ORGANOMETALLIC CHEMISTRY Main Group metals Dr S Danialdoss


![2) Zeise’s Salt synthesized in 1827 = K[Pt(C 2 H 4)Cl 3] • H 2) Zeise’s Salt synthesized in 1827 = K[Pt(C 2 H 4)Cl 3] • H](https://slidetodoc.com/presentation_image/5bc4e35c4abd22e5d7f5fef4bd3123c0/image-5.jpg)









- Slides: 50

ORGANOMETALLIC CHEMISTRY Main Group metals Dr. S. Danialdoss Assistant Professor Chemistry Department Loyola College, Chennai-34 1

What is Organometallic Chemistry ? An area which bridges organic and inorganic chemistry A class of compounds wherein one or more metal-carbon bonds exists 2

�Organometallic compounds around us Stainless steel (Fe-C ) Cyanocobalamin (Vitamin B-12) (Co – C bond) 3

A brief history of organometallic chemistry 1) Organometallic Chemistry has really been around for millions of years Naturally occurring Cobalimins contain Co—C bonds Vitamin B 12 4
![2 Zeises Salt synthesized in 1827 KPtC 2 H 4Cl 3 H 2) Zeise’s Salt synthesized in 1827 = K[Pt(C 2 H 4)Cl 3] • H](https://slidetodoc.com/presentation_image/5bc4e35c4abd22e5d7f5fef4bd3123c0/image-5.jpg)
2) Zeise’s Salt synthesized in 1827 = K[Pt(C 2 H 4)Cl 3] • H 2 O Confirmed to have H 2 C=CH 2 as a ligand in 1868 Structure not fully known until 1975 3) Ni(CO)4 synthesized in 1890 4) Grignard Reagents (XMg. R) synthesized about 1900 Accidentally produced while trying to make other compounds Utility to Organic Synthesis recognized early on 5

5) Ferrocene synthesized in 1951 � Modern Organometallic Chemistry begins with this discovery (Paulson and Miller) � 1952 Fischer and Wilkinson 6

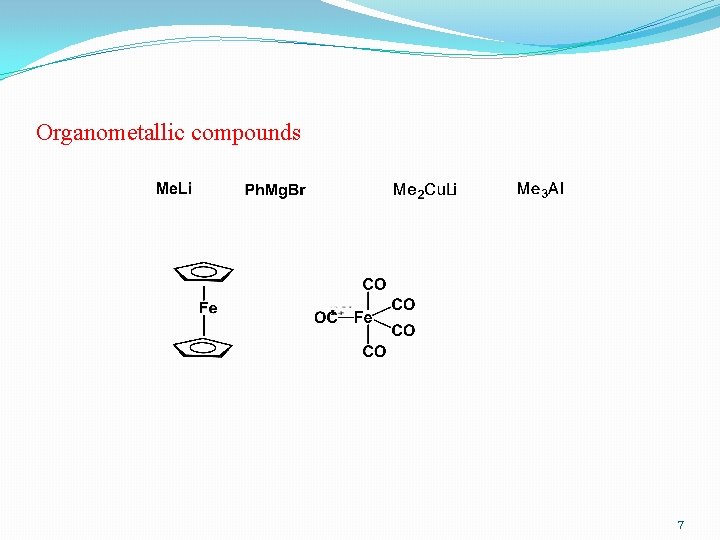
Organometallic compounds 7

Nobel -Prize Winners related to the area: Victor Grignard and Paul Sabatier (1912) Grignard reagent K. Ziegler, G. Natta (1963) Zieglar-Natta catalyst E. O. Fisher, G. Wilkinson (1973) Sandwich compounds K. B. Sharpless, R. Noyori (2001) Hydrogenation and oxidation Yves Chauvin, Robert H. Grubbs, Richard R. Schrock (2005) Metal-catalyzed alkene metathesis 8

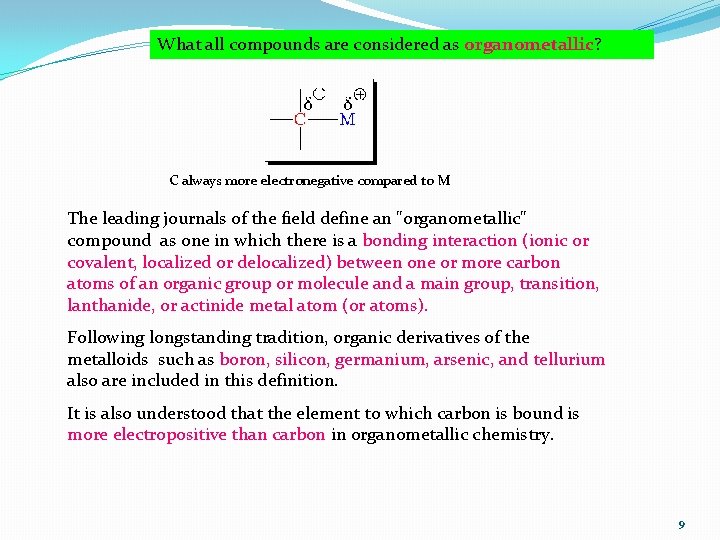
What all compounds are considered as organometallic? C always more electronegative compared to M The leading journals of the field define an "organometallic" compound as one in which there is a bonding interaction (ionic or covalent, localized or delocalized) between one or more carbon atoms of an organic group or molecule and a main group, transition, lanthanide, or actinide metal atom (or atoms). Following longstanding tradition, organic derivatives of the metalloids such as boron, silicon, germanium, arsenic, and tellurium also are included in this definition. It is also understood that the element to which carbon is bound is more electropositive than carbon in organometallic chemistry. 9

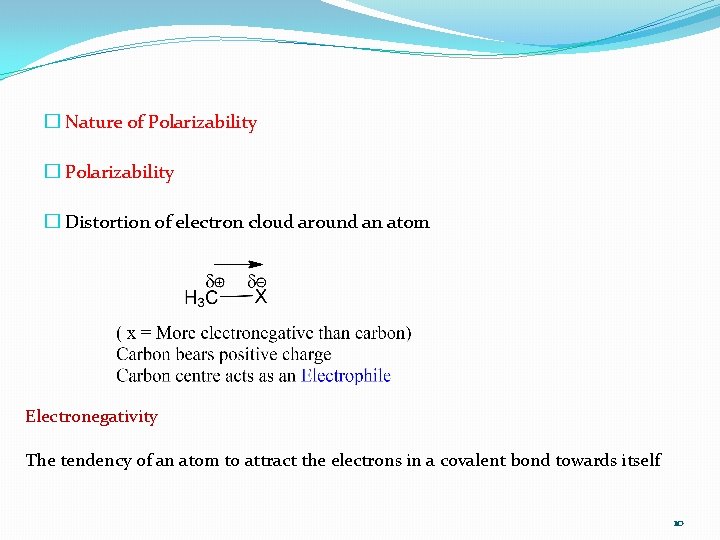
� Nature of Polarizability � Distortion of electron cloud around an atom Electronegativity The tendency of an atom to attract the electrons in a covalent bond towards itself 10

