Origin of mineral deposits part 01 Origin of











- Slides: 20

Origin of mineral deposits (part 01)

Origin of the mineral deposits A. Syngenetic mineral deposits B. Epigenetic mineral deposits

Origin of the mineral deposits The processes by which mineral deposits are formed: A. Endogenic processes: A. Magmatic concentration B. Hydrothermal C. Contact processes metasomatism D. Metamorphism B. Exogenic processes: A. Sedimentation B. Mechanical and evaporation concentration C. Volcanic-sedimentary processes

1 - Magmatic concentration mineral deposits which are formed during crystallization of a magma are called magmatic mineral deposits. They are massive and disseminated. massive ores are formed by: A. Gravitational settling B. Differentiation C. Immiscible separations

1 - Magmatic concentration A. Gravitational settling: gravitational settling causes early-formed minerals to sink to the bottom of a magma chamber Occurs in ultramafic and mafic magmas

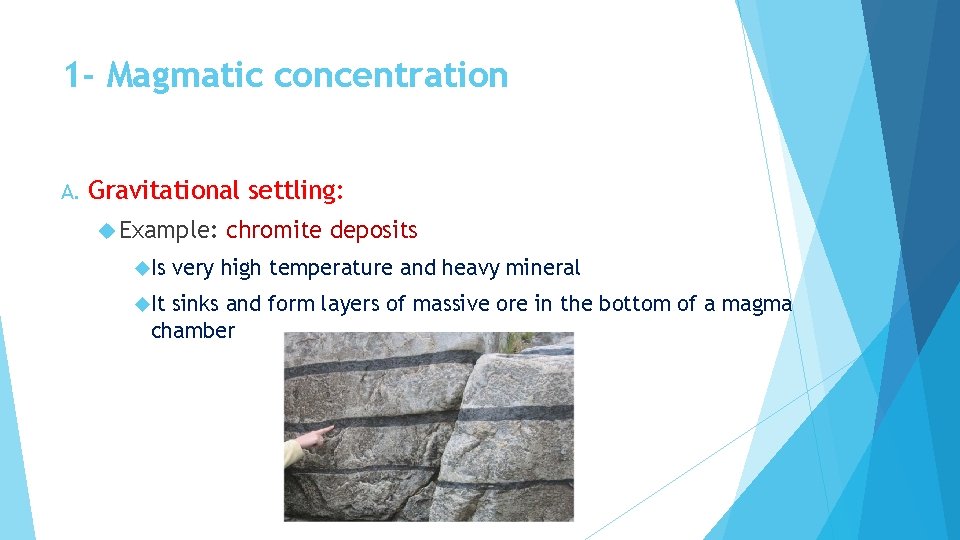
1 - Magmatic concentration A. Gravitational settling: Example: Is It chromite deposits very high temperature and heavy mineral sinks and form layers of massive ore in the bottom of a magma chamber

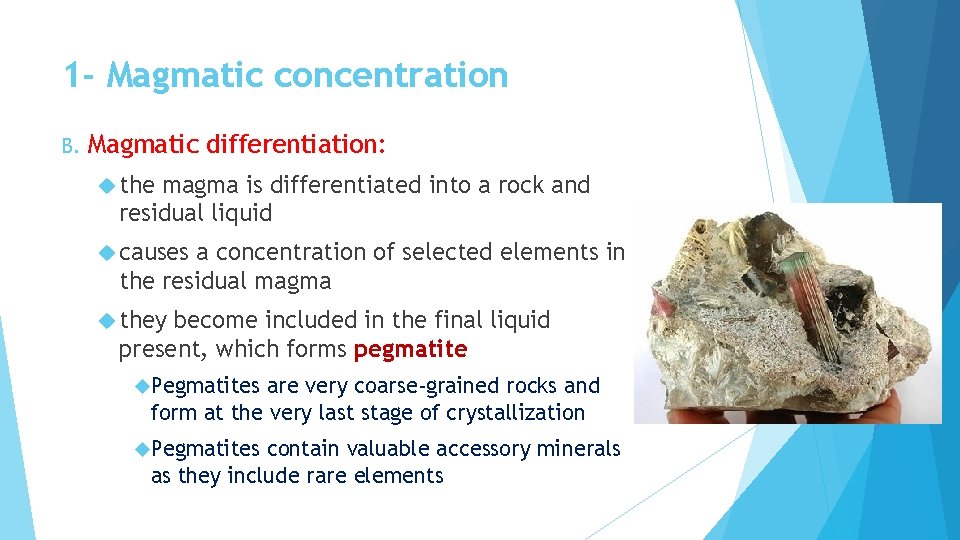
1 - Magmatic concentration B. Magmatic differentiation: the magma is differentiated into a rock and residual liquid causes a concentration of selected elements in the residual magma they become included in the final liquid present, which forms pegmatite Pegmatites are very coarse-grained rocks and form at the very last stage of crystallization Pegmatites contain valuable accessory minerals as they include rare elements

1 - Magmatic concentration C. Immiscible separation: Immiscibility is separation of an initially homogeneous magma liquid into two compositionally distinct immiscible liquids Immiscible melts form irregular shaped segregations or may be injected as a dike into previously crystallized material iron sulfide is the principal constituent of most immiscible magmas: Immiscible sulfide drops are separated and form immiscible magma layers in a magma chamber when layers of sulfide magma cool and crystallize, the result is a deposit of ore minerals of copper, nickel, and platinum-group metals in a gangue of an iron sulfide mineral.

