ORLISTAT 1 2 LIPASE FFA Orlistat lipase TG

























- Slides: 59




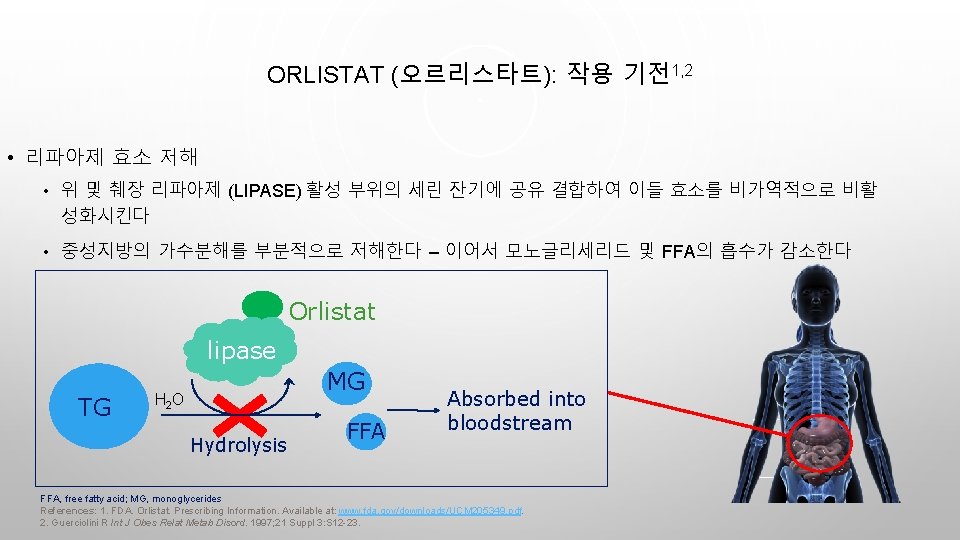
ORLISTAT (오르리스타트): 작용 기전1, 2 • 리파아제 효소 저해 • 위 및 췌장 리파아제 (LIPASE) 활성 부위의 세린 잔기에 공유 결합하여 이들 효소를 비가역적으로 비활 성화시킨다 • 중성지방의 가수분해를 부분적으로 저해한다 – 이어서 모노글리세리드 및 FFA의 흡수가 감소한다 Orlistat lipase TG MG H 2 O Hydrolysis FFA Absorbed into bloodstream FFA, free fatty acid; MG, monoglycerides References: 1. FDA. Orlistat. Prescribing Information. Available at: www. fda. gov/downloads/UCM 205349. pdf. 2. Guerciolini R Int J Obes Relat Metab Disord. 1997; 21 Suppl 3: S 12 -23.

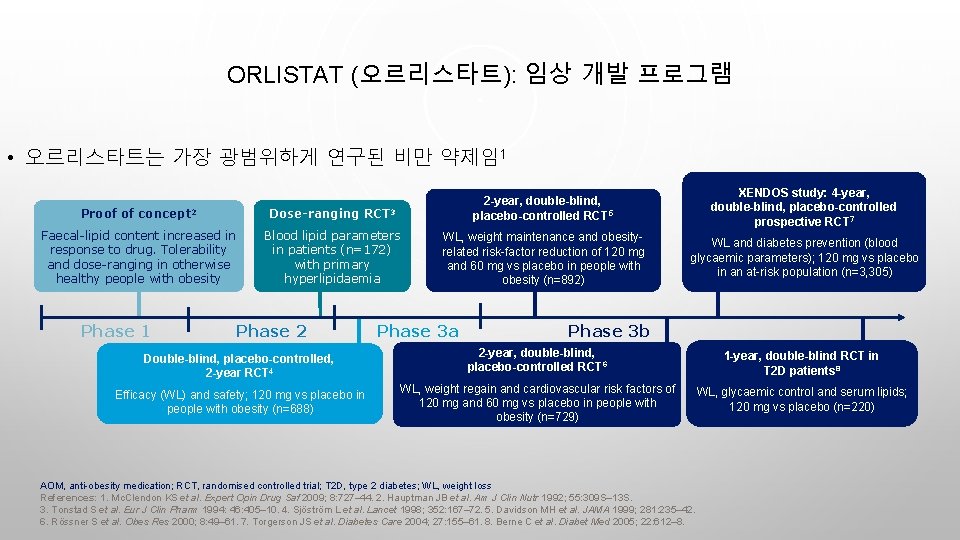
ORLISTAT (오르리스타트): 임상 개발 프로그램 • 오르리스타트는 가장 광범위하게 연구된 비만 약제임1 Proof of concept 2 Dose-ranging RCT 3 2 -year, double-blind, placebo-controlled RCT 5 Faecal-lipid content increased in response to drug. Tolerability and dose-ranging in otherwise healthy people with obesity Blood lipid parameters in patients (n=172) with primary hyperlipidaemia WL, weight maintenance and obesityrelated risk-factor reduction of 120 mg and 60 mg vs placebo in people with obesity (n=892) Phase 1 Phase 2 Double-blind, placebo-controlled, 2 -year RCT 4 Efficacy (WL) and safety; 120 mg vs placebo in people with obesity (n=688) Phase 3 a XENDOS study: 4 -year, double-blind, placebo-controlled prospective RCT 7 WL and diabetes prevention (blood glycaemic parameters); 120 mg vs placebo in an at-risk population (n=3, 305) Phase 3 b 2 -year, double-blind, placebo-controlled RCT 6 1 -year, double-blind RCT in T 2 D patients 8 WL, weight regain and cardiovascular risk factors of 120 mg and 60 mg vs placebo in people with obesity (n=729) WL, glycaemic control and serum lipids; 120 mg vs placebo (n=220) AOM, anti-obesity medication; RCT, randomised controlled trial; T 2 D, type 2 diabetes; WL, weight loss References: 1. Mc. Clendon KS et al. Expert Opin Drug Saf 2009; 8: 727– 44. 2. Hauptman JB et al. Am J Clin Nutr 1992; 55: 309 S– 13 S. 3. Tonstad S et al. Eur J Clin Pharm 1994: 46: 405– 10. 4. Sjöström L et al. Lancet 1998; 352: 167– 72. 5. Davidson MH et al. JAMA 1999; 281: 235– 42. 6. Rössner S et al. Obes Res 2000; 8: 49– 61. 7. Torgerson JS et al. Diabetes Care 2004; 27: 155– 61. 8. Berne C et al. Diabet Med 2005; 22: 612– 8.

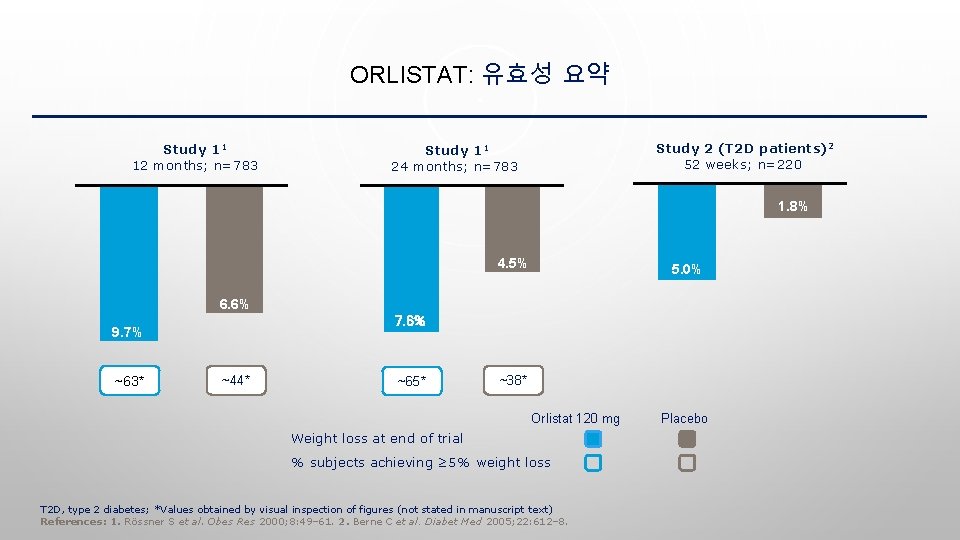
ORLISTAT: 유효성 요약 Study 11 12 months; n=783 Study 2 (T 2 D patients)2 52 weeks; n=220 Study 11 24 months; n=783 1. 8% 4. 5% 5. 0% 6. 6% 7. 6% 9. 7% ~63* ~44* ~65* ~38* Orlistat 120 mg Weight loss at end of trial % subjects achieving ≥ 5% weight loss T 2 D, type 2 diabetes; *Values obtained by visual inspection of figures (not stated in manuscript text) References: 1. Rössner S et al. Obes Res 2000; 8: 49– 61. 2. Berne C et al. Diabet Med 2005; 22: 612– 8. Placebo

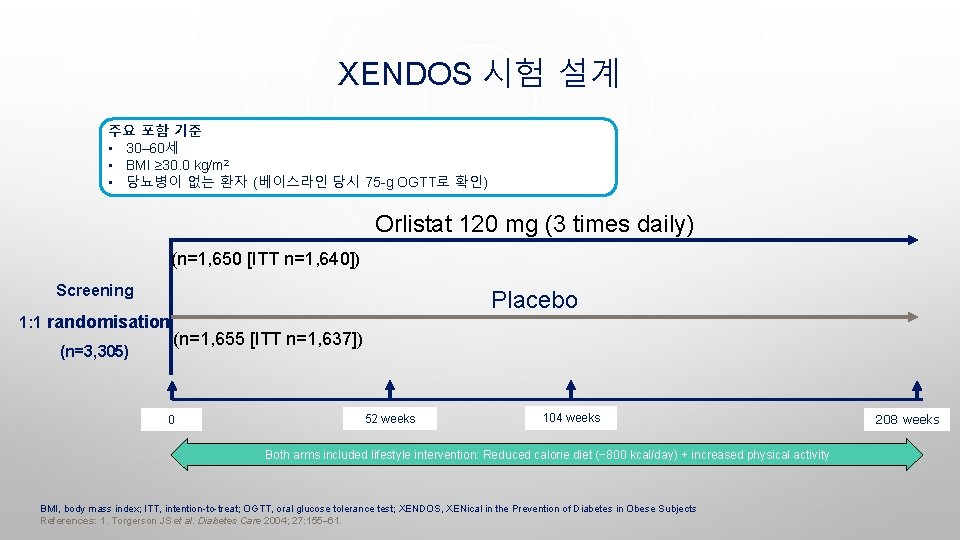
XENDOS 시험 설계 주요 포함 기준 • 30– 60세 • BMI ≥ 30. 0 kg/m 2 • 당뇨병이 없는 환자 (베이스라인 당시 75 -g OGTT로 확인) Orlistat 120 mg (3 times daily) (n=1, 650 [ITT n=1, 640]) Screening 1: 1 randomisation (n=3, 305) Placebo (n=1, 655 [ITT n=1, 637]) 0 52 weeks 104 weeks Both arms included lifestyle intervention: Reduced calorie diet (− 800 kcal/day) + increased physical activity BMI, body mass index; ITT, intention-to-treat; OGTT, oral glucose tolerance test; XENDOS, XENical in the Prevention of Diabetes in Obese Subjects References: 1. Torgerson JS et al. Diabetes Care 2004; 27: 155– 61. 208 weeks

체중 및 T 2 D 진행에 대한 영향 XENDOS 시험: 4년 T 2 D 누적 발생률 IGT 환자만 포함 − 3. 0 kg − 6. 2 kg − 5. 8 kg − 10. 6 kg Cumulative incidence of T 2 D (%) Change in weight (kg) 체중 변화 28. 8% − 45%* 18. 8% Time (weeks) Orlistat + lifestyle Placebo + lifestyle Baseline: weight, 110. 5 kg; BMI, 37 kg/m 2; *p=0. 0024 BMI, body mass index; IGT, impaired glucose tolerance; T 2 D, type 2 diabetes; XENDOS, XENical in the Prevention of Diabetes in Obese Subjects References: 1. Torgerson JS et al. Diabetes Care 2004; 27: 155– 61.