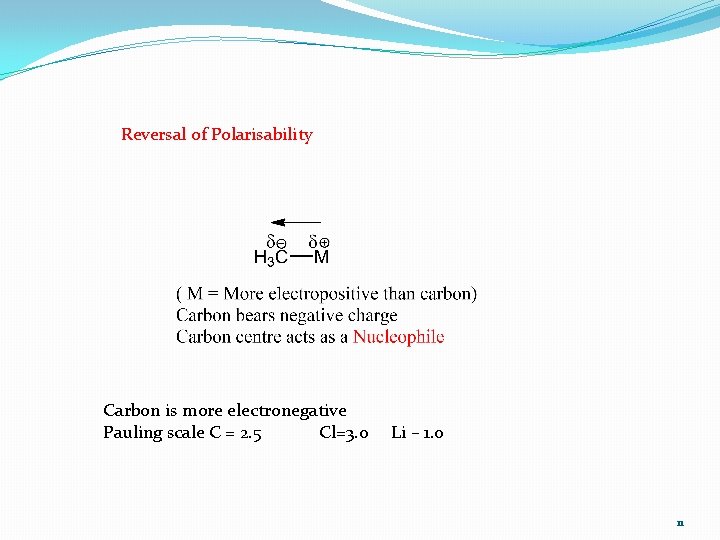
Reversal of Polarisability Carbon is more electronegative Pauling scale C = 2. 5 Cl=3. 0 Li – 1. 0 11

Application Carbanionic character of organometallic compounds is responsible for their usefulness as synthetic reagents 12

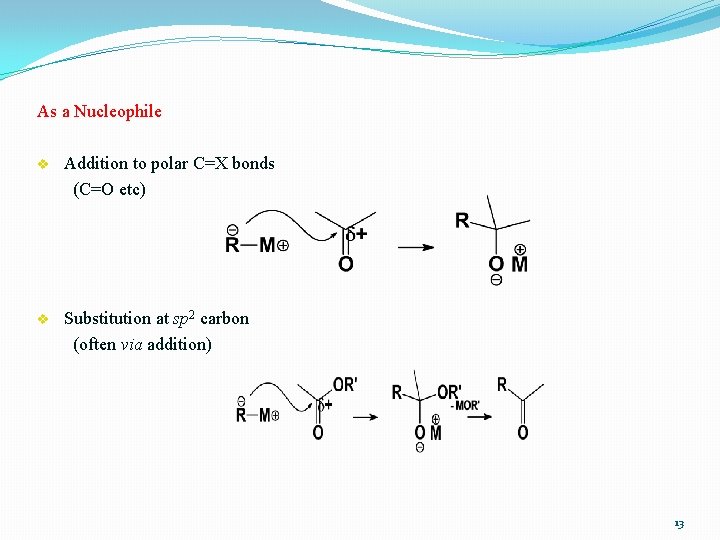
As a Nucleophile v Addition to polar C=X bonds (C=O etc) v Substitution at sp 2 carbon (often via addition) 13

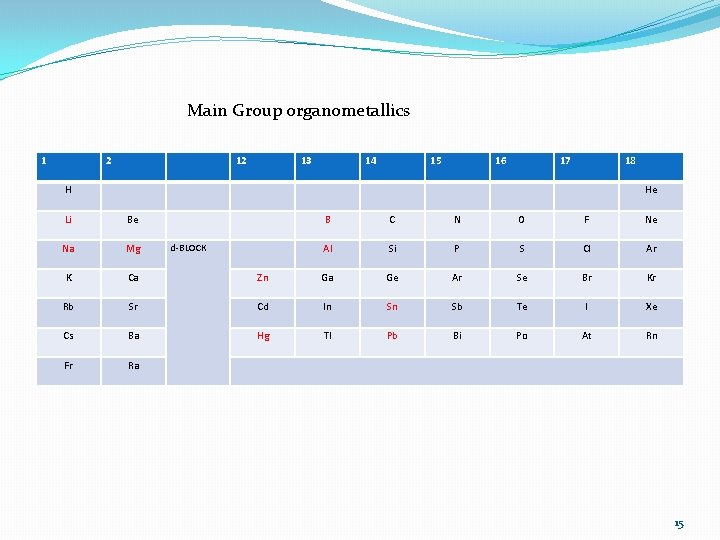
Main group organometallics at a glance � Structures � s bonds and 3 c-2 e (or even 4 c-2 e) bonds � Synthesis � the first M-C bond � Reactivity � nucleophilic and basic � auxiliaries in organic synthesis � source of organic groups for transition metals Main-Group Organometallics 14

Main Group organometallics 1 2 12 13 14 15 16 17 18 H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Zn Ga Ge Ar Se Br Kr Rb Sr Cd In Sn Sb Te I Xe Cs Ba Hg Tl Pb Bi Po At Rn Fr Ra d-BLOCK 15

Structures � Not always 8/18 e � 8/18 e "preference" rather than "rule" 16

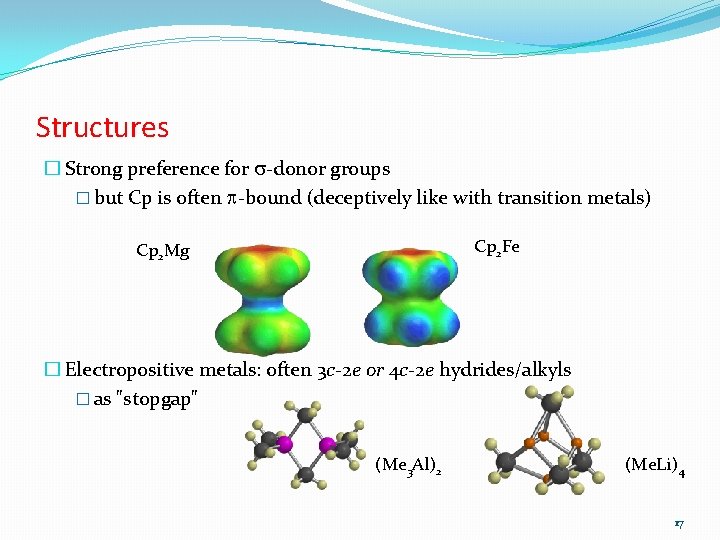
Structures � Strong preference for s-donor groups � but Cp is often p-bound (deceptively like with transition metals) Cp 2 Fe Cp 2 Mg � Electropositive metals: often 3 c-2 e or 4 c-2 e hydrides/alkyls � as "stopgap" (Me 3 Al)2 (Me. Li)4 17

