ORIGIN EVOLUTION OF ATMOSPHERES Atmospheric Basics Layer of

ORIGIN & EVOLUTION OF ATMOSPHERES

Atmospheric Basics Ø Layer of gas surrounding a planet. Ø Usually very thin for terrestrial planets (except for Venus). Ø Affects conditions on the planet. Ø We would like to understand how each of the terrestrial planets ended up having such different atmospheres. Venus’s thick atmosphere

Atmospheres are created by three processes: – Outgassing – Evaporation/sublimation – Impacts • All planets probably had minimal (primary. H, He) atmospheres at some point after they formed

The original primary atmospheres (H, He) were swept away from the terrestrial planets early in their life. Current atmospheres are secondary atmospheres, formed primarily by outgassing: composed mostly of carbon dioxide – CO 2 or, in the case of the Earth, a tertiary atmosphere of oxygen and nitrogen (secondary atmosphere modified by life and presence of liquid water)

Holding onto an atmosphere requires gravity Ø The strength of gravity determines the escape velocity from the planet. Ø The temperature and composition of an atmosphere determines the velocities of atoms and molecules in the atmosphere. (at a given temperature, H and He will have higher velocities than more massive elements or molecules)

Holding onto an atmosphere requires gravity Ø The strength of gravity determines the escape velocity from the planet. Ø The temperature and composition of an atmosphere determines the velocities of atoms and molecules in the atmosphere. Ø If the constituents of an atmosphere are moving faster than escape velocity, then a planet or moon will be unable to hold onto an atmosphere.

–Larger, cooler planets can hold onto atmospheres better than smaller hotter planets

• Which Terrestrial planets have atmospheres? – Venus has the most – Earth has some – Mars has a little – Mercury and the Moon have essentially none • By contrast, the Jovian planets (high gravity, cool/cold atmospheres) have very substantial atmospheres.

• Which moons have atmospheres? ØJupiter's Io (tenuous sulfur dioxide) ØJupiter's Europa (tenuous oxygen) ØSaturn's largest moon Titan (dense nitrogen & methane) ØNeptune's largest moon Triton (tenuous nitrogen & methane).

• Moon and Mercury “Airless” worlds Ø gravity too weak to hold onto an atmosphere Ø“black sky” Ø The little atmosphere that exists consists of particles of the solar wind that are temporarily trapped.

• Mars Ø Very little atmosphere today (CO 2) ØMars had standing and running water on its surface in the past. Ø Therefore, it must have had a more substantial atmosphere in the past Ø Does it have water today? Yes - frozen in polar ice caps and possibly beneath its soil

• Earth Ø A moderate atmosphere today Ø Mostly nitrogen (N 2), with some oxygen (O 2), carbon dioxide (CO 2), etc. Ø Enough to enable liquid water to exist (temperature and pressure adequate) Ø Together the air & water produce erosion

• Venus Ø Densest atmosphere of all Terrestrials Ø Mostly CO 2 Ø Temperature at surface hot enough to melt lead Ø Pressure at the surface ~ 90 times that on Earth Ø Perpetual cloud cover, sulfuric acid rain

How do we account for these differences? ØComposition of outgassed gasses The dominant gasses arising from outgassing were carbon dioxide and water vapor, with minor amounts of nitrogen, sulfer, argon, … Each terrestrial planet’s outgassed atmosphere was roughly the same.

How do we account for these differences? ØComposition of outgassed gasses (Carbon dioxide and water vapor) ØTemperature appropriate for liquid water? Distance from the sun, amount of greenhouse gasses in atmosphere. Oceans absorb carbon dioxide -> carbonate rocks

How do we account for these differences? ØComposition of outgassed gasses (Carbon dioxide and water vapor) ØTemperature appropriate for liquid water? (Carbon dioxide dissolves in oceans) ØInteraction of light and atmospheres Dissociation of water and ammonia molecules by UV light, and warming by the greenhouse effect
How do we account for these differences? ØComposition of outgassed gasses (Carbon dioxide and water vapor) ØTemperature appropriate for liquid water? (Carbon dioxide dissolves in oceans) ØInteraction of light and atmospheres (dissociation and the greenhouse effect) Ø Photosynthetic life - generation of oxygen