이차 유효성 평가변수† XENDOS 시험: 대사지표가 4년 기간 지속적으로 개선되었다 WC (cm) Baseline values (orlistat; placebo) DBP (mm. Hg) 115. 0; 115. 4 SBP (mm. Hg) 82. 0; 82. 3 130. 8; 130. 4 4. 6; 4. 6 1, 0 Change from baseline FBG (mmol/L) Total cholesterol (%) 5. 8; 5. 8 0, 2 0, 1 * * -1, 0 * -3, 0 * -5, 0 -4, 4 -11, 0 -1, 9 -2, 3 -3, 4 -3, 6 * -5. 2 -4, 9 * -7, 0 -9, 0 -2, 6 -1, 3 -7. 0 -6, 4 * -7, 3 * * * -9, 6 Orlistat (Year 1) Placebo (Year 1) -8, 8 *p<0. 01 for comparison between orlistat and placebo; †figure does not show all secondary endpoint parameters (see original publication for complete set) DBP, diastolic blood pressure; FBG, fasting blood glucose; NS, not significant; SBP, systolic blood pressure; WC, waist circumference; XENDOS, XENical in the Prevention of Diabetes in Obese Subjects References: 1. Torgerson JS et al. Diabetes Care 2004; 27: 155– 61. -7, 9

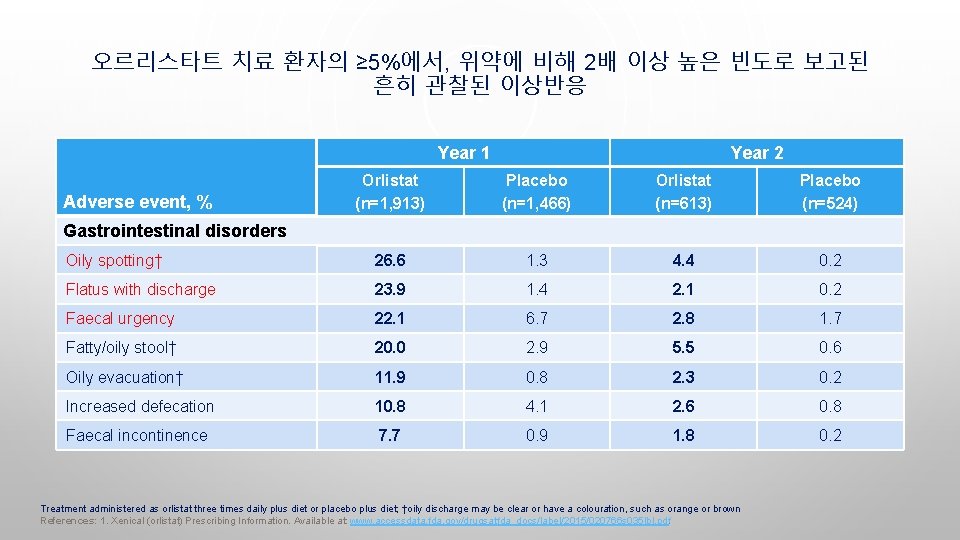
오르리스타트 치료 환자의 ≥ 5%에서, 위약에 비해 2배 이상 높은 빈도로 보고된 흔히 관찰된 이상반응 Year 1 Year 2 Orlistat (n=1, 913) Placebo (n=1, 466) Orlistat (n=613) Placebo (n=524) Oily spotting† 26. 6 1. 3 4. 4 0. 2 Flatus with discharge 23. 9 1. 4 2. 1 0. 2 Faecal urgency 22. 1 6. 7 2. 8 1. 7 Fatty/oily stool† 20. 0 2. 9 5. 5 0. 6 Oily evacuation† 11. 9 0. 8 2. 3 0. 2 Increased defecation 10. 8 4. 1 2. 6 0. 8 Faecal incontinence 7. 7 0. 9 1. 8 0. 2 Adverse event, % Gastrointestinal disorders Treatment administered as orlistat three times daily plus diet or placebo plus diet; †oily discharge may be clear or have a colouration, such as orange or brown References: 1. Xenical (orlistat) Prescribing Information. Available at: www. accessdata. fda. gov/drugsatfda_docs/label/2015/020766 s 035 lbl. pdf.



날트렉손 (NALTREXONE) 및 부프로피온 (BUPROPION) 식욕 조절에서 작용 기전 • 날트렉손/부프로피온 병용은 에너지 섭취 감소와 관련이 있는 두 가지 경로에 영향을 미친다 POMC: ↓ Appetite ↑ Energy expenditure • Satiety signal • Increased firing leads to weight loss α-MSH + β-endorphin: Weight loss: Second-order neuron MC 4 -R • Activated by bupropion Bupropion: Dopamine reuptake inhibition POMC neuron stimulus + POMC β-endorphin (POMC auto-inhibitory loop) ‒ Hypothalamus (ARC) ARC, arcuate nucleus of the hypothalamus; MC 4 -R, melanocortin-4 receptor; α-MSH, alpha-melanocyte stimulating hormone; NB, naltrexone/bupropion; μOR, μ-opioid receptor; POMC, pro-opiomelanocortin; PR, prolonged release References: 1. Ornellas T et al. P&T 2011; 36: 255– 62. 2. Padwal RS et al. Curr Opin Investig Drugs 2009; 10: 1117– 25. 3. Stahl S. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge: Cambridge University Press, 2013. • Released with α-MSH (α-melanocycte stimulating hormone) • Inhibits POMC firing • Effect blocked by naltrexone Naltrexone: blocks POMC inhibition

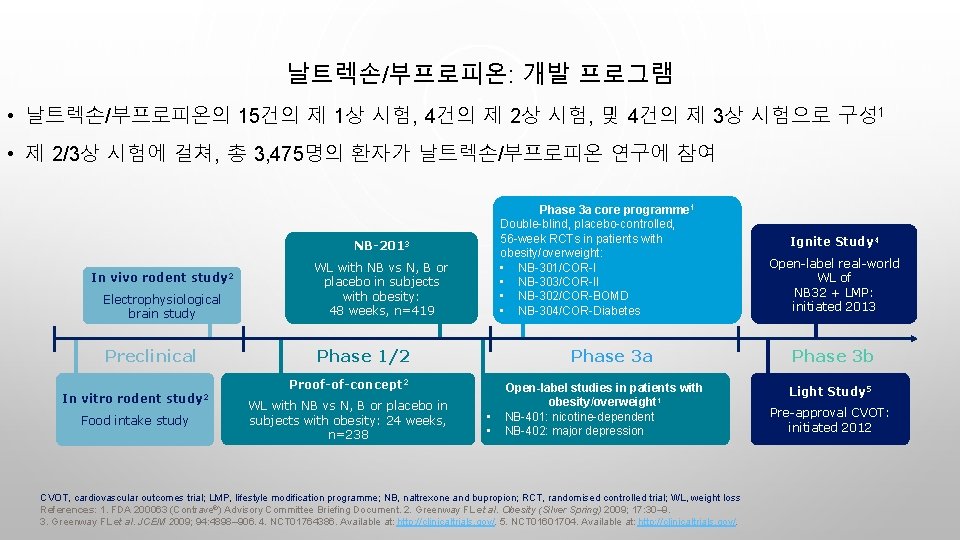
날트렉손/부프로피온: 개발 프로그램 • 날트렉손/부프로피온의 15건의 제 1상 시험, 4건의 제 2상 시험, 및 4건의 제 3상 시험으로 구성 1 • 제 2/3상 시험에 걸쳐, 총 3, 475명의 환자가 날트렉손/부프로피온 연구에 참여 Phase 3 a core programme 1 Double-blind, placebo-controlled, 56 -week RCTs in patients with obesity/overweight: • NB-301/COR-I • NB-303/COR-II • NB-302/COR-BOMD • NB-304/COR-Diabetes NB-2013 Electrophysiological brain study WL with NB vs N, B or placebo in subjects with obesity: 48 weeks, n=419 Preclinical Phase 1/2 In vivo rodent study 2 Phase 3 a Proof-of-concept 2 In vitro rodent study 2 Food intake study WL with NB vs N, B or placebo in subjects with obesity: 24 weeks, n=238 • • Open-label studies in patients with obesity/overweight 1 NB-401: nicotine-dependent NB-402: major depression CVOT, cardiovascular outcomes trial; LMP, lifestyle modification programme; NB, naltrexone and bupropion; RCT, randomised controlled trial; WL, weight loss References: 1. FDA 200063 (Contrave®) Advisory Committee Briefing Document. 2. Greenway FL et al. Obesity (Silver Spring) 2009; 17: 30– 9. 3. Greenway FL et al. JCEM 2009; 94: 4898– 906. 4. NCT 01764386. Available at: http: //clinicaltrials. gov/. 5. NCT 01601704. Available at: http: //clinicaltrials. gov/. Ignite Study 4 Open-label real-world WL of NB 32 + LMP: initiated 2013 Phase 3 b Light Study 5 Pre-approval CVOT: initiated 2012

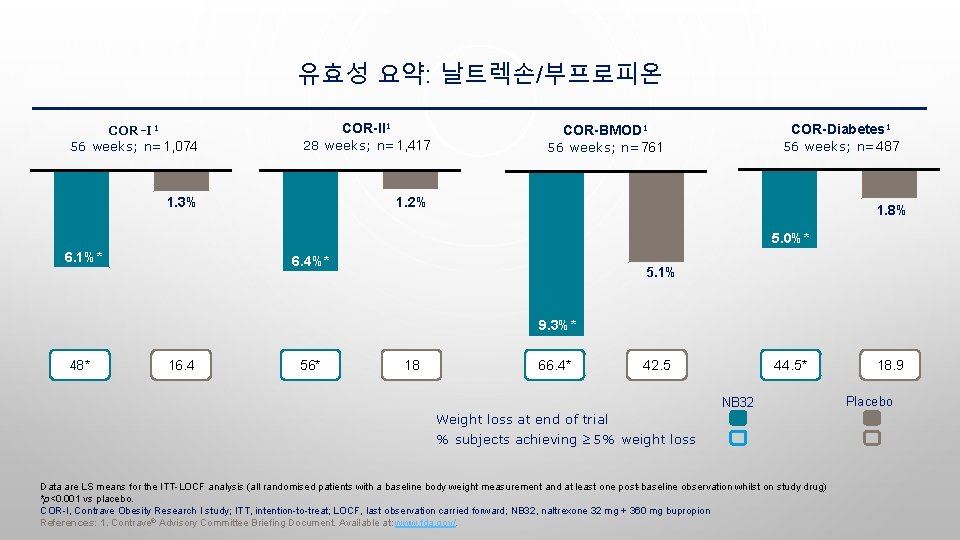
유효성 요약: 날트렉손/부프로피온 COR-I 1 56 weeks; n=1, 074 COR-II 1 28 weeks; n=1, 417 1. 3% 1. 2% COR-Diabetes 1 56 weeks; n=487 COR-BMOD 1 56 weeks; n=761 1. 8% 5. 0%* 6. 1%* 6. 4%* 5. 1% 9. 3%* 48* 16. 4 56* 18 66. 4* 42. 5 44. 5* NB 32 Weight loss at end of trial % subjects achieving ≥ 5% weight loss Data are LS means for the ITT-LOCF analysis (all randomised patients with a baseline body weight measurement and at least one post-baseline observation whilst on study drug) *p<0. 001 vs placebo. COR-I, Contrave Obesity Research I study; ITT, intention-to-treat; LOCF, last observation carried forward; NB 32, naltrexone 32 mg + 360 mg bupropion References: 1. Contrave® Advisory Committee Briefing Document. Available at: www. fda. gov/. 18. 9 Placebo