Organometallic compounds as bases and Nuceophiles 18

Organolithium Reagents � Organolithium reagents are strong bases and nucleophiles. � Due to their thermal instability and extremely high reactivity they require elaborate precautions during use. Many organolithium reagents are commercially available as dilute solution in hydrocarbon solvents. � In such solvents they are polymeric species with n = 4 to 6. � In ethers, however, they are mostly tetrameric in nature. � In the presence of strong chelating ligands such as HMPA and DMPU, the degree of association decreases and they exist as monomeric species. 19

Preparation Simple organolithium reagents are prepared by the reaction of organic halides with lithium metal. The order of reactivity of the organic halides decreases in the following order RI > RBr > RCl. 20

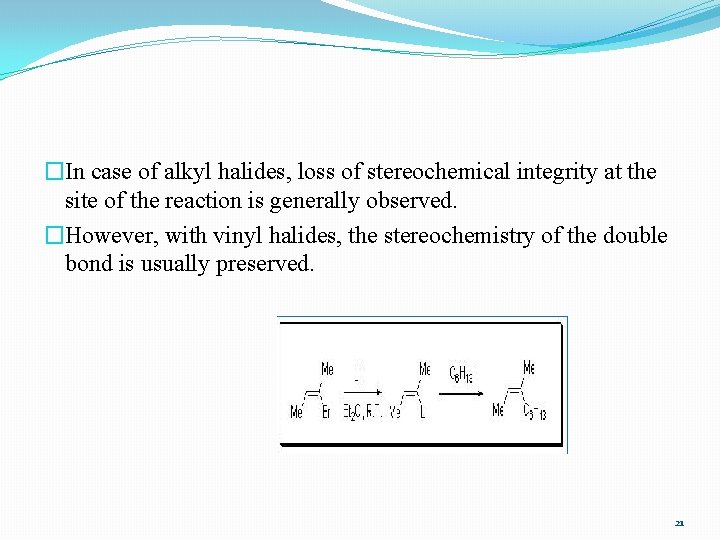
�In case of alkyl halides, loss of stereochemical integrity at the site of the reaction is generally observed. �However, with vinyl halides, the stereochemistry of the double bond is usually preserved. 21

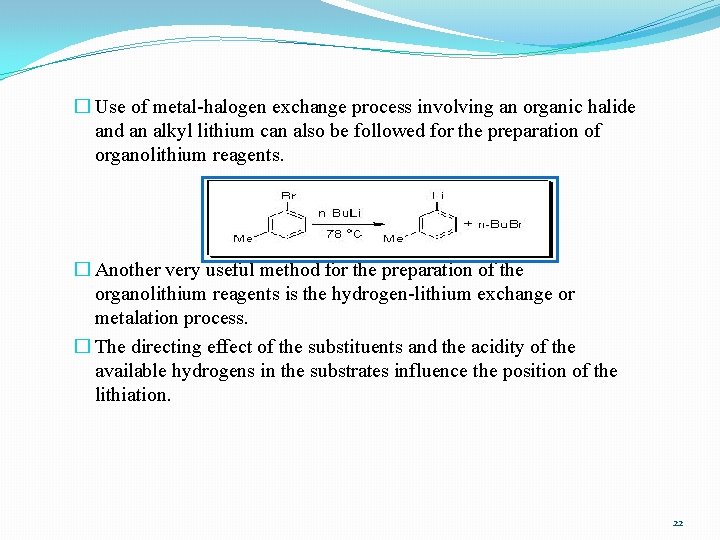
� Use of metal-halogen exchange process involving an organic halide and an alkyl lithium can also be followed for the preparation of organolithium reagents. � Another very useful method for the preparation of the organolithium reagents is the hydrogen-lithium exchange or metalation process. � The directing effect of the substituents and the acidity of the available hydrogens in the substrates influence the position of the lithiation. 22

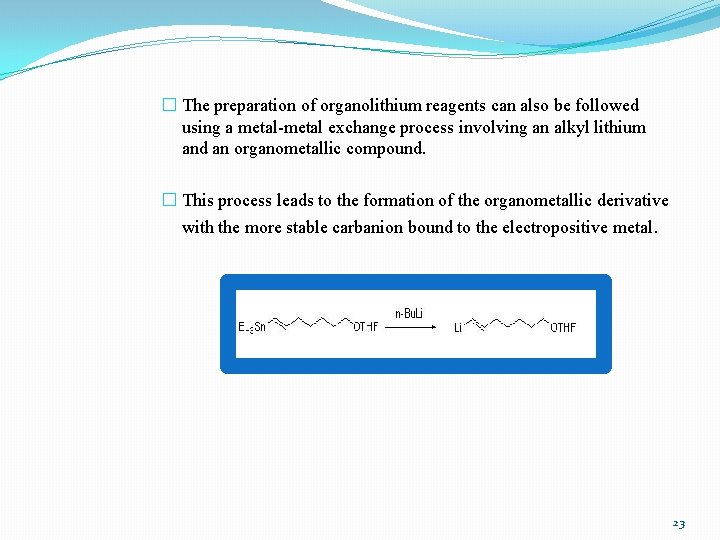
� The preparation of organolithium reagents can also be followed using a metal-metal exchange process involving an alkyl lithium and an organometallic compound. � This process leads to the formation of the organometallic derivative with the more stable carbanion bound to the electropositive metal. 23

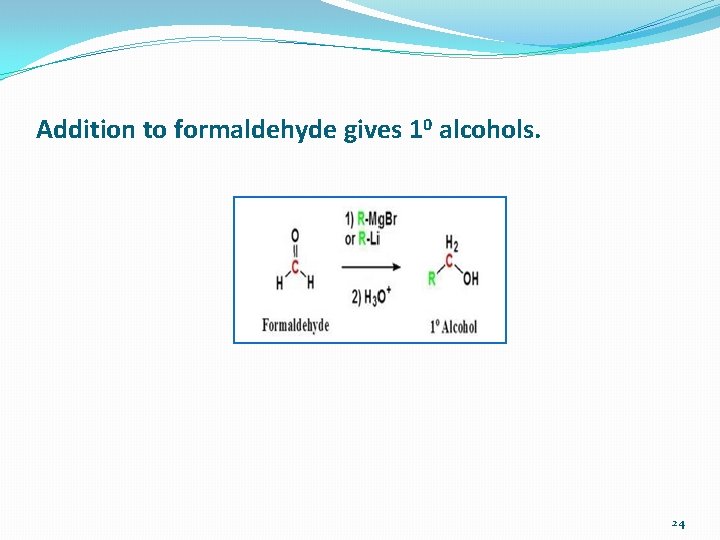
Addition to formaldehyde gives 10 alcohols. 24