LAYERING OF ATMOSPHERES

Structure is created within an atmosphere through interactions of atmospheric gasses with light
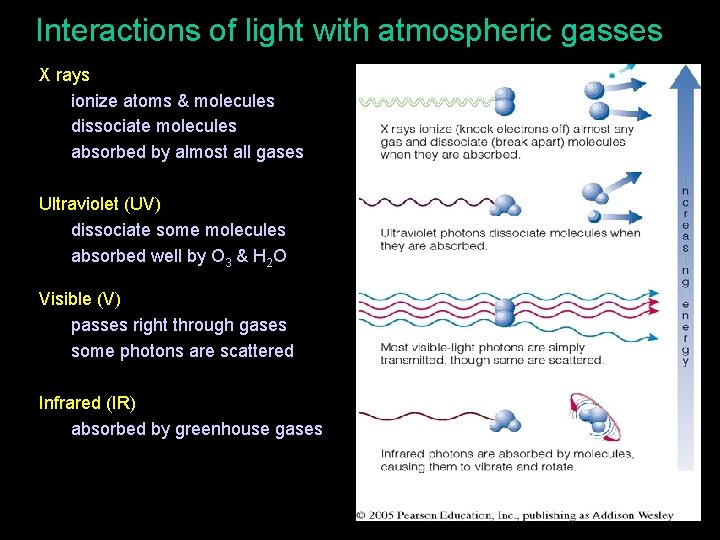
Interactions of light with atmospheric gasses • X rays • ionize atoms & molecules • dissociate molecules • absorbed by almost all gases • Ultraviolet (UV) • dissociate some molecules • absorbed well by O 3 & H 2 O • Visible (V) • passes right through gases • some photons are scattered • Infrared (IR) • absorbed by greenhouse gases
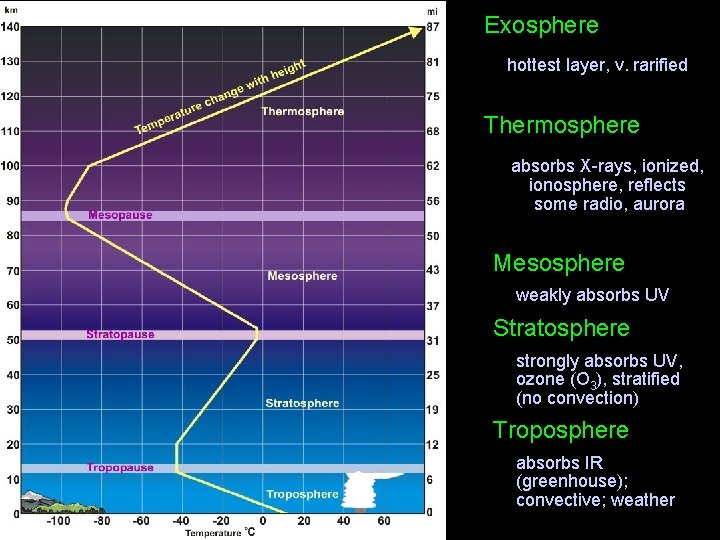
Exosphere hottest layer, v. rarified Thermosphere absorbs X-rays, ionized, ionosphere, reflects some radio, aurora Mesosphere • weakly absorbs UV Stratosphere • strongly absorbs UV, ozone (O 3), stratified (no convection) Troposphere • absorbs IR (greenhouse); convective; weather