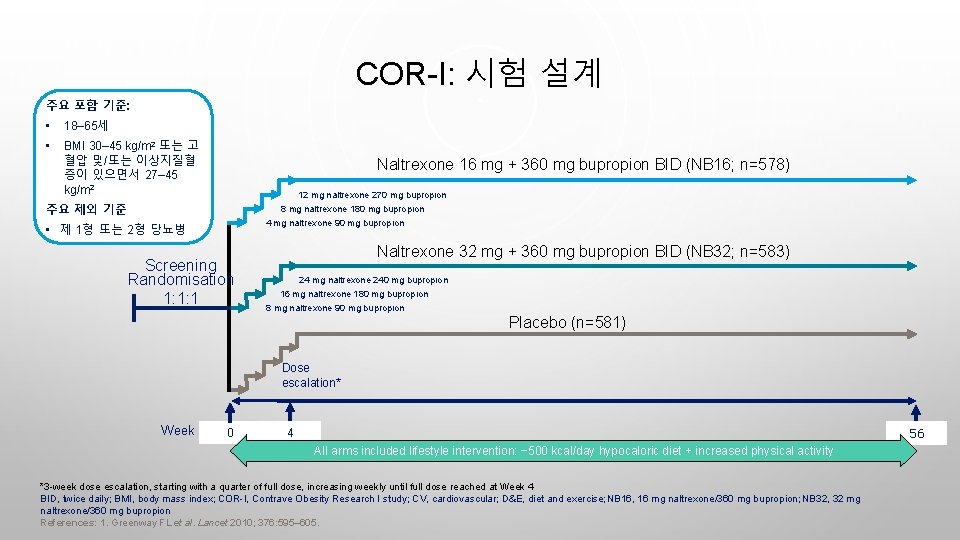
COR-I: 시험 설계 주요 포함 기준: • 18– 65세 • BMI 30– 45 kg/m 2 또는 고 혈압 및/또는 이상지질혈 증이 있으면서 27– 45 kg/m 2 Naltrexone 16 mg + 360 mg bupropion BID (NB 16; n=578) 12 mg naltrexone 270 mg bupropion 주요 제외 기준 8 mg naltrexone 180 mg bupropion 4 mg naltrexone 90 mg bupropion • 제 1형 또는 2형 당뇨병 Screening Randomisation 1: 1: 1 Naltrexone 32 mg + 360 mg bupropion BID (NB 32; n=583) 24 mg naltrexone 240 mg bupropion 16 mg naltrexone 180 mg bupropion 8 mg naltrexone 90 mg bupropion Placebo (n=581) Dose escalation* Week 0 56 4 All arms included lifestyle intervention: − 500 kcal/day hypocaloric diet + increased physical activity *3 -week dose escalation, starting with a quarter of full dose, increasing weekly until full dose reached at Week 4 BID, twice daily; BMI, body mass index; COR-I, Contrave Obesity Research I study; CV, cardiovascular; D&E, diet and exercise; NB 16, 16 mg naltrexone/360 mg bupropion; NB 32, 32 mg naltrexone/360 mg bupropion References: 1. Greenway FL et al. Lancet 2010; 376: 595– 605.

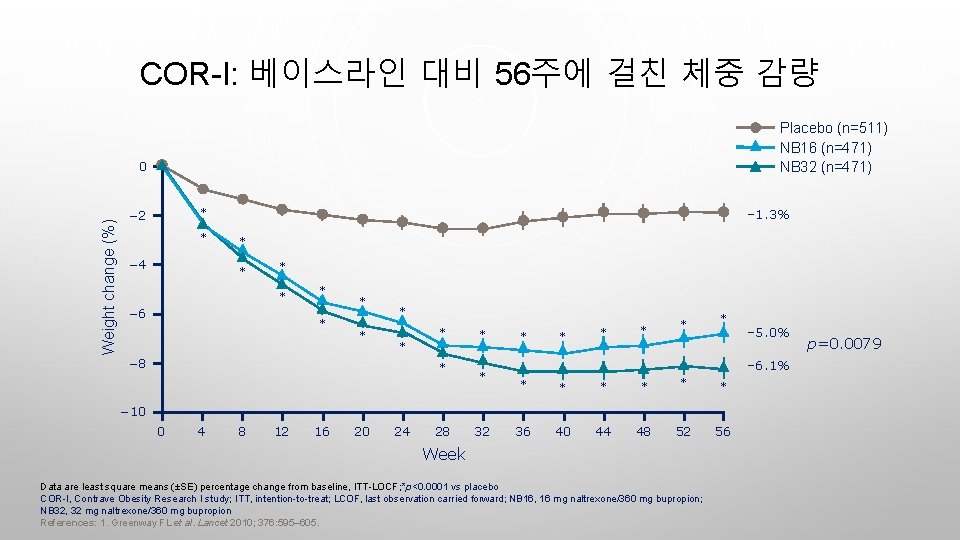
COR-I: 베이스라인 대비 56주에 걸친 체중 감량 Placebo (n=511) NB 16 (n=471) NB 32 (n=471) Weight change (%) 0 – 1. 3% * − 2 * − 4 * * − 6 * * * * − 8 * * * * * – 6. 1% * * * 36 40 44 48 52 56 − 10 0 4 8 12 16 20 24 28 32 – 5. 0% Week Data are least square means (±SE) percentage change from baseline, ITT-LOCF; *p<0. 0001 vs placebo COR-I, Contrave Obesity Research I study; ITT, intention-to-treat; LCOF, last observation carried forward; NB 16, 16 mg naltrexone/360 mg bupropion; NB 32, 32 mg naltrexone/360 mg bupropion References: 1. Greenway FL et al. Lancet 2010; 376: 595– 605. p=0. 0079

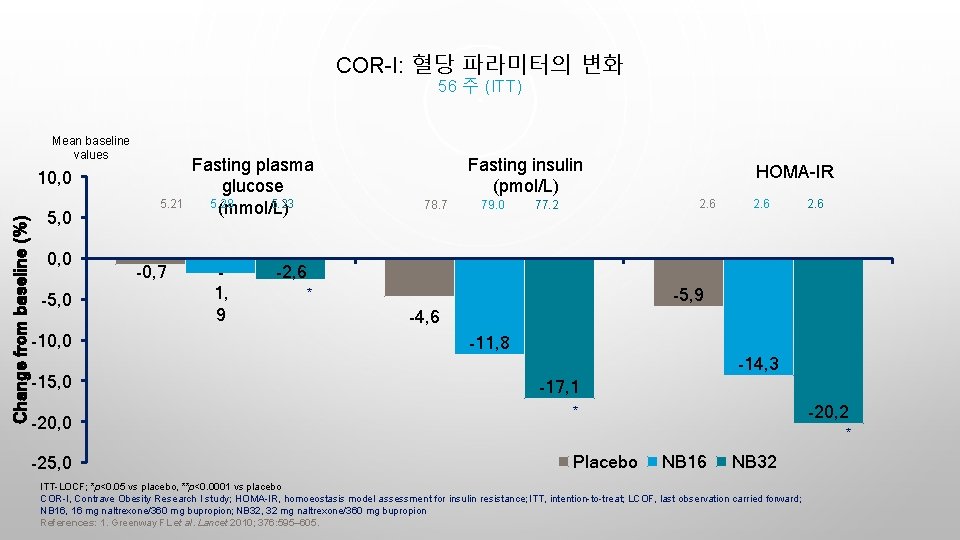
COR-I: 혈당 파라미터의 변화 56 주 (ITT) Mean baseline values Change from baseline (%) 10, 0 5, 0 0, 0 -5, 0 -10, 0 -15, 0 -20, 0 -25, 0 5. 21 -0, 7 Fasting plasma glucose 5. 28 5. 23 (mmol/L) 1, 9 Fasting insulin (pmol/L) 78. 7 79. 0 HOMA-IR 2. 6 77. 2 2. 6 -2, 6 -5, 9 * -4, 6 -11, 8 -14, 3 -17, 1 -20, 2 * * Placebo NB 16 NB 32 ITT-LOCF; *p<0. 05 vs placebo, **p<0. 0001 vs placebo COR-I, Contrave Obesity Research I study; HOMA-IR, homoeostasis model assessment for insulin resistance; ITT, intention-to-treat; LCOF, last observation carried forward; NB 16, 16 mg naltrexone/360 mg bupropion; NB 32, 32 mg naltrexone/360 mg bupropion References: 1. Greenway FL et al. Lancet 2010; 376: 595– 605.

날트렉손/부프로피온 치료 환자의 ≥ 2%에서, 위약에 비해 높은 빈도로 보고된 이상반응 (1) Naltrexone/bupropion (n=2, 545) Placebo (n=1, 515) Nausea 32. 5 6. 7 Constipation 19. 2 7. 2 Headache 17. 6 10. 4 Vomiting 10. 7 2. 9 Dizziness 9. 9 3. 4 Insomnia 9. 2 5. 9 Dry mouth 8. 1 2. 3 Diarrhoea 7. 1 5. 2 Anxiety 4. 2 2. 8 Hot flush 4. 2 1. 2 Fatigue 4. 0 3. 4 Tremor 4. 0 0. 7 Upper abdominal pain 3. 5 1. 3 Adverse event, % References: 1. Contrave (naltrexone HCl and bupropion HCl). Prescribing Information. Available at: www. accessdata. fda. gov/drugsatfda_docs/label/2014/200063 s 000 lbl. pdf.




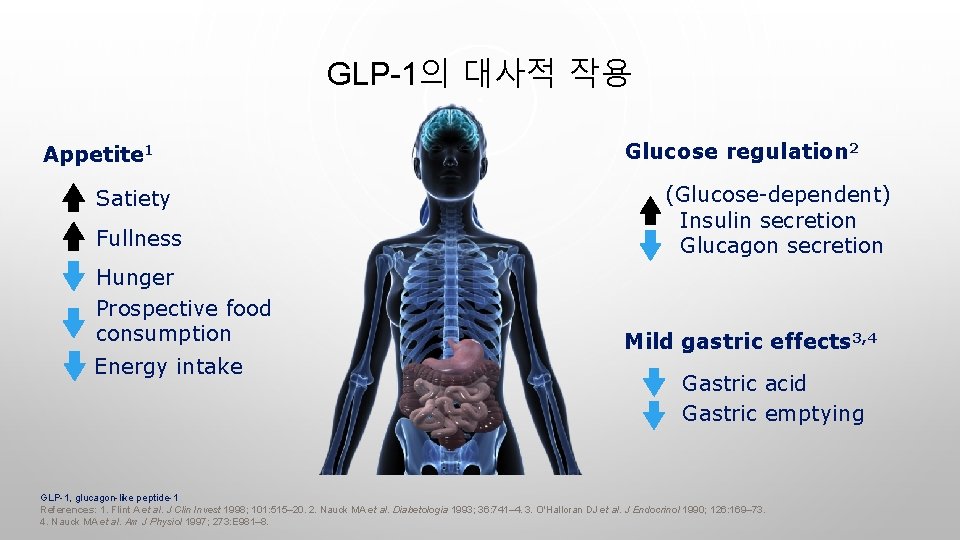
GLP-1의 대사적 작용 Appetite 1 Satiety Fullness Hunger Prospective food consumption Energy intake Glucose regulation 2 (Glucose-dependent) Insulin secretion Glucagon secretion Mild gastric effects 3, 4 Gastric acid Gastric emptying GLP-1, glucagon-like peptide-1 References: 1. Flint A et al. J Clin Invest 1998; 101: 515– 20. 2. Nauck MA et al. Diabetologia 1993; 36: 741– 4. 3. O'Halloran DJ et al. J Endocrinol 1990; 126: 169– 73. 4. Nauck MA et al. Am J Physiol 1997; 273: E 981– 8.

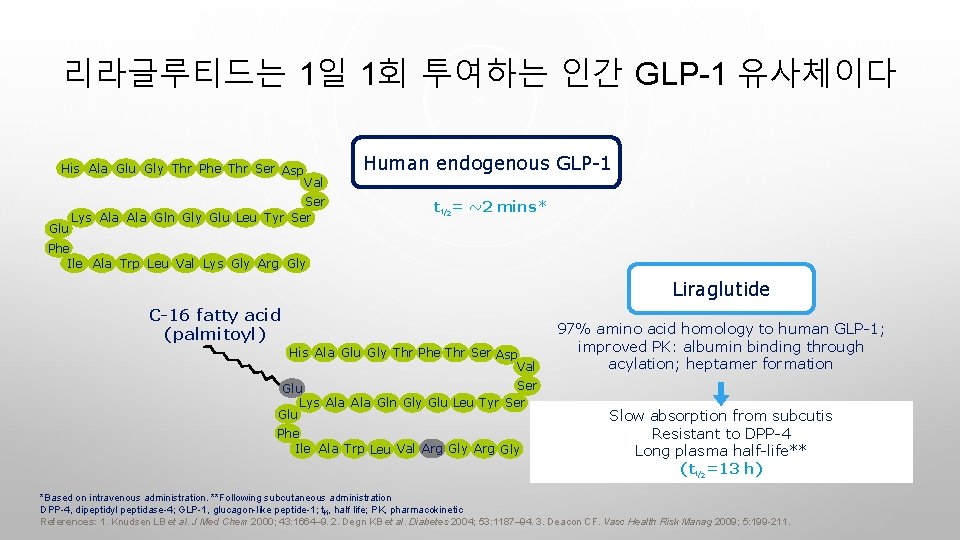
리라글루티드는 1일 1회 투여하는 인간 GLP-1 유사체이다 His Ala Glu Gly Thr Phe Thr Ser Asp Glu Human endogenous GLP-1 Val Ser Lys Ala Gln Gly Glu Leu Tyr Ser t½= ~2 mins* Phe Ile Ala Trp Leu Val Lys Gly Arg Gly Liraglutide C-16 fatty acid (palmitoyl) His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Glu Lys Ala Gln Gly Glu Leu Tyr Ser Glu Phe Ile Ala Trp Leu Val Arg Gly 97% amino acid homology to human GLP-1; improved PK: albumin binding through acylation; heptamer formation Slow absorption from subcutis Resistant to DPP-4 Long plasma half-life** (t½=13 h) *Based on intravenous administration. **Following subcutaneous administration DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; t½, half life; PK, pharmacokinetic References: 1. Knudsen LB et al. J Med Chem 2000; 43: 1664– 9. 2. Degn KB et al. Diabetes 2004; 53: 1187– 94. 3. Deacon CF. Vasc Health Risk Manag 2009; 5: 199 -211.