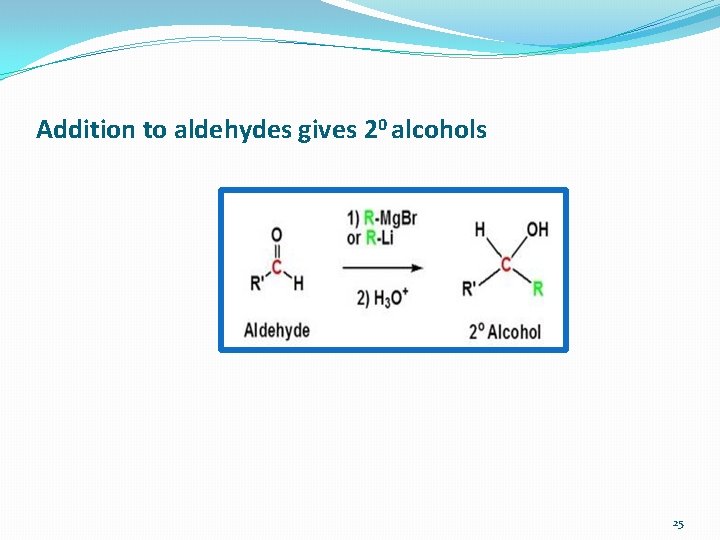
Addition to aldehydes gives 20 alcohols 25

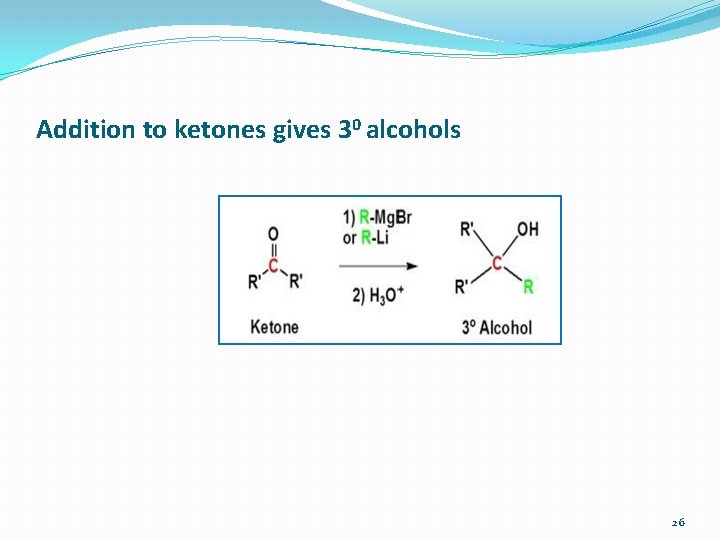
Addition to ketones gives 30 alcohols 26

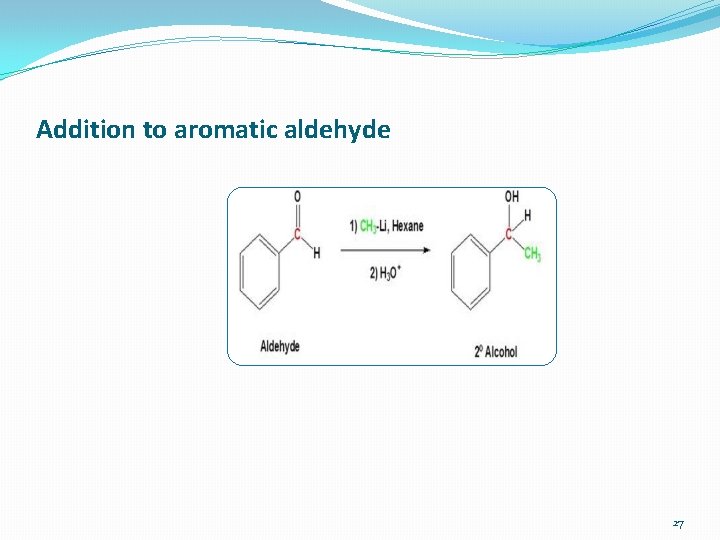
Addition to aromatic aldehyde 27

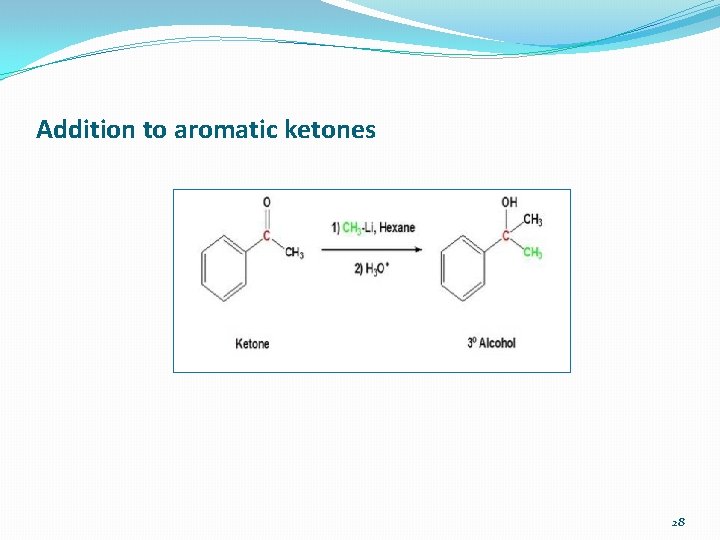
Addition to aromatic ketones 28

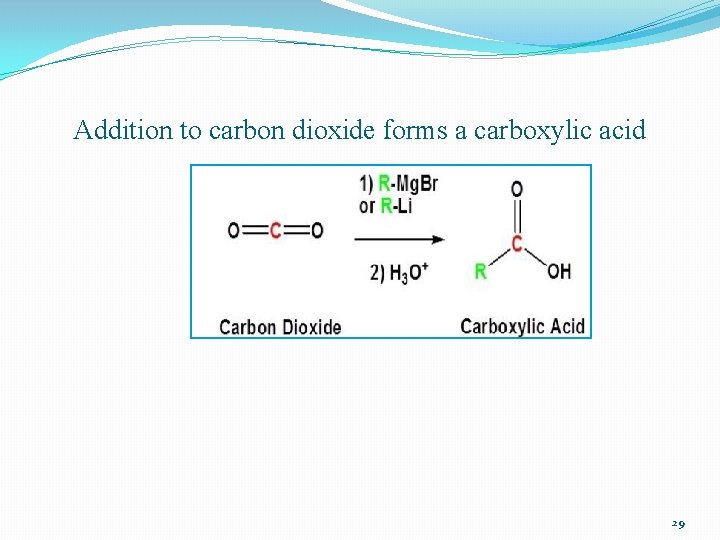
Addition to carbon dioxide forms a carboxylic acid 29

