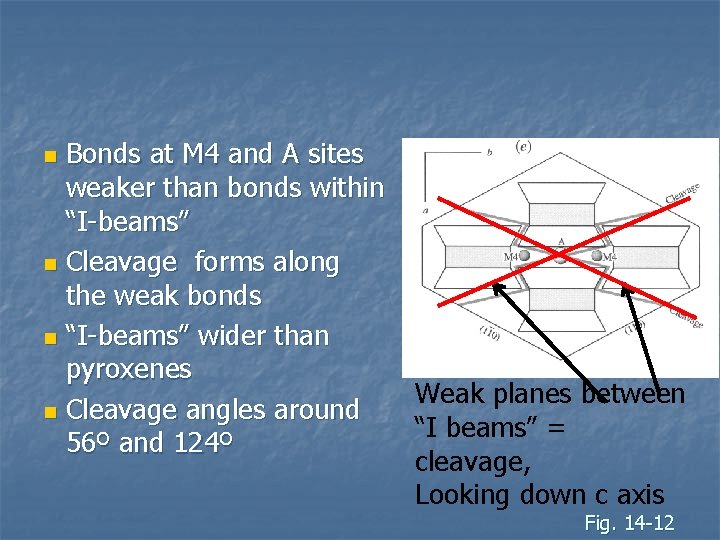
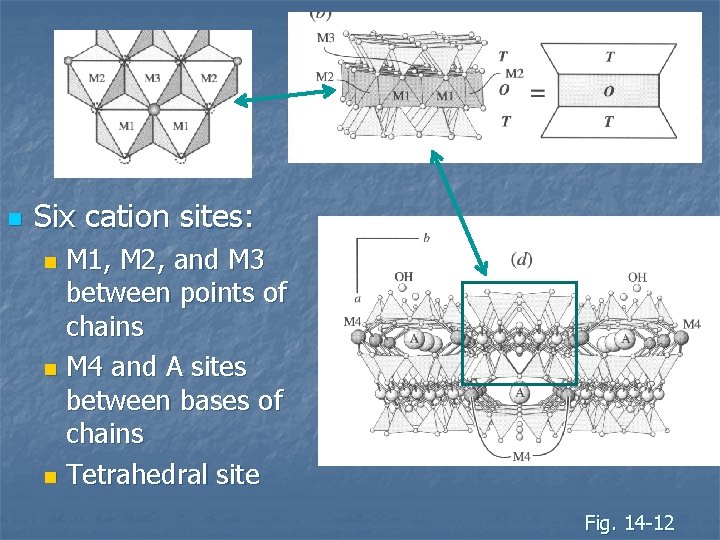
n Geometry produces six different structure sites M 1, M 2, and M 3 between points of chains n M 4 and A sites between bases of chains n Tetrahedral site n Fig. 14 -12

Bonds at M 4 and A sites weaker than bonds within “I-beams” n Cleavage forms along the weak bonds n “I-beams” wider than pyroxenes n Cleavage angles around 56º and 124º n Weak planes between “I beams” = cleavage, Looking down c axis Fig. 14 -12

n Six cation sites: M 1, M 2, and M 3 between points of chains n M 4 and A sites between bases of chains n Tetrahedral site n Fig. 14 -12

Composition W 0 -1 X 2 Y 5 Z 8 O 22(OH)2 n n n Note: Z/O ratio 4/11 Each cation fits a particular site W cation Occurs in A site n Has ~10 fold coordination n Generally large, usually Na+ n

W 0 -1 X 2 Y 5 Z 8 O 22(OH)2 n X cations Located in M 4 sites n Analogous to M 2 sites in pyroxenes n Have 6 or 8 fold coordination depending on arrangement of chains n If 8 -fold, X usually Ca n If 6 -fold, X usually Fe or Mg n

W 0 -1 X 2 Y 5 Z 8 O 22(OH)2 n Y cations Located in M 1, M 2, and M 3 sites; Octahedral cations in TOT strips n Similar to M 1 sites in pyroxenes n Usually Mg, Fe 2+, Fe 3+, Al n n Z cations n Usually Si and Al

W 0 -1 X 2 Y 5 Z 8 O 22(OH)2 n Water – hydrous phase Form from magma that contains water n Form from weathering of pyroxenes at surface n

n Composition Most common amphiboles shown on ternary diagram n Wide variety of substitution, simple and coupled n Divided into ortho and clino amphiboles n Depends on X cations in M 4 site (largely amount of Ca), distorts structure n Reduces symmetry from orthorhombic to monoclinic n