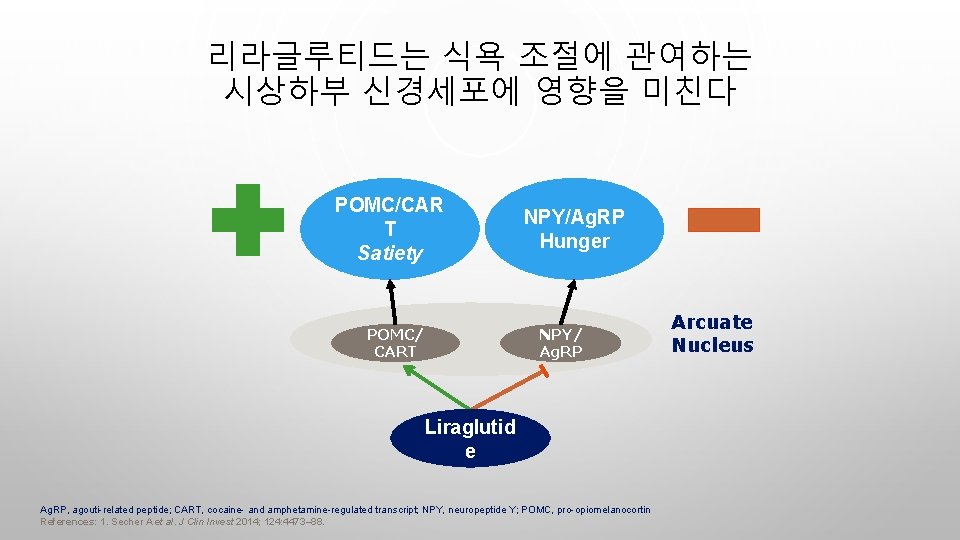
리라글루티드는 식욕 조절에 관여하는 시상하부 신경세포에 영향을 미친다 POMC/CAR T Satiety POMC/ CART NPY/Ag. RP Hunger NPY/ Ag. RP Liraglutid e Ag. RP, agouti-related peptide; CART, cocaine- and amphetamine-regulated transcript; NPY, neuropeptide Y; POMC, pro-opiomelanocortin References: 1. Secher A et al. J Clin Invest 2014; 124: 4473– 88. Arcuate Nucleus

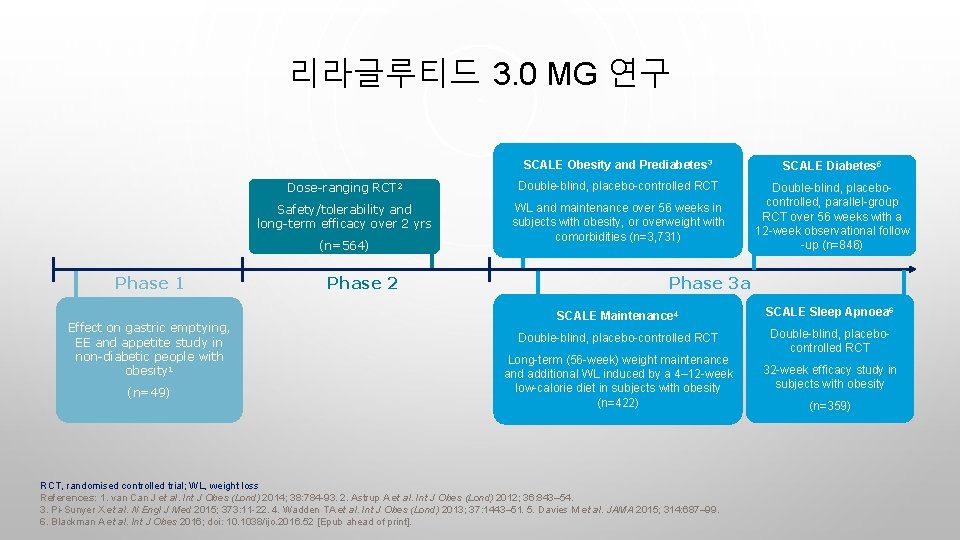
리라글루티드 3. 0 MG 연구 SCALE Obesity and Prediabetes 3 SCALE Diabetes 5 Dose-ranging RCT 2 Double-blind, placebo-controlled RCT Safety/tolerability and long-term efficacy over 2 yrs WL and maintenance over 56 weeks in subjects with obesity, or overweight with comorbidities (n=3, 731) Double-blind, placebocontrolled, parallel-group RCT over 56 weeks with a 12 -week observational follow -up (n=846) (n=564) Phase 1 Effect on gastric emptying, EE and appetite study in non-diabetic people with obesity 1 (n=49) Phase 2 Phase 3 a SCALE Maintenance 4 SCALE Sleep Apnoea 6 Double-blind, placebo-controlled RCT Double-blind, placebocontrolled RCT Long-term (56 -week) weight maintenance and additional WL induced by a 4– 12 -week low-calorie diet in subjects with obesity (n=422) RCT, randomised controlled trial; WL, weight loss References: 1. van Can J et al. Int J Obes (Lond) 2014; 38: 784 -93. 2. Astrup A et al. Int J Obes (Lond) 2012; 36: 843– 54. 3. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11 -22. 4. Wadden TA et al. Int J Obes (Lond) 2013; 37: 1443– 51. 5. Davies M et al. JAMA 2015; 314: 687– 99. 6. Blackman A et al. Int J Obes 2016; doi: 10. 1038/ijo. 2016. 52 [Epub ahead of print]. 32 -week efficacy study in subjects with obesity (n=359)

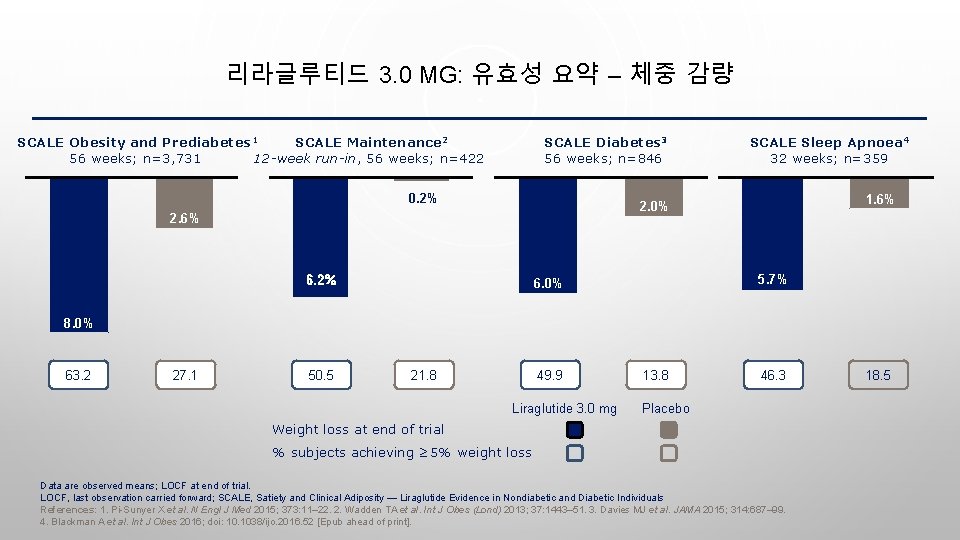
리라글루티드 3. 0 MG: 유효성 요약 – 체중 감량 SCALE Obesity and Prediabetes 1 SCALE Maintenance 2 56 weeks; n=3, 731 12 -week run-in, 56 weeks; n=422 SCALE Diabetes 3 56 weeks; n=846 0. 2% SCALE Sleep Apnoea 4 32 weeks; n=359 1. 6% 2. 0% 2. 6% 6. 2% 5. 7% 6. 2% 6. 0% 8. 0% 63. 2 27. 1 50. 5 21. 8 49. 9 Liraglutide 3. 0 mg 13. 8 46. 3 Placebo Weight loss at end of trial % subjects achieving ≥ 5% weight loss Data are observed means; LOCF at end of trial. LOCF, last observation carried forward; SCALE, Satiety and Clinical Adiposity — Liraglutide Evidence in Nondiabetic and Diabetic Individuals References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22. 2. Wadden TA et al. Int J Obes (Lond) 2013; 37: 1443– 51. 3. Davies MJ et al. JAMA 2015; 314: 687– 99. 4. Blackman A et al. Int J Obes 2016; doi: 10. 1038/ijo. 2016. 52 [Epub ahead of print]. 18. 5

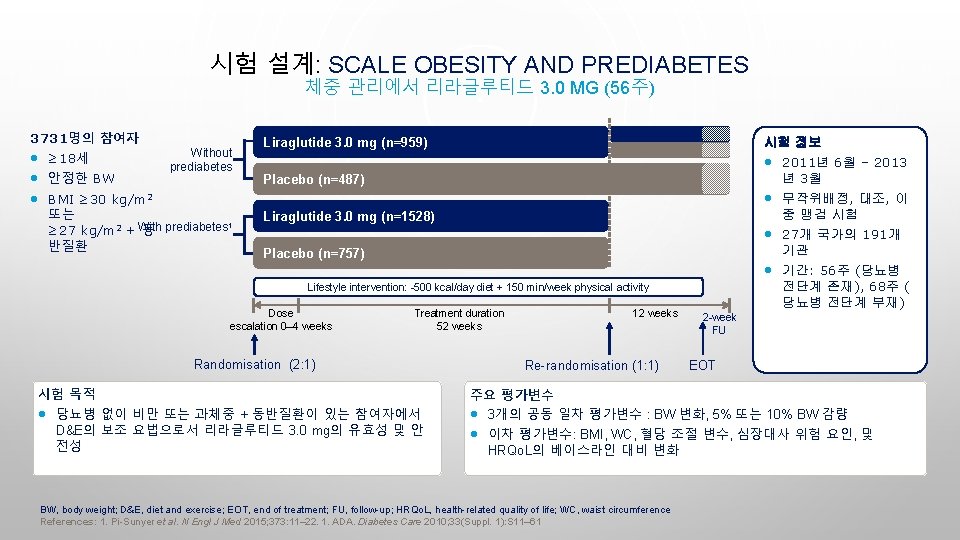
시험 설계: SCALE OBESITY AND PREDIABETES 체중 관리에서 리라글루티드 3. 0 MG (56주) 3731명의 참여자 • ≥ 18세 • 안정한 BW • BMI ≥ 30 kg/m 2 Without prediabetes 또는 1 ≥ 27 kg/m 2 + With 동 prediabetes 반질환 Liraglutide 3. 0 mg (n=959) 시험 정보 • 2011년 6월 – 2013 년 3월 Placebo (n=487) • 무작위배정, 대조, 이 중 맹검 시험 Liraglutide 3. 0 mg (n=1528) • 27개 국가의 191개 기관 Placebo (n=757) • 기간: 56주 (당뇨병 전단계 존재), 68주 ( 당뇨병 전단계 부재) Lifestyle intervention: -500 kcal/day diet + 150 min/week physical activity Dose escalation 0– 4 weeks Treatment duration 52 weeks Randomisation (2: 1) 시험 목적 • 당뇨병 없이 비만 또는 과체중 + 동반질환이 있는 참여자에서 D&E의 보조 요법으로서 리라글루티드 3. 0 mg의 유효성 및 안 전성 12 weeks Re-randomisation (1: 1) 2 -week FU EOT 주요 평가변수 • 3개의 공동 일차 평가변수 : BW 변화, 5% 또는 10% BW 감량 • 이차 평가변수: BMI, WC, 혈당 조절 변수, 심장대사 위험 요인, 및 HRQo. L의 베이스라인 대비 변화 BW, body weight; D&E, diet and exercise; EOT, end of treatment; FU, follow-up; HRQo. L, health-related quality of life; WC, waist circumference References: 1. Pi-Sunyer et al. N Engl J Med 2015; 373: 11– 22. 1. ADA. Diabetes Care 2010; 33(Suppl. 1): S 11– 61