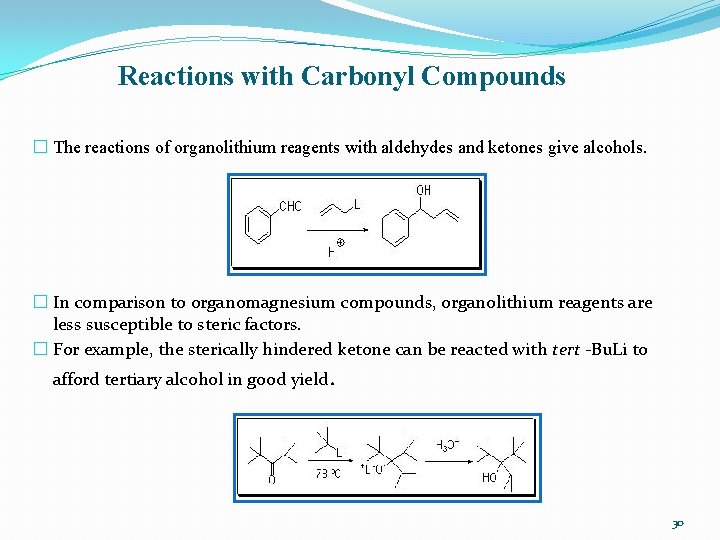
Reactions with Carbonyl Compounds � The reactions of organolithium reagents with aldehydes and ketones give alcohols. � In comparison to organomagnesium compounds, organolithium reagents are less susceptible to steric factors. � For example, the sterically hindered ketone can be reacted with tert -Bu. Li to afford tertiary alcohol in good yield. 30

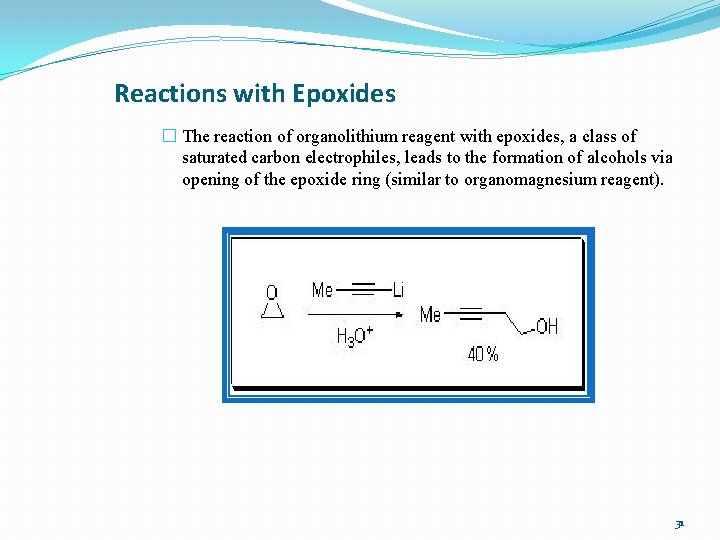
Reactions with Epoxides � The reaction of organolithium reagent with epoxides, a class of saturated carbon electrophiles, leads to the formation of alcohols via opening of the epoxide ring (similar to organomagnesium reagent). 31

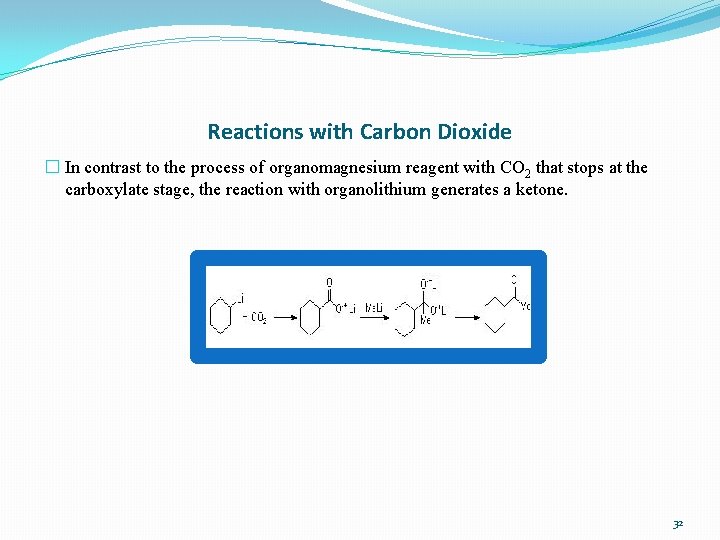
Reactions with Carbon Dioxide � In contrast to the process of organomagnesium reagent with CO 2 that stops at the carboxylate stage, the reaction with organolithium generates a ketone. 32

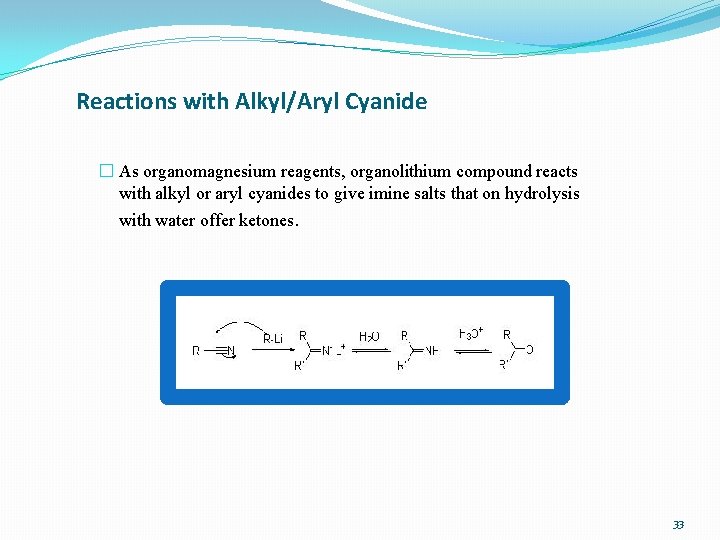
Reactions with Alkyl/Aryl Cyanide � As organomagnesium reagents, organolithium compound reacts with alkyl or aryl cyanides to give imine salts that on hydrolysis with water offer ketones. 33