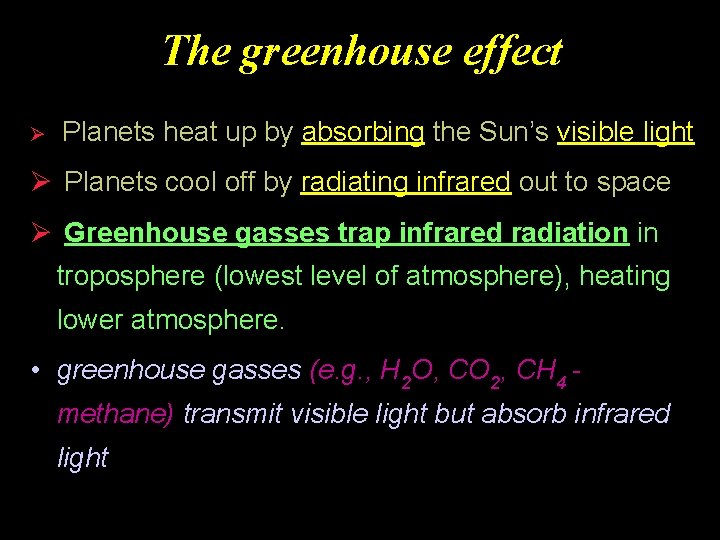
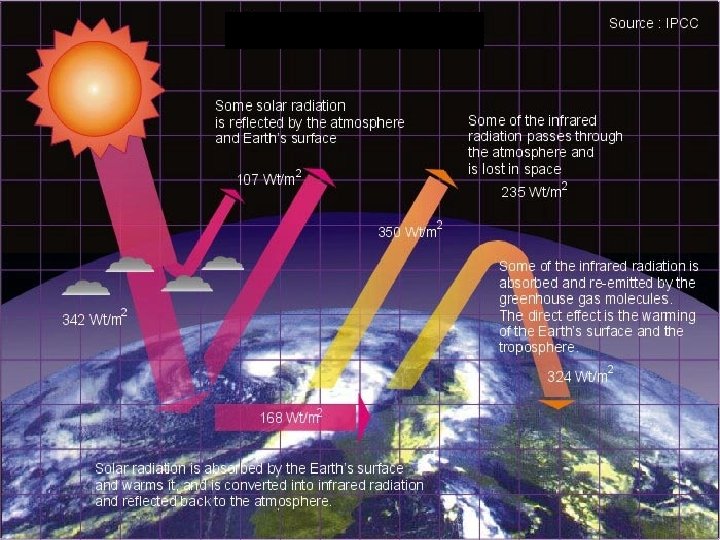
The greenhouse effect Ø Planets heat up by absorbing the Sun’s visible light Ø Planets cool off by radiating infrared out to space Ø Greenhouse gasses trap infrared radiation in troposphere (lowest level of atmosphere), heating lower atmosphere. • greenhouse gasses (e. g. , H 2 O, CO 2, CH 4 methane) transmit visible light but absorb infrared light
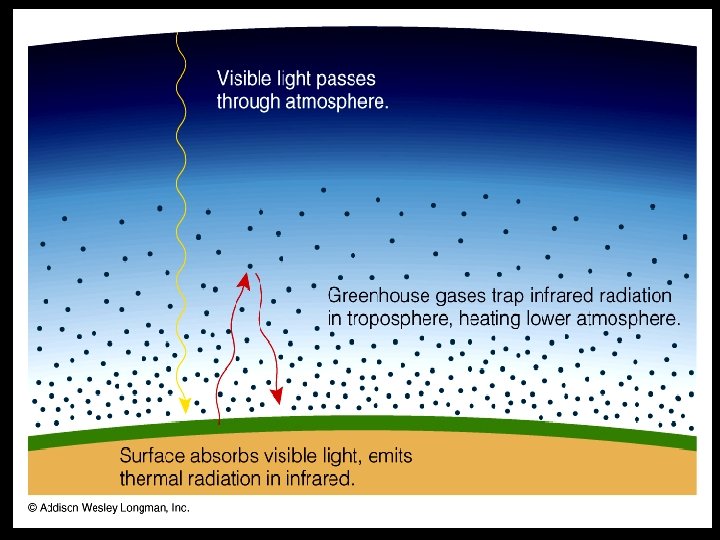
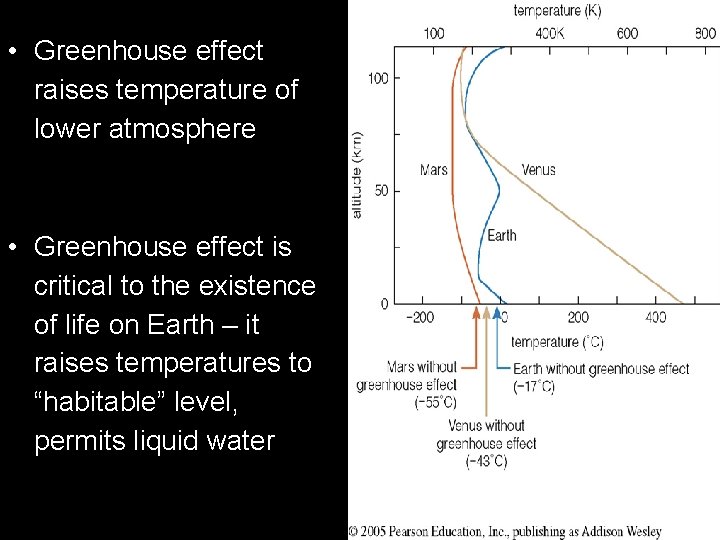
• Greenhouse effect raises temperature of lower atmosphere • Greenhouse effect is critical to the existence of life on Earth – it raises temperatures to “habitable” level, permits liquid water