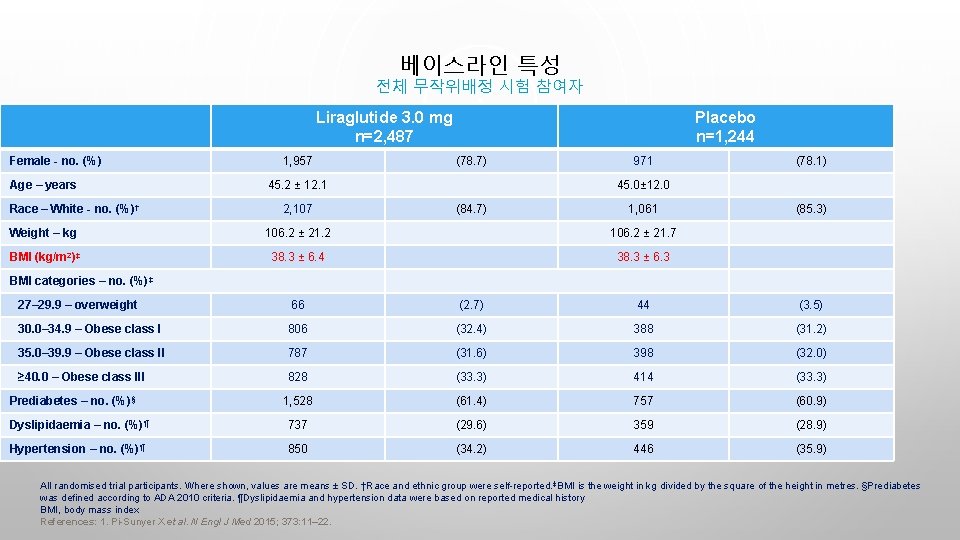
베이스라인 특성 전체 무작위배정 시험 참여자 Liraglutide 3. 0 mg n=2, 487 Female - no. (%) Age – years Race – White - no. (%) † 1, 957 Placebo n=1, 244 (78. 7) 45. 2 ± 12. 1 2, 107 971 (78. 1) 45. 0± 12. 0 (84. 7) 1, 061 Weight – kg 106. 2 ± 21. 2 106. 2 ± 21. 7 BMI (kg/m 2)‡ 38. 3 ± 6. 4 38. 3 ± 6. 3 (85. 3) BMI categories – no. (%)‡ 27– 29. 9 – overweight 66 (2. 7) 44 (3. 5) 30. 0– 34. 9 – Obese class I 806 (32. 4) 388 (31. 2) 35. 0– 39. 9 – Obese class II 787 (31. 6) 398 (32. 0) ≥ 40. 0 – Obese class III 828 (33. 3) 414 (33. 3) 1, 528 (61. 4) 757 (60. 9) Dyslipidaemia – no. (%)¶ 737 (29. 6) 359 (28. 9) Hypertension – no. (%)¶ 850 (34. 2) 446 (35. 9) Prediabetes – no. (%)§ All randomised trial participants. Where shown, values are means ± SD. †Race and ethnic group were self-reported. ‡BMI is the weight in kg divided by the square of the height in metres. §Prediabetes was defined according to ADA 2010 criteria. ¶Dyslipidaemia and hypertension data were based on reported medical history BMI, body mass index References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22.

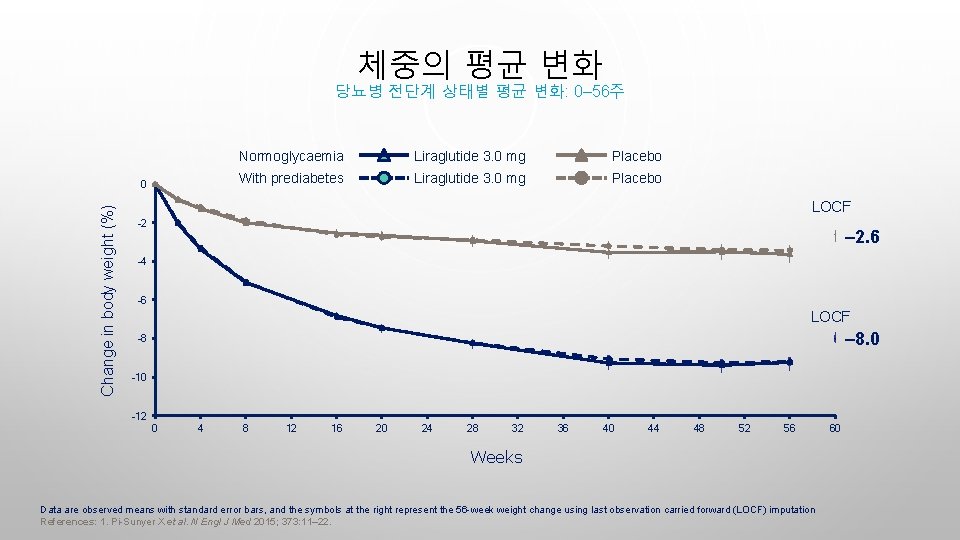
체중의 평균 변화 당뇨병 전단계 상태별 평균 변화: 0– 56주 Change in body weight (%) 0 Normoglycaemia Liraglutide 3. 0 mg Placebo With prediabetes Liraglutide 3. 0 mg Placebo LOCF -2 – 2. 6 -4 -6 LOCF – 8. 0 -8 -10 -12 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 Weeks Data are observed means with standard error bars, and the symbols at the right represent the 56 -week weight change using last observation carried forward (LOCF) imputation References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22. 60

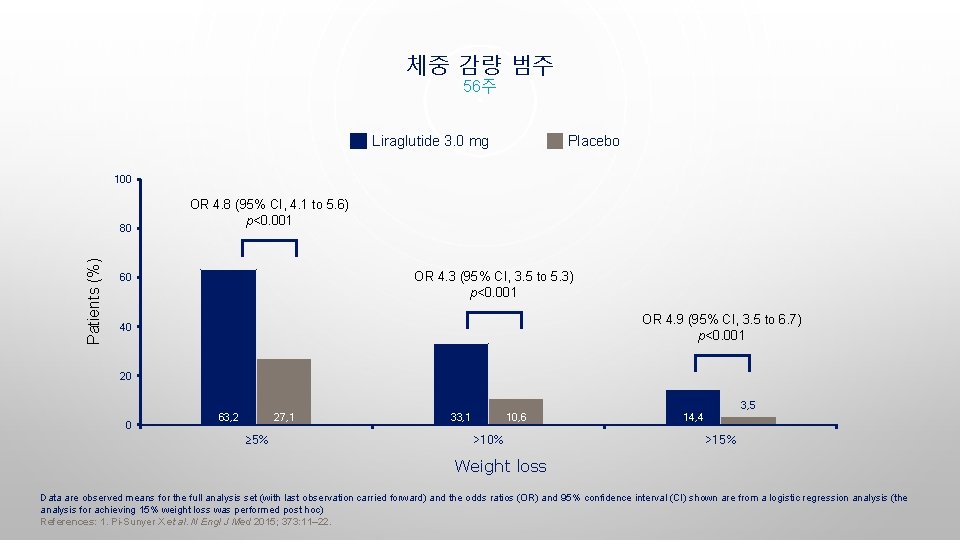
체중 감량 범주 56주 Liraglutide 3. 0 mg Placebo 100 Patients (%) 80 OR 4. 8 (95% CI, 4. 1 to 5. 6) p<0. 001 OR 4. 3 (95% CI, 3. 5 to 5. 3) p<0. 001 60 OR 4. 9 (95% CI, 3. 5 to 6. 7) p<0. 001 40 20 3, 5 0 63, 2 27, 1 ≥ 5% 33, 1 10, 6 >10% 14, 4 >15% Weight loss Data are observed means for the full analysis set (with last observation carried forward) and the odds ratios (OR) and 95% confidence interval (CI) shown are from a logistic regression analysis (the analysis for achieving 15% weight loss was performed post hoc) References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22.

SCALE OBESITY AND PREDIABETES: 공복 지질의 변화 Total cholesterol Baseline (mg/d. L): 193. 7 194. 3 LDL-C 111. 6 112. 2 HDL-C 51. 4 51. 0 Triglycerides 126. 3 129. 3 p=0. 001 Change from baseline (%) 4 2. 3 2 0. 7 0 -1. 0 -2 -4 -6 -3. 1 p<0. 001 -1. 0 -3. 0 p=0. 002 -5. 5 -8 -10 -12 Liraglutide 3. 0 mg -14 Placebo -13. 3 p=0. 001 Full analysis set, last observation carried forward. Statistical analysis are log-transformed ANCOVA (analysis of covariance) at Week 56. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SCALE, Satiety and Clinical Adiposity — Liraglutide Evidence in Nondiabetic and Diabetic Individuals References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22.

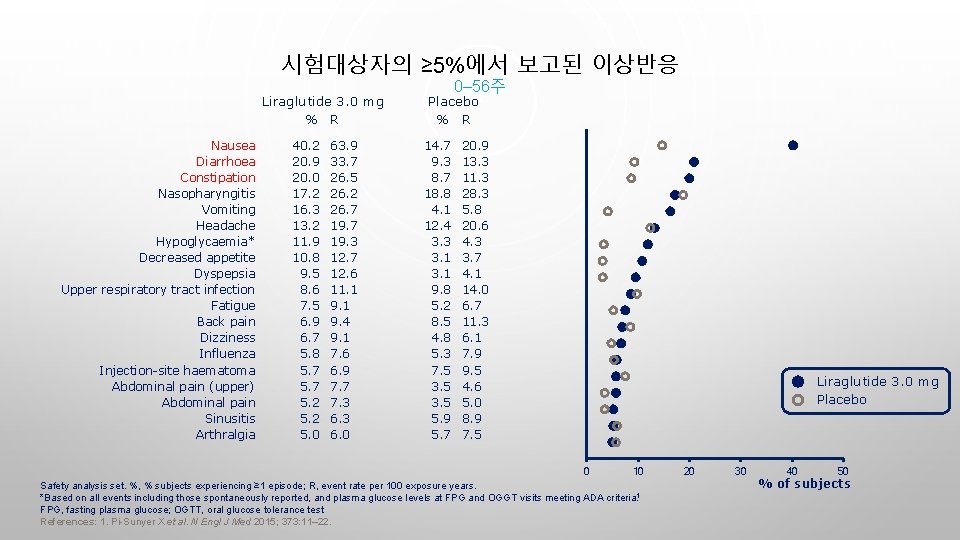
시험대상자의 ≥ 5%에서 보고된 이상반응 Liraglutide 3. 0 mg % R Nausea Diarrhoea Constipation Nasopharyngitis Vomiting Headache Hypoglycaemia* Decreased appetite Dyspepsia Upper respiratory tract infection Fatigue Back pain Dizziness Influenza Injection-site haematoma Abdominal pain (upper) Abdominal pain Sinusitis Arthralgia 40. 2 20. 9 20. 0 17. 2 16. 3 13. 2 11. 9 10. 8 9. 5 8. 6 7. 5 6. 9 6. 7 5. 8 5. 7 5. 2 5. 0 63. 9 33. 7 26. 5 26. 2 26. 7 19. 3 12. 7 12. 6 11. 1 9. 4 9. 1 7. 6 6. 9 7. 7 7. 3 6. 0 0– 56주 Placebo % R 14. 7 9. 3 8. 7 18. 8 4. 1 12. 4 3. 3 3. 1 9. 8 5. 2 8. 5 4. 8 5. 3 7. 5 3. 5 5. 9 5. 7 20. 9 13. 3 11. 3 28. 3 5. 8 20. 6 4. 3 3. 7 4. 1 14. 0 6. 7 11. 3 6. 1 7. 9 9. 5 4. 6 5. 0 8. 9 7. 5 Liraglutide 3. 0 mg Placebo 0 10 Safety analysis set. %, % subjects experiencing ≥ 1 episode; R, event rate per 100 exposure years. *Based on all events including those spontaneously reported, and plasma glucose levels at FPG and OGGT visits meeting ADA criteria. 1 FPG, fasting plasma glucose; OGTT, oral glucose tolerance test References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22. 20 30 40 50 % of subjects