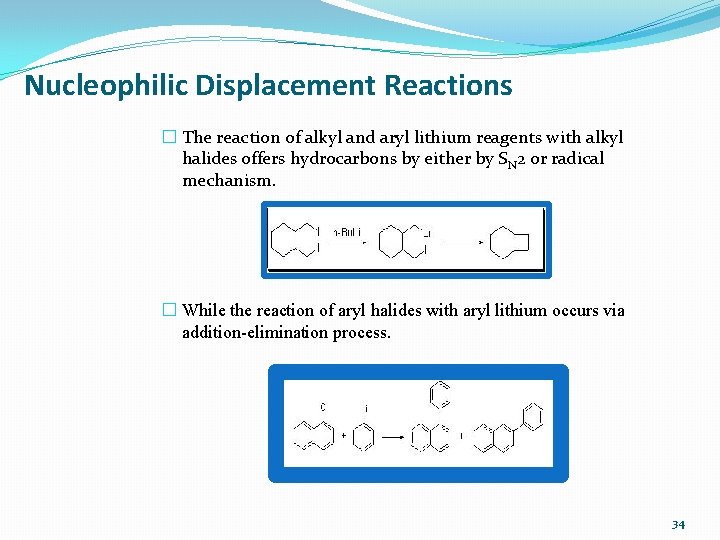
Nucleophilic Displacement Reactions � The reaction of alkyl and aryl lithium reagents with alkyl halides offers hydrocarbons by either by SN 2 or radical mechanism. � While the reaction of aryl halides with aryl lithium occurs via addition-elimination process. 34

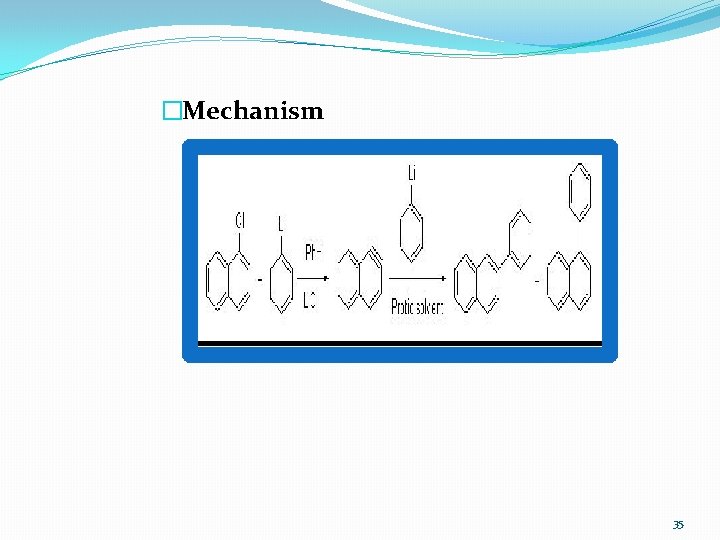
�Mechanism 35

Grignard Reagents • • Discovered by Victor Grignard in 1900 – Key factors are ethereal solvent and water-free conditions Awarded Nobel Prize in 1912 36

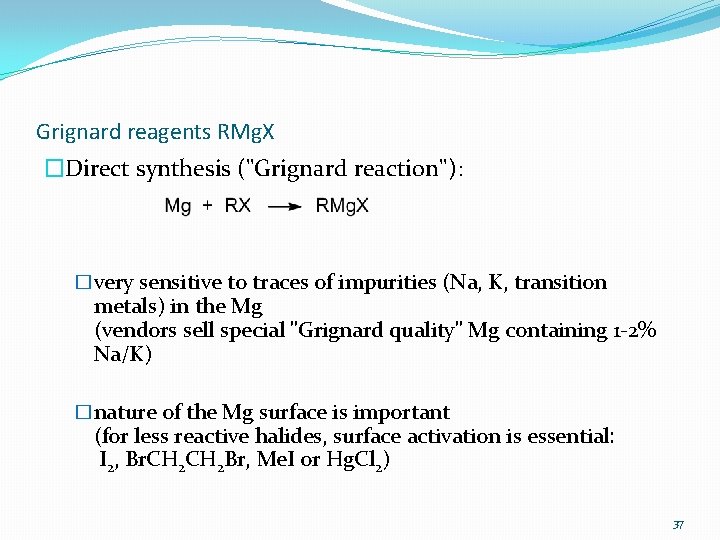
Grignard reagents RMg. X �Direct synthesis ("Grignard reaction"): �very sensitive to traces of impurities (Na, K, transition metals) in the Mg (vendors sell special "Grignard quality" Mg containing 1 -2% Na/K) �nature of the Mg surface is important (for less reactive halides, surface activation is essential: I 2, Br. CH 2 Br, Me. I or Hg. Cl 2) 37

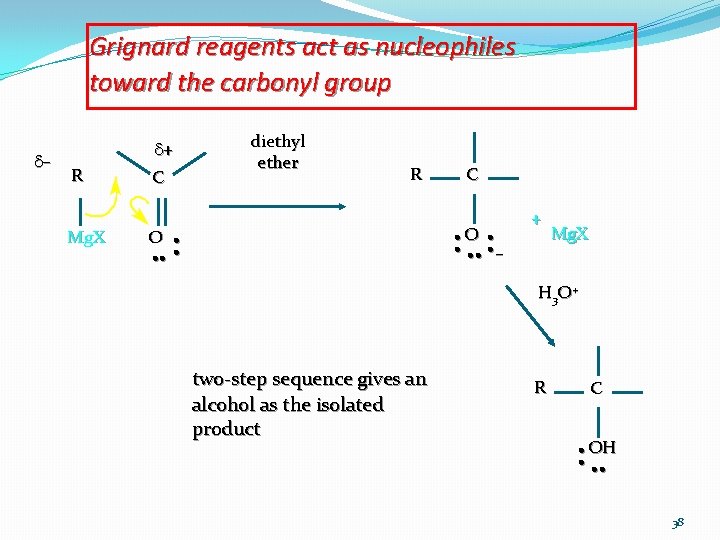
Grignard reagents act as nucleophiles toward the carbonyl group d– d+ R Mg. X C diethyl ether R C • O • • • – O • • + Mg. X H 3 O+ two-step sequence gives an alcohol as the isolated product R C • OH • • • 38

Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols ketones to give tertiary alcohols esters to give tertiary alcohols 39