Think about it. Keeping in mind that H 2 O is a strong greenhouse gas, why is it that …

During the winter, the coldest nights are cloudless?

During the summer, violent storms are associated with high humidity?

Deserts experience large temperature swings between daytime and night time?

Terrestrial planets: atmospheres • Question: Why does Venus have so much more atmosphere than Earth? • The answer is found in what Venus’ atmosphere is made of: CO 2 (carbon monoxide)

• What happens if there is a lot of CO 2 in a planet’s atmosphere? ● Due to the large amount of CO 2 in its atmosphere, the surface temperature on Venus is over 700 K, instead of the 230 K that it should be at this distance from the Sun. ● ● Does this also explain why Venus has so much atmosphere? ? ? YES!

Evolution of Atmospheres: Earth vs. Venus because water can exist in liquid form On Earth there are oceans Original CO 2 has dissolved into oceans, rocks (carbonates) which keeps levels of CO 2 just balanced in atmosphere keeps planet WARM but not HOT if planet were hotter, CO 2, H 2 O would be boiled out of oceans and baked out of rocks more CO 2, H 2 O enter Atmosphere
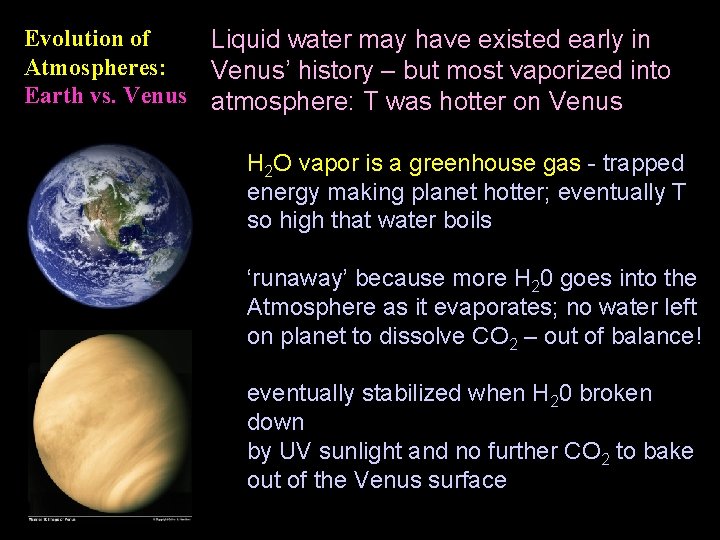
Evolution of Liquid water may have existed early in Atmospheres: Venus’ history – but most vaporized into Earth vs. Venus atmosphere: T was hotter on Venus H 2 O vapor is a greenhouse gas - trapped energy making planet hotter; eventually T so high that water boils ‘runaway’ because more H 20 goes into the Atmosphere as it evaporates; no water left on planet to dissolve CO 2 – out of balance! eventually stabilized when H 20 broken down by UV sunlight and no further CO 2 to bake out of the Venus surface

• Earth has about the same amount of CO 2 as Venus • Much of the Earth’s CO 2 is ‘frozen’ into the rocks • However, if we could raise the temperature of our atmosphere a little bit, it would release a little bit more CO 2 into the air • This would trap a little bit more heat, raising the temperature a little bit more… • This would release a little bit more CO 2… • …which would trap a little bit more heat…

• …which would raise the temperature a little bit more… • …which would release a little bit more CO 2… • …which would trap a little bit more heat… • …which would raise the temperature a little bit more… • …which would release a little bit more CO 2… You get the idea!