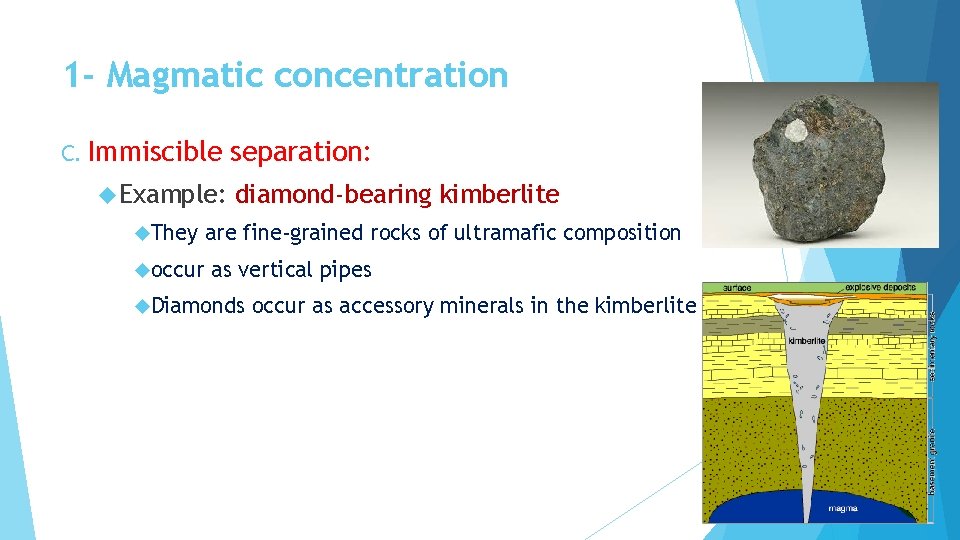
1 - Magmatic concentration C. Immiscible Example: They occur separation: diamond-bearing kimberlite are fine-grained rocks of ultramafic composition as vertical pipes Diamonds occur as accessory minerals in the kimberlite

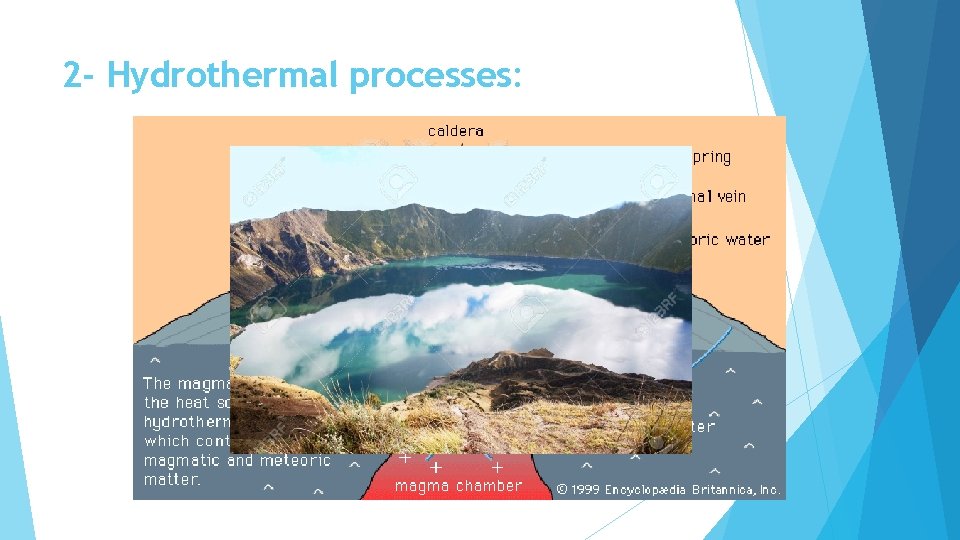
2 - Hydrothermal processes: Hydrothermal solutions are hot, aqueous, metal-saturated fluids that react chemically with crustal rocks They are effective solvents of many sulfide and oxide ore minerals hydrothermal mineral deposits

2 - Hydrothermal processes:

2 - Hydrothermal processes:

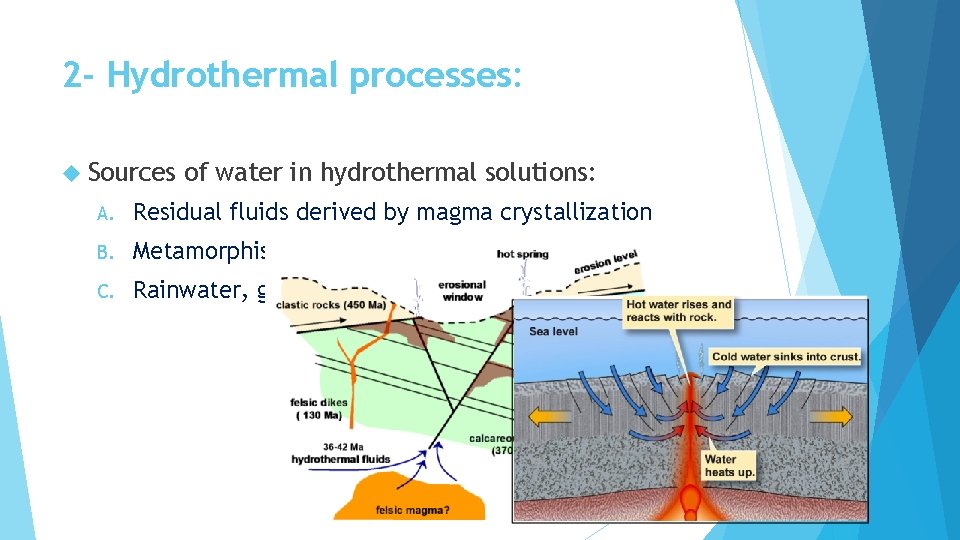
2 - Hydrothermal processes: Sources of water in hydrothermal solutions: A. Residual fluids derived by magma crystallization B. Metamorphism C. Rainwater, groundwater or seawater

2 - Hydrothermal processes: the final compositions of all hydrothermal solutions are due to reactions between solutions and the rocks they encounter The metallic elements are deposited to form the ore and gangue, in response to a change in the solution and very sharp decrease in temperature

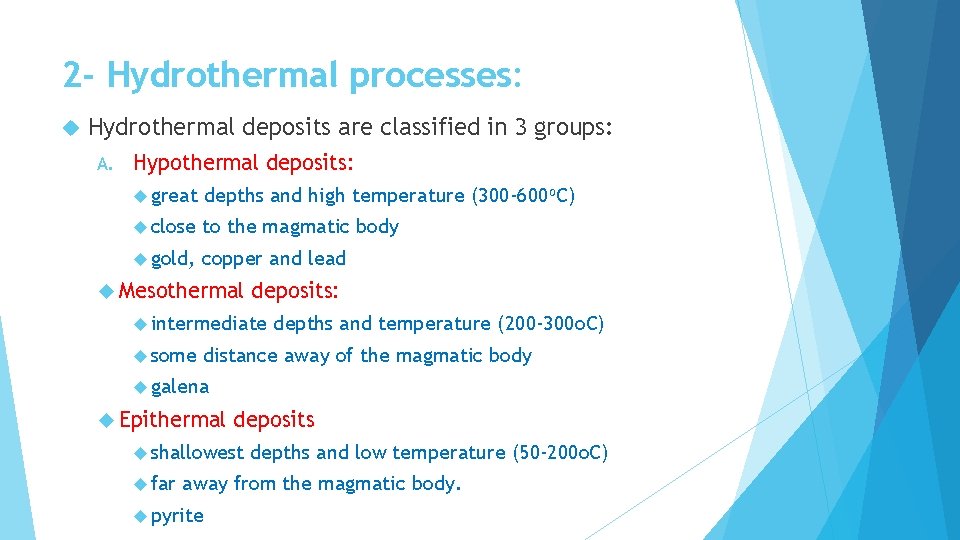
2 - Hydrothermal processes: Hydrothermal deposits are classified in 3 groups: A. Hypothermal deposits: great depths and high temperature (300 -600 o. C) close to the magmatic body gold, copper and lead Mesothermal deposits: intermediate some depths and temperature (200 -300 o. C) distance away of the magmatic body galena Epithermal deposits shallowest far depths and low temperature (50 -200 o. C) away from the magmatic body. pyrite

2 - Hydrothermal processes: Mineral deposits are formed by hydrothermal solutions by two processes Cavity filling Replacement

2 - Hydrothermal processes: 1. Cavity filling: ore deposits that get deposited from hydrothermal solutions in rock openings (cavities). Epithermal deposits veins hydrothermal vein deposits

2 - Hydrothermal processes: 1. Cavity filling: When the hydrothermal solution interacts with carbonate rocks they form caves Solution cavity filling Barite

2 - Hydrothermal processes: 1. Cavity filling: also occur as layered deposits known as stratabound deposits

2 - Hydrothermal processes: 2. Replacement: the minerals dissolved in hydrothermal solutions are substituted for one or more earlier formed minerals in the encountered rocks mesothermal and hypothermal deposits