Grignard reagents RMg. X � Reactivity: I > Br > Cl >> F � Selectivity: I gives largest amounts of side products � In ethers: � In Et 2 O cleaner than in THF, but also slower � Also in ether/hydrocarbon mixtures � Side reactions: � Wurtz coupling, especially for allylic halides � Radical rearrangements 40

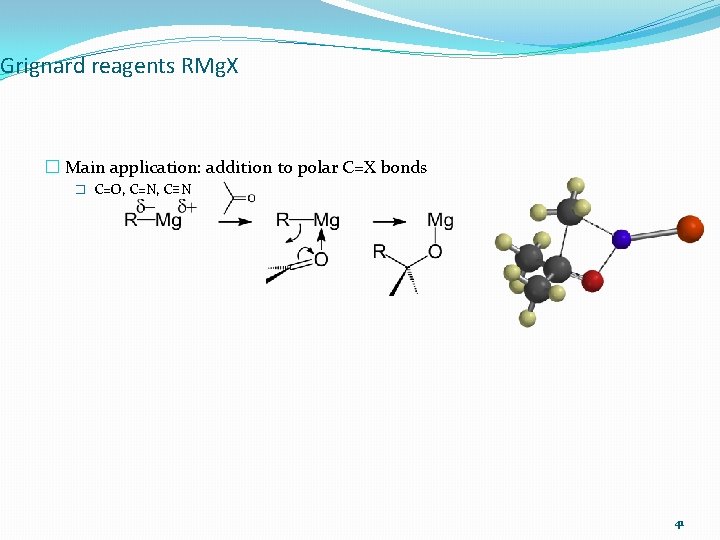
Grignard reagents RMg. X � Main application: addition to polar C=X bonds � C=O, C=N, C≡N 41

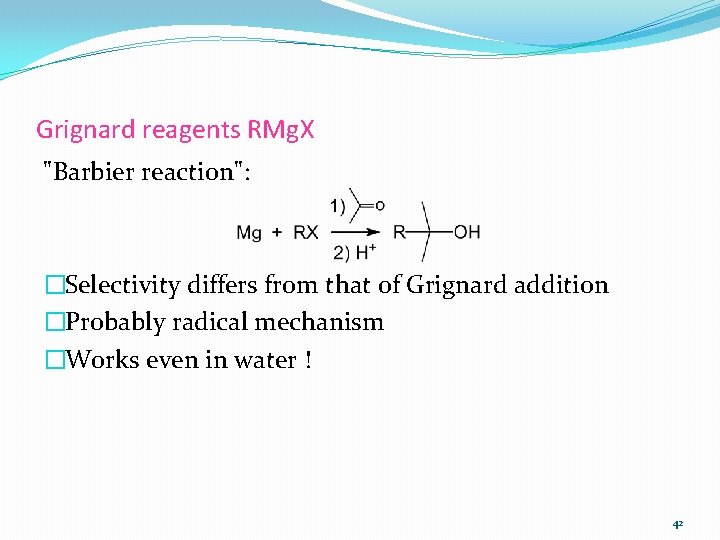
Grignard reagents RMg. X "Barbier reaction": �Selectivity differs from that of Grignard addition �Probably radical mechanism �Works even in water ! 42

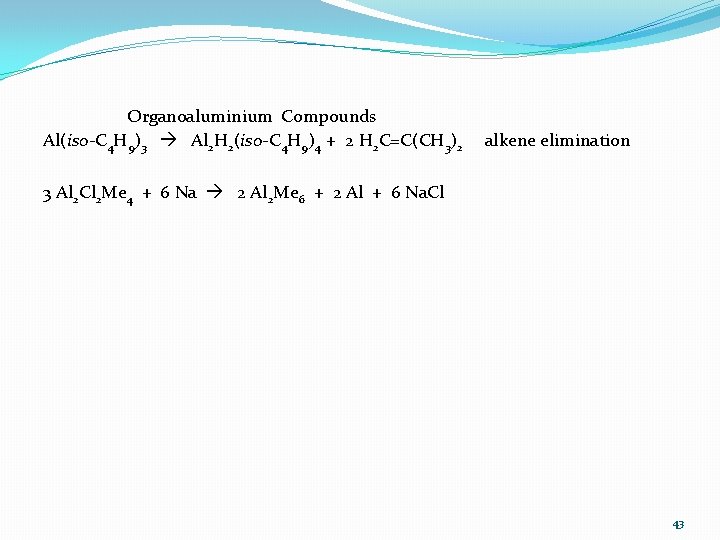
Organoaluminium Compounds Al(iso-C 4 H 9)3 Al 2 H 2(iso-C 4 H 9)4 + 2 H 2 C=C(CH 3)2 alkene elimination 3 Al 2 Cl 2 Me 4 + 6 Na 2 Al 2 Me 6 + 2 Al + 6 Na. Cl 43

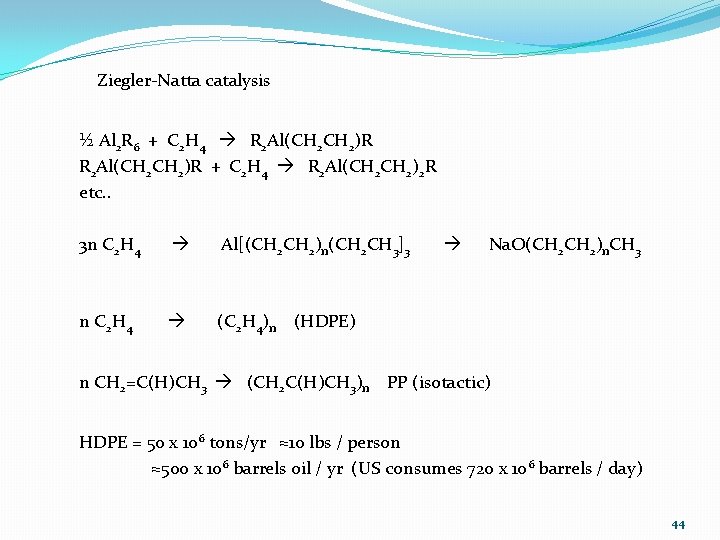
Ziegler-Natta catalysis ½ Al 2 R 6 + C 2 H 4 R 2 Al(CH 2 CH 2)R + C 2 H 4 R 2 Al(CH 2)2 R etc. . 3 n C 2 H 4 Al[(CH 2)n(CH 2 CH 3]3 Na. O(CH 2)n. CH 3 n C 2 H 4 (C 2 H 4)n (HDPE) n CH 2=C(H)CH 3 (CH 2 C(H)CH 3)n PP (isotactic) HDPE = 50 x 106 tons/yr ≈10 lbs / person ≈500 x 106 barrels oil / yr (US consumes 720 x 106 barrels / day) 44