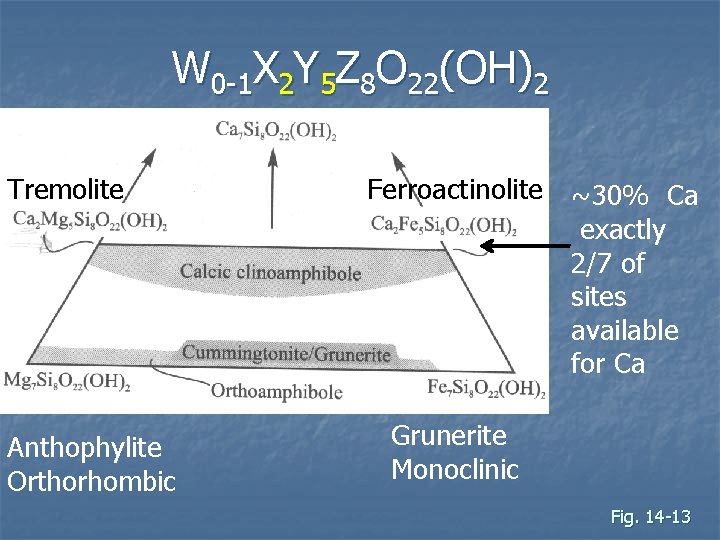
W 0 -1 X 2 Y 5 Z 8 O 22(OH)2 Tremolite Anthophylite Orthorhombic Ferroactinolite ~30% Ca exactly 2/7 of sites available for Ca Grunerite Monoclinic Fig. 14 -13

Pyroxenes and Amphiboles

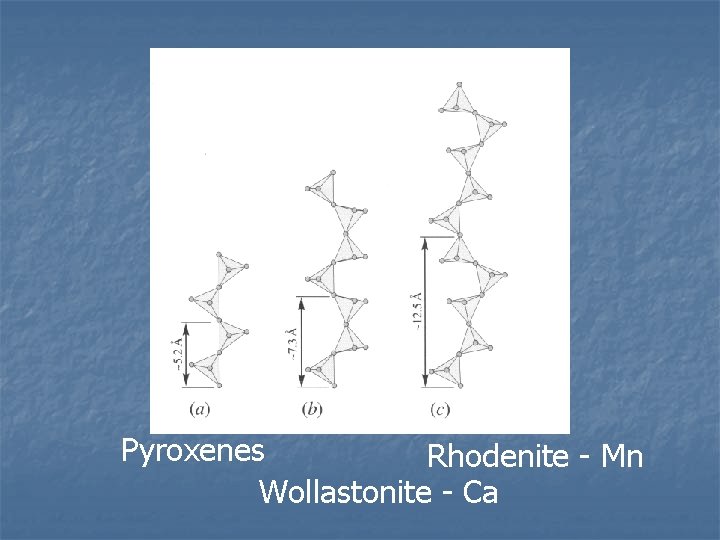
Pyroxenoid Group n Similar to pyroxenes Single chains n Z/O ratio 1/3 n n Differ in repeat distance along c axis Pyroxene – 2 tetrahedron repeat (5. 2 Å) n Pyroxenoid – 3 or more repeat (more than 7. 3 Å) n Difference is the pyroxenes are straight pyroxenoids are kinked n n Cased by larger linking cations

Pyroxenes Rhodenite - Mn Wollastonite - Ca

n n Only a few minerals Most common have Ca, Mn, or Ca plus Na filling the M 1 and M 2 sites Wollastonite – Ca, fairly common, metamorphosed qtz and carbonate systems n Rhodonite – Mn n Pectolite – Ca and Na n

n Wollastonite Composition: Ca with some Mn and Fe substitution n Common in altered carbonate rocks, particularly with reaction with qtz n Useful industrial mineral, replacing asbestos, also used in paints and plastics n
 Formule plagioclase
Formule plagioclase Olivine group of minerals
Olivine group of minerals Homogeneous minerals
Homogeneous minerals Olivine melting point
Olivine melting point Tetrahedron fire triangle
Tetrahedron fire triangle Surface area of a square-based pyramid
Surface area of a square-based pyramid Tetrahedron volume
Tetrahedron volume Tom suk
Tom suk Elements of fire tetrahedron
Elements of fire tetrahedron Probability vocabulary
Probability vocabulary How to find planes of symmetry
How to find planes of symmetry Sessile dislocation
Sessile dislocation Nature of roots
Nature of roots Foam tetrahedron
Foam tetrahedron Composition of fire
Composition of fire Tetrahedron faces edges vertices
Tetrahedron faces edges vertices Tetrahedron mesh ansys
Tetrahedron mesh ansys Fire tetrahedron
Fire tetrahedron Pulsating vasi
Pulsating vasi Basalt tetrahedron
Basalt tetrahedron Geogebra tetrahedron
Geogebra tetrahedron Octahedral voids in bcc
Octahedral voids in bcc Chemical sedimentary rocks formed
Chemical sedimentary rocks formed Sara toogood
Sara toogood What is isolated i/o
What is isolated i/o Is the earth a closed system
Is the earth a closed system Isolated versus memory mapped i/o
Isolated versus memory mapped i/o Isolated diene
Isolated diene Special-purpose op-amp circuits
Special-purpose op-amp circuits Isolated thrombocytopenia
Isolated thrombocytopenia Isolated thrombocytopenia
Isolated thrombocytopenia Isolated feature combined feature effects
Isolated feature combined feature effects Every denture design
Every denture design Ikatan kovalen polar
Ikatan kovalen polar Naphthalene chemical name
Naphthalene chemical name Lumpy cloud
Lumpy cloud Isolation habitat
Isolation habitat Isolated system
Isolated system Momentum
Momentum What is nozzle in thermodynamics
What is nozzle in thermodynamics When do clouds form?
When do clouds form? Isolated vertical load bearing member
Isolated vertical load bearing member