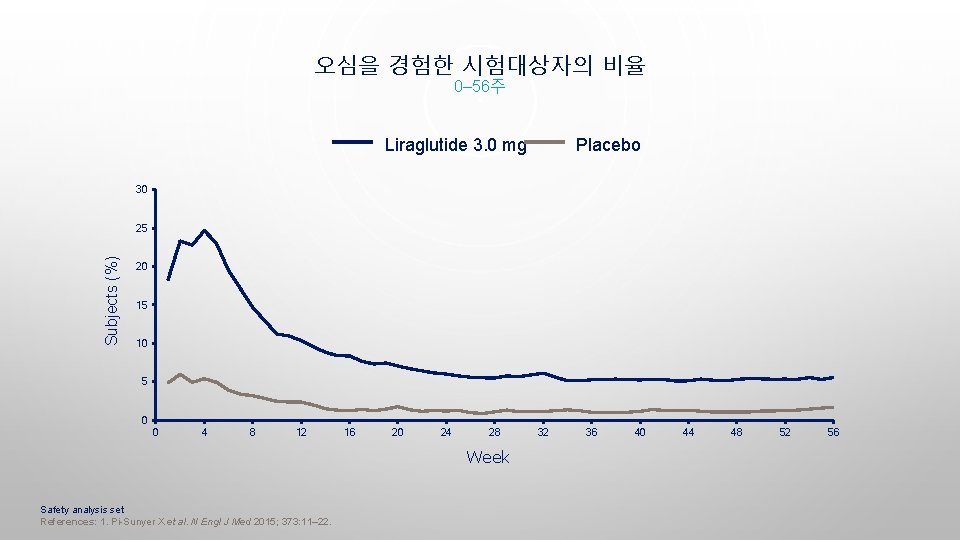
오심을 경험한 시험대상자의 비율 0– 56주 Liraglutide 3. 0 mg Placebo 30 Subjects (%) 25 20 15 10 5 0 0 4 8 12 16 20 24 28 Week Safety analysis set References: 1. Pi-Sunyer X et al. N Engl J Med 2015; 373: 11– 22. 32 36 40 44 48 52 56


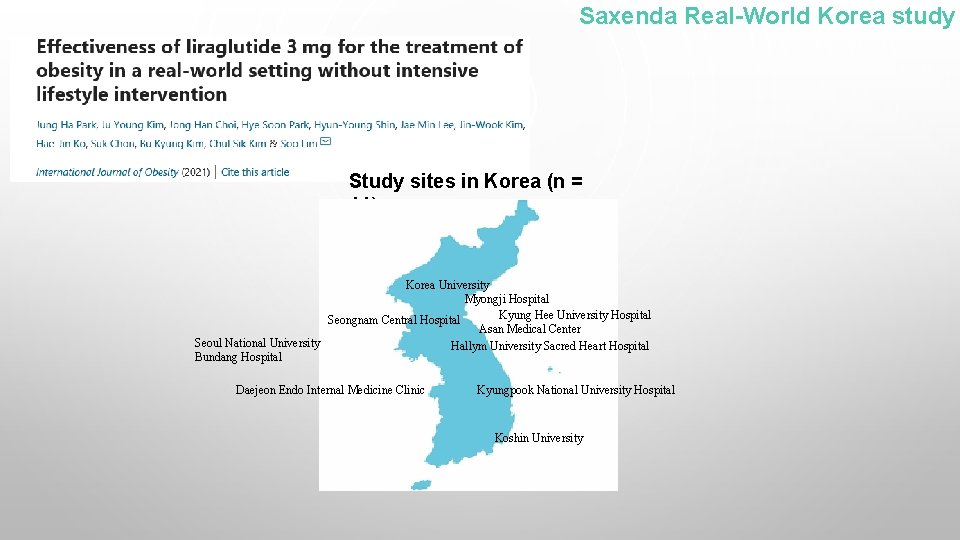
Saxenda Real-World Korea study Study sites in Korea (n = 11) Korea University Myongji Hospital Kyung Hee University Hospital Seongnam Central Hospital Asan Medical Center Seoul National University Hallym University Sacred Heart Hospital Bundang Hospital Daejeon Endo Internal Medicine Clinic Kyungpook National University Hospital Koshin University

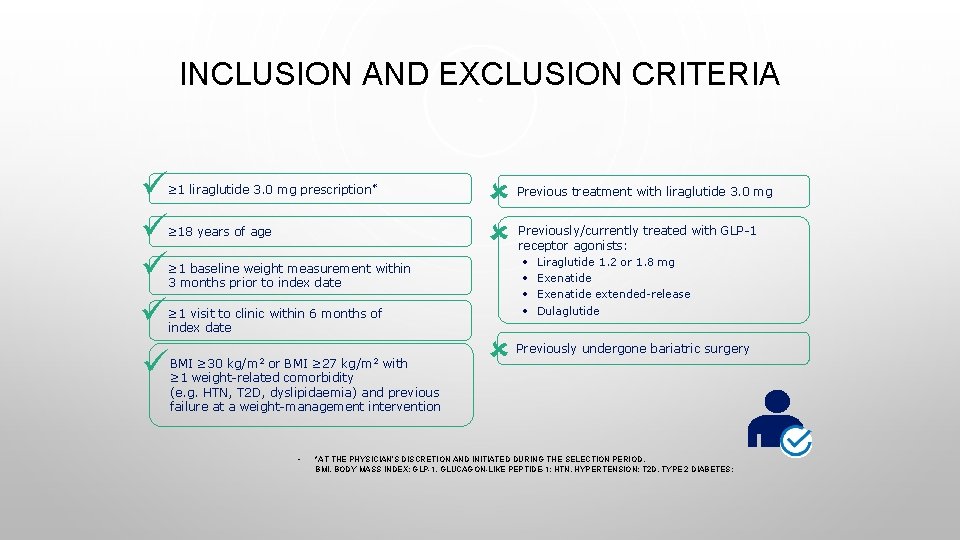
INCLUSION AND EXCLUSION CRITERIA ≥ 1 liraglutide 3. 0 mg prescription* ≥ 18 years of age ≥ 1 baseline weight measurement within 3 months prior to index date ≥ 1 visit to clinic within 6 months of index date BMI ≥ 30 kg/m 2 or BMI ≥ 27 kg/m 2 with ≥ 1 weight-related comorbidity (e. g. HTN, T 2 D, dyslipidaemia) and previous failure at a weight-management intervention • Previous treatment with liraglutide 3. 0 mg Previously/currently treated with GLP-1 receptor agonists: • Liraglutide 1. 2 or 1. 8 mg • Exenatide extended-release • Dulaglutide Previously undergone bariatric surgery *AT THE PHYSICIAN’S DISCRETION AND INITIATED DURING THE SELECTION PERIOD. BMI, BODY MASS INDEX; GLP-1, GLUCAGON-LIKE PEPTIDE-1; HTN, HYPERTENSION; T 2 D, TYPE 2 DIABETES;

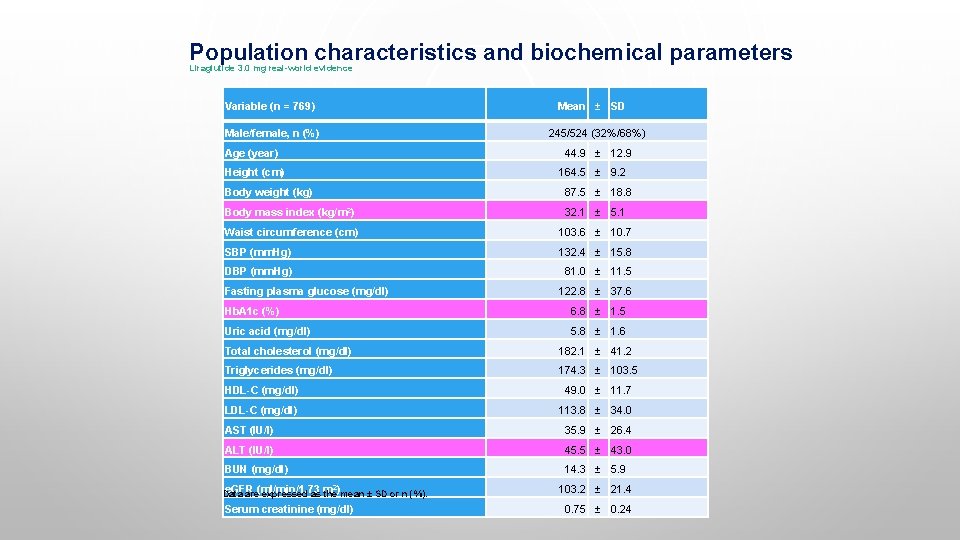
Population characteristics and biochemical parameters Liraglutide 3. 0 mg real-world evidence Variable (n = 769) Mean ± SD Male/female, n (%) 245/524 (32%/68%) Age (year) 44. 9 ± 12. 9 Height (cm) 164. 5 ± 9. 2 Body weight (kg) 87. 5 ± 18. 8 Body mass index (kg/m 2) 32. 1 ± 5. 1 Waist circumference (cm) 103. 6 ± 10. 7 SBP (mm. Hg) 132. 4 ± 15. 8 DBP (mm. Hg) 81. 0 ± 11. 5 Fasting plasma glucose (mg/dl) 122. 8 ± 37. 6 Hb. A 1 c (%) 6. 8 ± 1. 5 Uric acid (mg/dl) 5. 8 ± 1. 6 Total cholesterol (mg/dl) 182. 1 ± 41. 2 Triglycerides (mg/dl) 174. 3 ± 103. 5 HDL-C (mg/dl) 49. 0 ± 11. 7 LDL-C (mg/dl) 113. 8 ± 34. 0 AST (IU/l) 35. 9 ± 26. 4 ALT (IU/l) 45. 5 ± 43. 0 BUN (mg/dl) 14. 3 ± 5. 9 e. GFR Data are(ml/min/1. 73 expressed as m the)mean ± SD or n (%). 2 Serum creatinine (mg/dl) 103. 2 ± 21. 4 0. 75 ± 0. 24

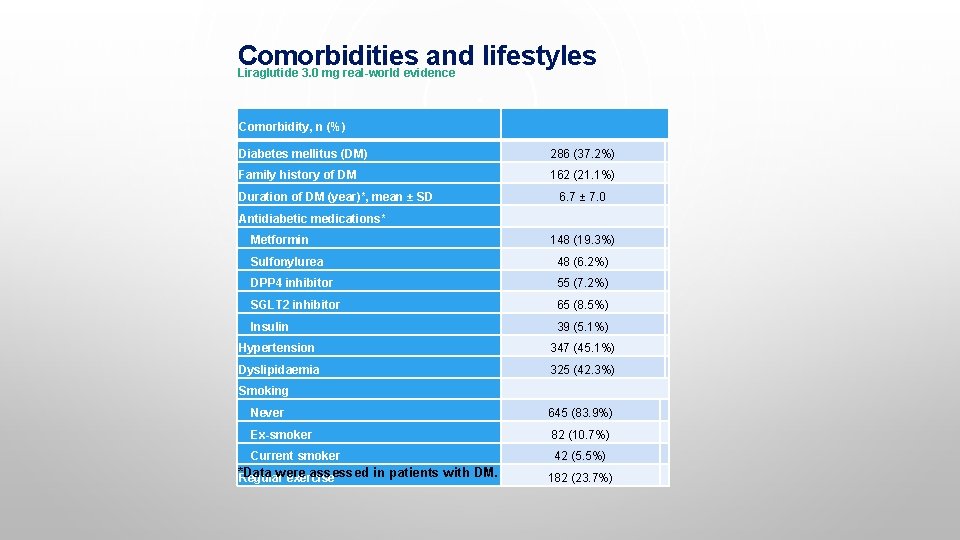
Comorbidities and lifestyles Liraglutide 3. 0 mg real-world evidence Comorbidity, n (%) Diabetes mellitus (DM) 286 (37. 2%) Family history of DM 162 (21. 1%) Duration of DM (year)*, mean ± SD 6. 7 ± 7. 0 Antidiabetic medications* Metformin 148 (19. 3%) Sulfonylurea 48 (6. 2%) DPP 4 inhibitor 55 (7. 2%) SGLT 2 inhibitor 65 (8. 5%) Insulin 39 (5. 1%) Hypertension 347 (45. 1%) Dyslipidaemia 325 (42. 3%) Smoking Never 645 (83. 9%) Ex-smoker 82 (10. 7%) Current smoker 42 (5. 5%) *Data were assessed in patients with DM. Regular exercise 182 (23. 7%)