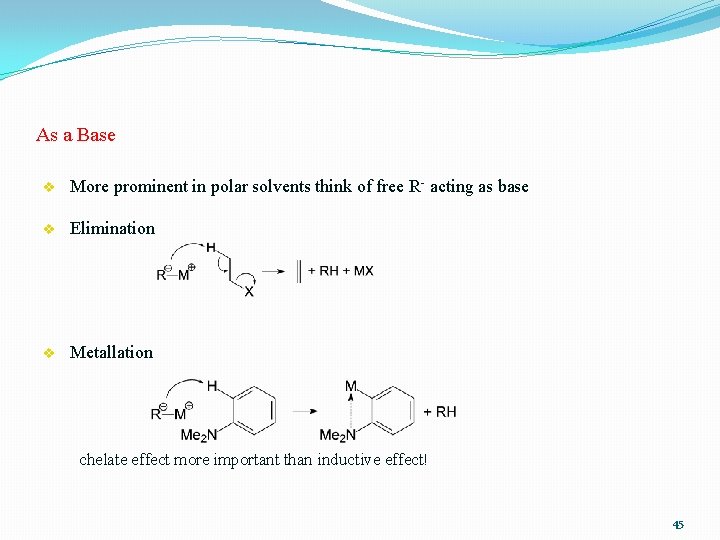
As a Base v More prominent in polar solvents think of free R- acting as base v Elimination v Metallation chelate effect more important than inductive effect! 45

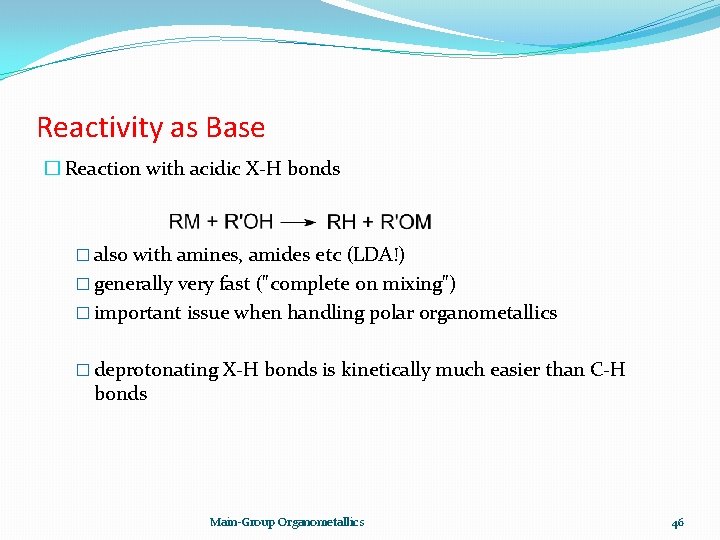
Reactivity as Base � Reaction with acidic X-H bonds � also with amines, amides etc (LDA!) � generally very fast ("complete on mixing") � important issue when handling polar organometallics � deprotonating X-H bonds is kinetically much easier than C-H bonds Main-Group Organometallics 46

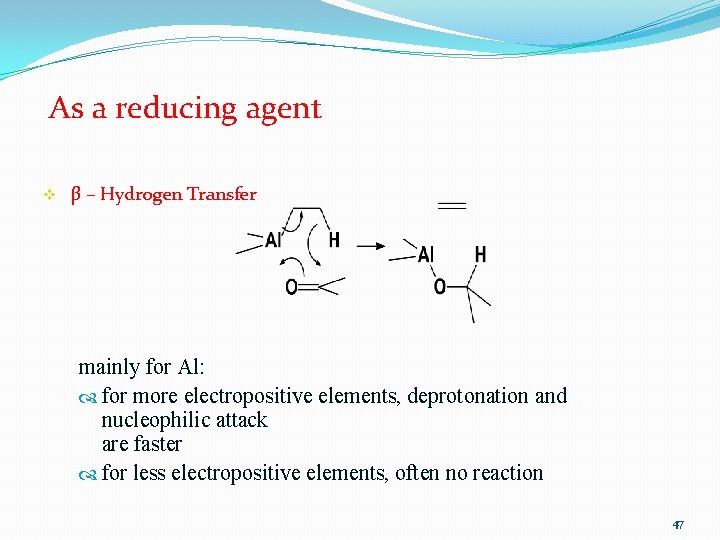
As a reducing agent v β – Hydrogen Transfer mainly for Al: for more electropositive elements, deprotonation and nucleophilic attack are faster for less electropositive elements, often no reaction 47

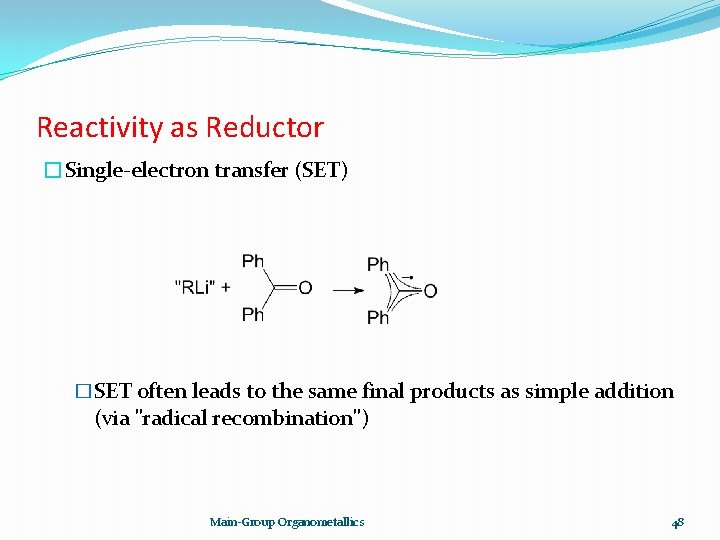
Reactivity as Reductor �Single-electron transfer (SET) �SET often leads to the same final products as simple addition (via "radical recombination") Main-Group Organometallics 48

Summary An overview of organometallics of main group metals These class of compounds are used as nucleophiles and bases They are very reactive compounds Mostly air and moisture sensitive Used extensivley in organic synthesis for functional group transformations Organoaluminium compounds are used in Zieger-Natta catalyst which is extensively used in polymer industry. 49

References: 1. Organic chemistry, 7 th edition, Francis. A. Carey, 2008 2. Organic chemistry, 7 th edition, Jonathan Cayden et al, 2 nd edition, 2017 50