• This is called the runaway greenhouse effect • It happened on Venus because Venus is closer to the Sun • So - Earth has less atmosphere because most of our CO 2 is still frozen in rocks
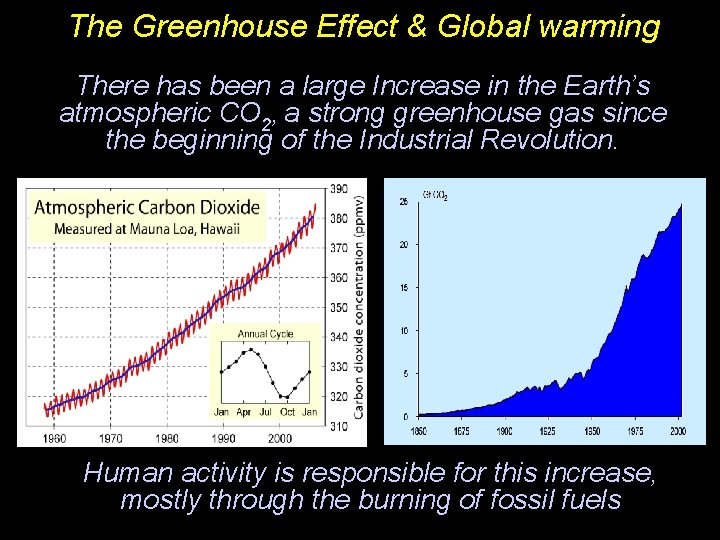
The Greenhouse Effect & Global warming There has been a large Increase in the Earth’s atmospheric CO 2, a strong greenhouse gas since the beginning of the Industrial Revolution. Human activity is responsible for this increase, mostly through the burning of fossil fuels
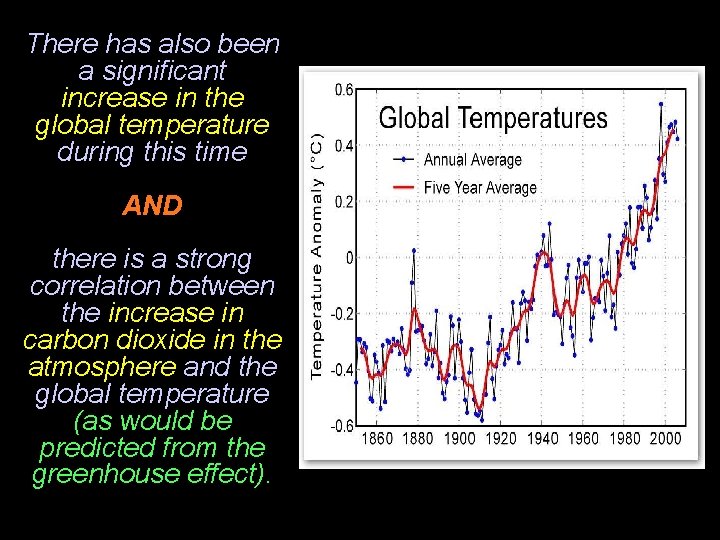
There has also been a significant increase in the global temperature during this time AND there is a strong correlation between the increase in carbon dioxide in the atmosphere and the global temperature (as would be predicted from the greenhouse effect).
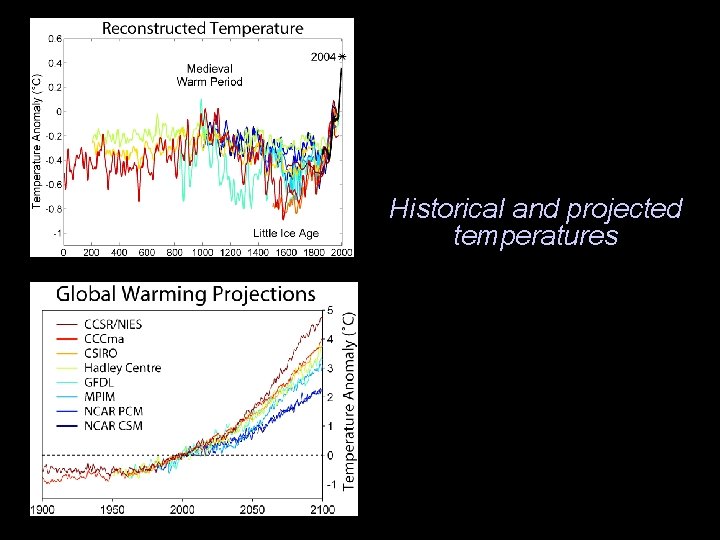
Historical and projected temperatures

Essentially all evidence indicates that human activity is a significant contributor (50 - 100%) to this trend.

What Determines a Planet’s Surface Temperature? In the absence of the Greenhouse Effect: Ø the planet's distance from the Sun Ø the planet’s overall reflectivity • the higher the albedo (reflectivity), the less light absorbed planet cooler

What Determines a Planet’s Surface Temperature? • Greenhouse Effect. Ø cannot change incoming Sunlight, so it cannot change the total energy returned to space Ø it increases the energy (heat) in lower atmosphere, keeping the surface warmer Ø It works like a blanket

Greenhouse Effect on the Planets • Greenhouse Effect warms Venus, Earth, & Mars Øon Venus: it is very strong Øon Earth: it is moderate Øon Mars: it is weak Øavg. temp. on Venus & Earth would be freezing without it
- Slides: 43