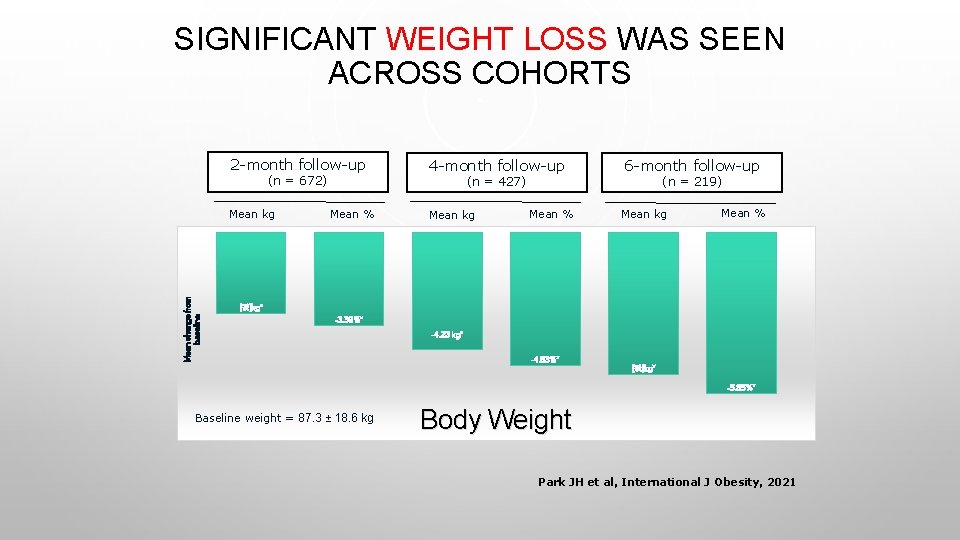
SIGNIFICANT WEIGHT LOSS WAS SEEN ACROSS COHORTS 2 -month follow-up (n = 672) Mean change from baseline Mean kg 4 -month follow-up (n = 427) Mean % Mean kg Mean % 6 -month follow-up (n = 219) Mean kg Mean % [값]kg* -3. 39%* -4. 23 kg* -4. 83%* [값]kg* -5. 85%* Baseline weight = 87. 3 ± 18. 6 kg Body Weight Park JH et al, International J Obesity, 2021

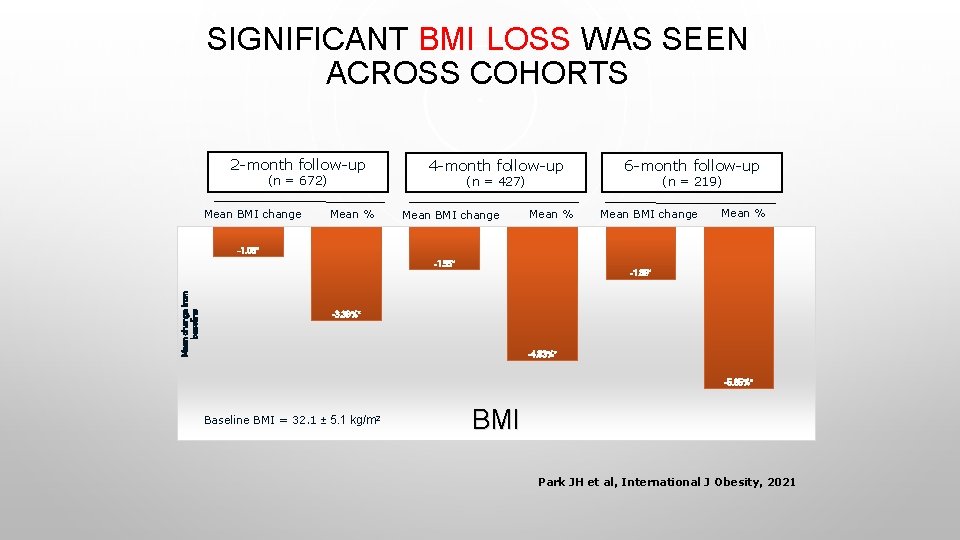
SIGNIFICANT BMI LOSS WAS SEEN ACROSS COHORTS 2 -month follow-up (n = 672) Mean BMI change 4 -month follow-up (n = 427) Mean % Mean BMI change Mean % 6 -month follow-up (n = 219) Mean BMI change Mean % -1. 08* -1. 55* Mean change from baseline -1. 89* -3. 39%* -4. 83%* -5. 85%* Baseline BMI = 32. 1 ± 5. 1 kg/m 2 BMI Park JH et al, International J Obesity, 2021

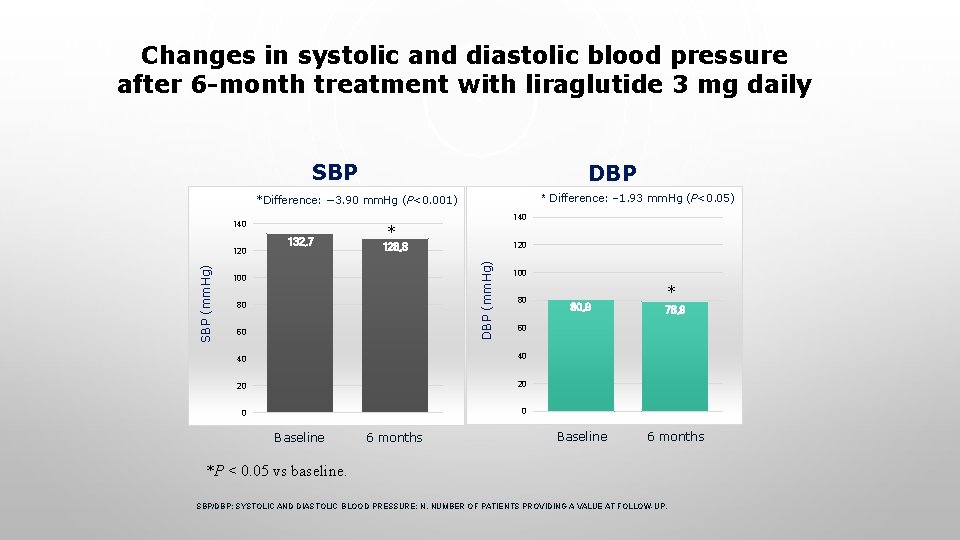
Changes in systolic and diastolic blood pressure after 6 -month treatment with liraglutide 3 mg daily SBP DBP * Difference: − 1. 93 mm. Hg (P<0. 05) *Difference: − 3. 90 mm. Hg (P<0. 001) 140 SBP * 120 128, 8 DBP (mm. Hg) SBP (mm. Hg) 120 132, 7 140 100 80 60 100 80 40 20 20 0 0 6 months 78, 9 Baseline 6 months 60 40 Baseline * 80, 9 *P < 0. 05 vs baseline. SBP/DBP; SYSTOLIC AND DIASTOLIC BLOOD PRESSURE; N, NUMBER OF PATIENTS PROVIDING A VALUE AT FOLLOW-UP.

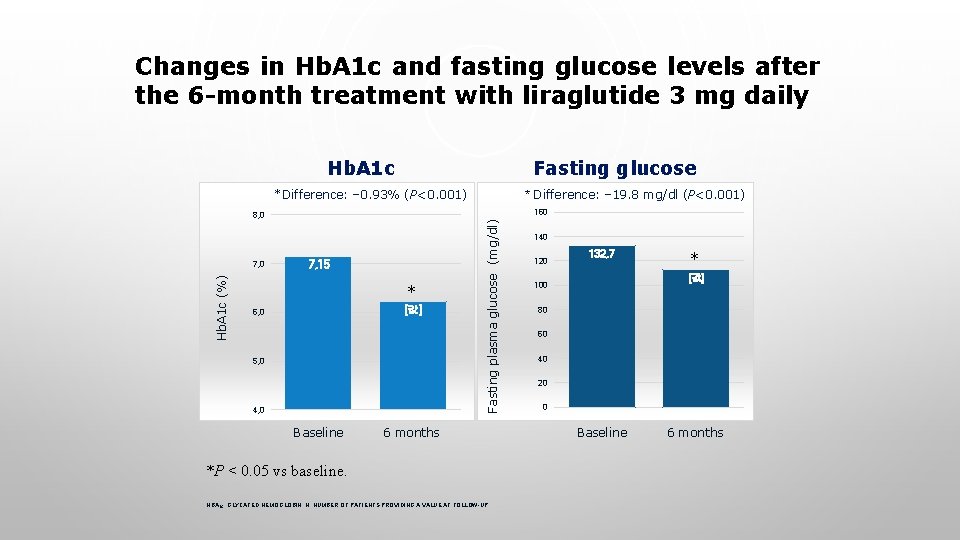
Changes in Hb. A 1 c and fasting glucose levels after the 6 -month treatment with liraglutide 3 mg daily Hb. A 1 c Fasting glucose *Difference: − 0. 93% (P<0. 001) Hb. A 1 c (%) 7, 15 * [값] 6, 0 5, 0 4, 0 Baseline Fasting plasma glucose (mg/dl) 160 8, 0 7, 0 * Difference: − 19. 8 mg/dl (P<0. 001) 6 months *P < 0. 05 vs baseline. HBA 1 C, GLYCATED HEMOGLOBIN; N, NUMBER OF PATIENTS PROVIDING A VALUE AT FOLLOW-UP. 140 120 132, 7 * [값] 100 80 60 40 20 0 Baseline 6 months

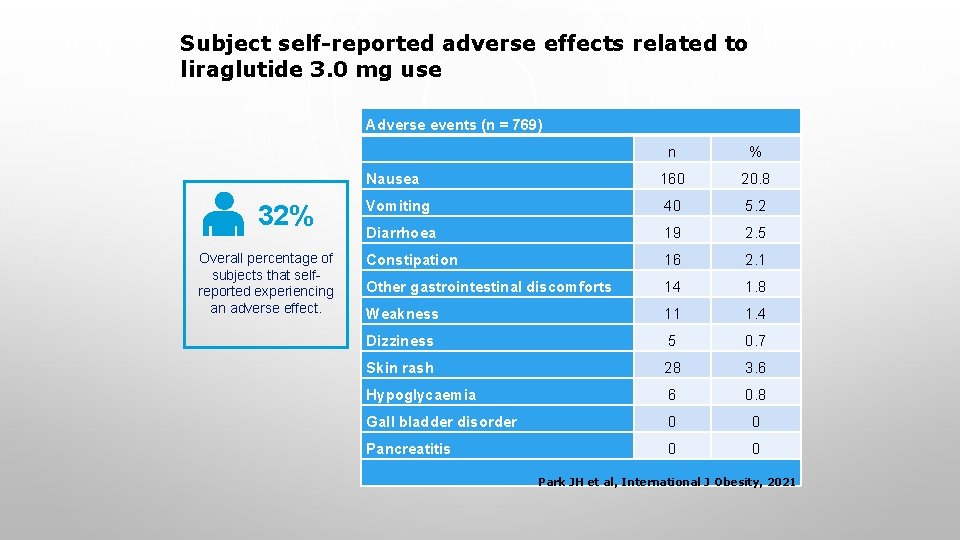
Subject self-reported adverse effects related to liraglutide 3. 0 mg use Adverse events (n = 769) 32% Overall percentage of subjects that selfreported experiencing an adverse effect. n % Nausea 160 20. 8 Vomiting 40 5. 2 Diarrhoea 19 2. 5 Constipation 16 2. 1 Other gastrointestinal discomforts 14 1. 8 Weakness 11 1. 4 Dizziness 5 0. 7 Skin rash 28 3. 6 Hypoglycaemia 6 0. 8 Gall bladder disorder 0 0 Pancreatitis 0 0 Park JH et al, International J Obesity, 2021

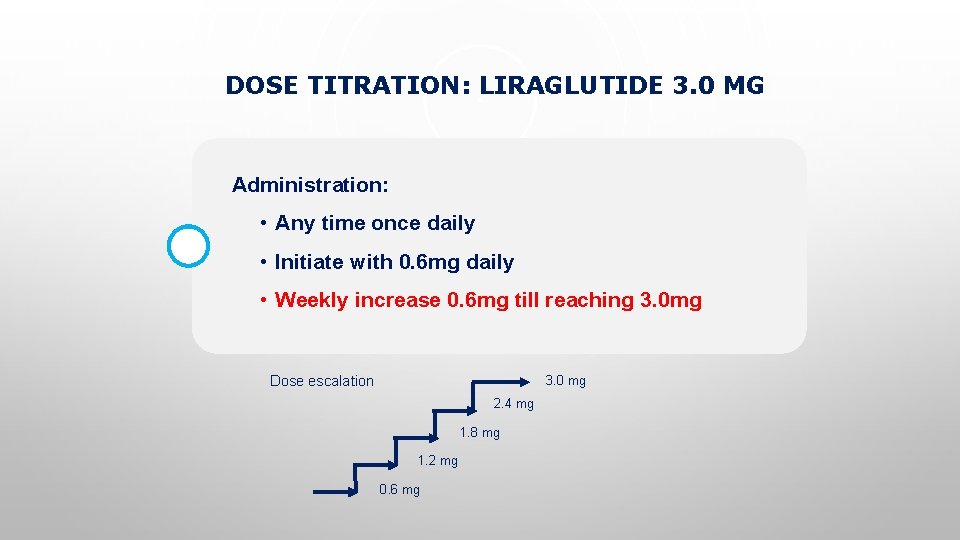
DOSE TITRATION: LIRAGLUTIDE 3. 0 MG Administration: • Any time once daily • Initiate with 0. 6 mg daily • Weekly increase 0. 6 mg till reaching 3. 0 mg Dose escalation 3. 0 mg 2. 4 mg 1. 8 mg 1. 2 mg 0. 6 mg

ADHERENCE Adherence Self-reported subject adherence was recorded for 87% in first 2 months, 55% in next 2 months, 29% in next 2 months

LIRAGLUTIDE 3. 0 MG WAS ASSOCIATED WITH CLINICALLY MEANINGFUL WEIGHT LOSS IN KOREANS WHEN COMBINED WITHOUT INTENSIVE LIFESTYLE MODIFICATION IN A REAL-WORLD SETTING, LIRAGLUTIDE 3. 0 MG DEMONSTRATED: 5. 14 kg 5. 9% average weight loss between baseline and 6 months in the 6 -month cohort average percentage weight loss between baseline and 6 months in the 6 -month cohort 52% of patients lost ≥ 5% body weight 32% of patients lost ≥ 7% body weight Decreases in Hb. A 1 c and BP were also observed No serious side events in Korean people with obesity Park JH et al, International J Obesity, 2021

KEY MESSAGES Indication: Weight management in ≥ 18 yrs old, * BMI≥ 27 + comorbidities e. g. Pre-DM, diabetes, dislipidemia/hypertension * BMI≥ 30 Pregnant, breast-feeding, fertility • Not recommended (Category X) Contraindications • Personal or family history of medullary thyroid carcinoma or MEN 2 • Hypersensitivity to liraglutide or any product components

CORONAVIRUS RELATED THREE EPIDEMIC DISEASES How to manage patients with obesity and DM During the COVID-19 pandemic



Jae Hyun Bae Hyuk-Sang Kwon Michael A. Nauck Lim S et al, Nature Reviews Endocrinology, 2021

SARS-Co. V-2 infection & glucose metabolism Infection with SARS-Co. V-2 ↑Metabolic rate ↑Tissue hypoxia Dysregulation of glucose metabolism ↑Glucotoxicity Aggravation of inflammation Endothelial damage Immune modulation ↑Oxidative stress ↑Cytokine production ↑Interstitial lung damage ↑Thromboembolic risk Damage to vital organs Acute respiratory distress syndrome Deep vein thrombosis & pulmonary embolism Multi-organ dysfunction Potential accentuated clinical process (red) after SARS-Co. V-2 entry in people with DM ↑Severity of COVID-19 and rapid progression to cardiorespiratory failure ↑Mortality Lim S et al, Nature Reviews Endocrinology, 2021

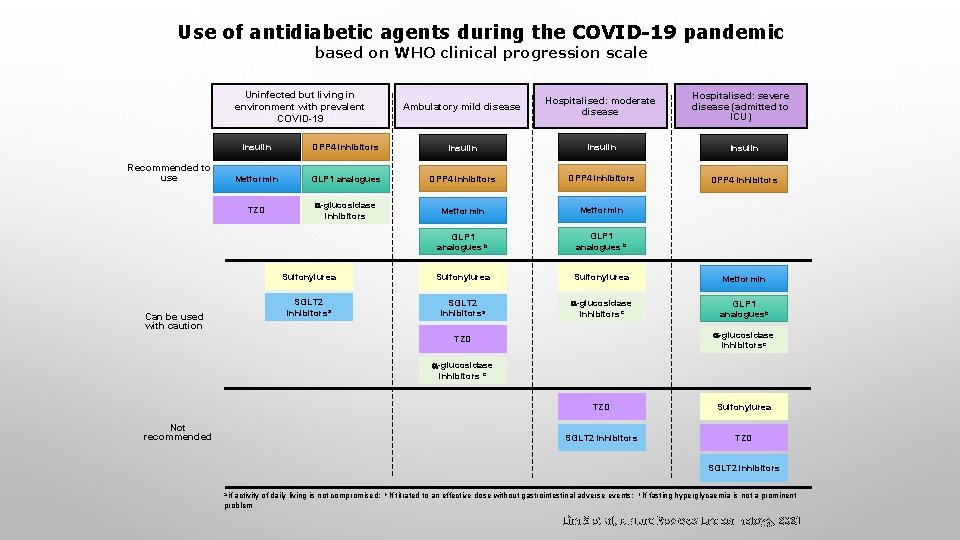
Use of antidiabetic agents during the COVID-19 pandemic based on WHO clinical progression scale Uninfected but living in environment with prevalent COVID-19 Recommended to use Can be used with caution Ambulatory mild disease Hospitalised: moderate disease Hospitalised: severe disease (admitted to ICU) Insulin DPP 4 inhibitors Insulin Metformin GLP 1 analogues DPP 4 inhibitors TZD -glucosidase inhibitors Metformin GLP 1 analogues b Sulfonylurea Metformin SGLT 2 inhibitorsa -glucosidase inhibitorsc GLP 1 analoguesb -glucosidase inhibitorsc TZD -glucosidase inhibitors c Not recommended TZD Sulfonylurea SGLT 2 inhibitors TZD SGLT 2 inhibitors a if activity of daily living is not compromised; problem b If titrated to an effective dose without gastrointestinal adverse events; c If fasting hyperglycaemia is not a prominent Lim S et al, Nature Reviews Endocrinology, 2021

Factors promoting unhealthy lifestyle and recommendations to overcome these barriers during the COVID-19 pandemic Lim Hyunjung Despres JP Lim S, Lim H, Despres JP. Obesity (Silver Spring) 2020

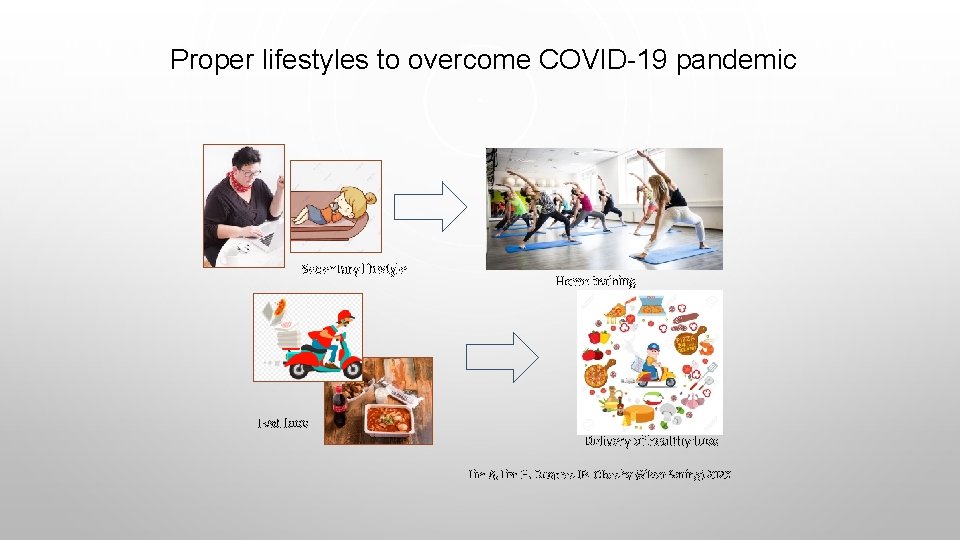
Proper lifestyles to overcome COVID-19 pandemic Sedentary lifestyle Home training Fast food Delivery of healthy food Lim S, Lim H, Despres JP. Obesity (Silver Spring) 2020

C-CELL THYROID CANCER: CONTRAINDICATION THE THYROID: RODENTS VS. HUMANS Humans Rodents Humans have fewer C-cells than rodents 1 GLP-1 receptor GLP-1 R is consistently expressed in C cells in rodents 2 C-cell GLP-1 receptor GLP-1 R expression is low or absent in normal human C cells 2, 3 1. Bjerre Knudsen Endocrinology 2010; 2. Körner J Nucl Med 2007; 3. Waser Neuroendocrinology 2011 C-cell


THANK YOU for listening. LIFESTYLE MODIFICATION
 Fasting
Fasting فریبرز فرساد
فریبرز فرساد Orlistat
Orlistat Pepsin renin
Pepsin renin Lingual lipase
Lingual lipase Lipase enzyme source
Lipase enzyme source Lacteal
Lacteal Lipase enzyme source
Lipase enzyme source Lingual lipase
Lingual lipase Uji aktivitas enzim lipase kacang tanah
Uji aktivitas enzim lipase kacang tanah Henry c groseclose
Henry c groseclose When was ffa creed adopted
When was ffa creed adopted Dripping springs ffa
Dripping springs ffa Randall waller instagram
Randall waller instagram Ffa creed 1st paragraph
Ffa creed 1st paragraph Blue and gold basics what is ffa
Blue and gold basics what is ffa Parts of the ffa emblem
Parts of the ffa emblem Ffa tool id
Ffa tool id What ffa officer is stationed by the door
What ffa officer is stationed by the door Ffa speech examples
Ffa speech examples Ffa history timeline
Ffa history timeline 3rd paragraph creed
3rd paragraph creed Em tiffany facts
Em tiffany facts Ffa official dress female pants
Ffa official dress female pants Agriscience fair project ideas
Agriscience fair project ideas Postpone definitely ffa definition
Postpone definitely ffa definition What are the four common methods of voting in ffa
What are the four common methods of voting in ffa Ffa greenhand degree requirements
Ffa greenhand degree requirements Spring branch ffa
Spring branch ffa Parts of the ffa emblem
Parts of the ffa emblem Javier moreno ffa
Javier moreno ffa Ffa colors meaning
Ffa colors meaning The fccla creed
The fccla creed Ffa 3 circle model
Ffa 3 circle model How to remember the ffa creed
How to remember the ffa creed When was the ffa foundation formed
When was the ffa foundation formed What are the official ffa colors
What are the official ffa colors Cinco ranch ffa
Cinco ranch ffa What is the ffa creed
What is the ffa creed Ffa back in the day
Ffa back in the day Ffa chapter degree pin
Ffa chapter degree pin Greenhand degree
Greenhand degree Ffa crossword puzzle
Ffa crossword puzzle Ffa symbols and meanings
Ffa symbols and meanings Ffa brotherhood pledge
Ffa brotherhood pledge Public law 740 passed by congress
Public law 740 passed by congress What are the official ffa colors? *
What are the official ffa colors? * Ffa unison
Ffa unison Ffa history
Ffa history Write the ffa mission in green
Write the ffa mission in green Brooksville sr ffa
Brooksville sr ffa Ffa emblem symbols
Ffa emblem symbols Ffa official dress
Ffa official dress 1926 ffa
1926 ffa Ffa creed 2 paragraph
Ffa creed 2 paragraph Ffa to practice brotherhood
Ffa to practice brotherhood Ffa officers and symbols
Ffa officers and symbols What are the ffa colors
What are the ffa colors Places and faces of the ffa
Places and faces of the ffa What are the ten essentials of a successful ffa chapter
What are the ten essentials of a successful ffa chapter