Organizing the Elements The Beginning Before written history

Organizing the Elements

The Beginning • Before written history, people were aware of some of the elements in the periodic table. Elements such as gold (Au), silver (Ag), copper (Cu), lead (Pb), tin (Sn), and mercury (Hg). • It wasn't until 1649, however, until the first element was discovered through scientific inquiry by Hennig Brand. That element was phosphorous (P).

1800 s • Electricity – used to break down compounds into element components • Spectometer – used to identify the isolated elements • Industrial revolution – making soaps, dyes, fertilizers By 1869, 63 elements had been discovered.

Putting it together…
John Newlands (1864) • Arranged elements by increasing atomic mass English chemist
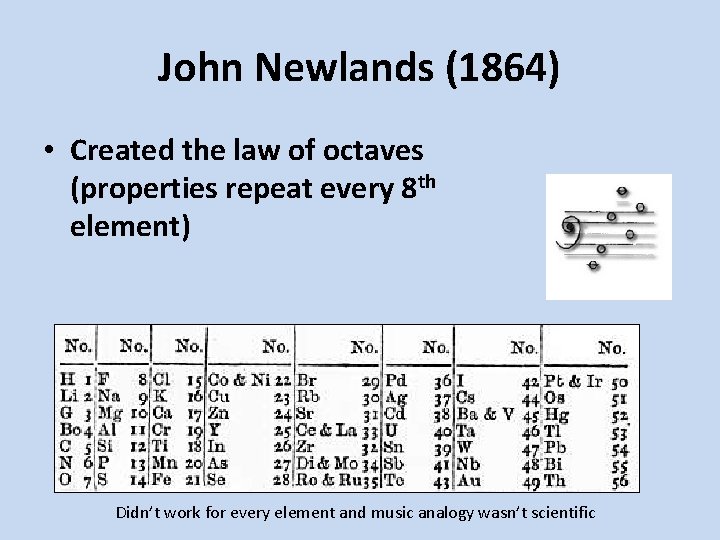
John Newlands (1864) • Created the law of octaves (properties repeat every 8 th element) Didn’t work for every element and music analogy wasn’t scientific

Meyer and Mendeleev (1869) • Arranged elements by increasing atomic mass German chemist Mendeleev published his findings first so he usually gets more credit Russian chemist
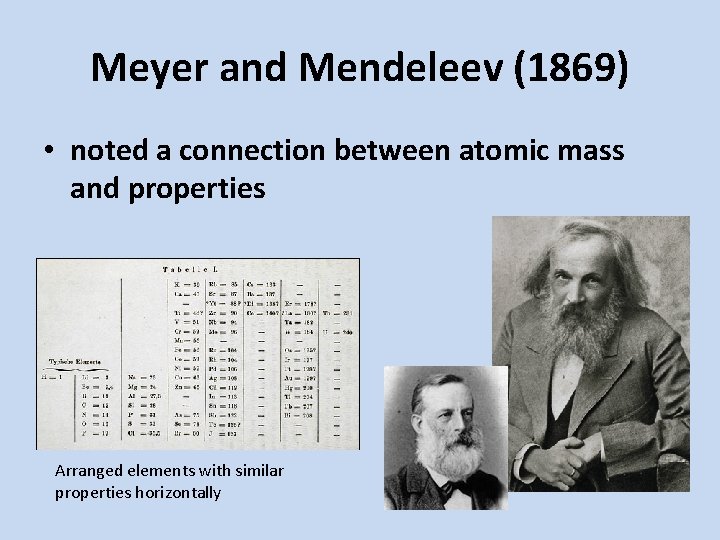
Meyer and Mendeleev (1869) • noted a connection between atomic mass and properties Arranged elements with similar properties horizontally
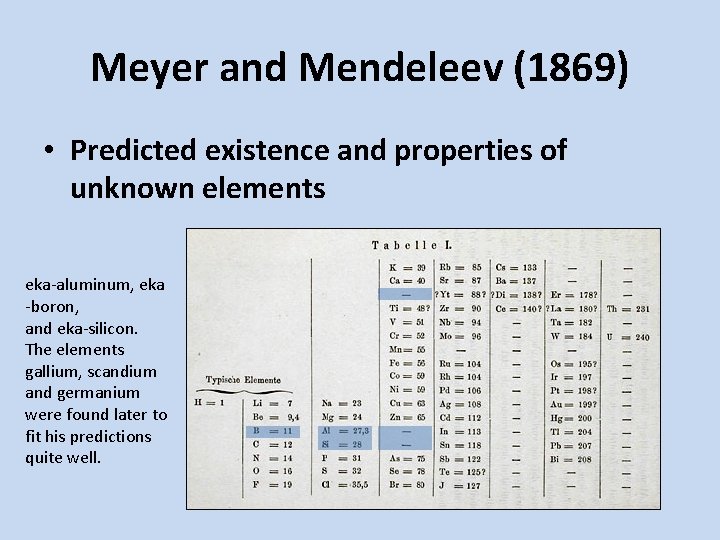
Meyer and Mendeleev (1869) • Predicted existence and properties of unknown elements eka-aluminum, eka -boron, and eka-silicon. The elements gallium, scandium and germanium were found later to fit his predictions quite well.

Problems • Mendeleev’s table wasn’t completely correct – As new elements were discovered, arranging the elements by atomic mass put some elements with groups that had different properties The atomic weight of the gas argon, which does not react readily with other elements, would place it in the same group as the chemically very active solids lithium and sodium.
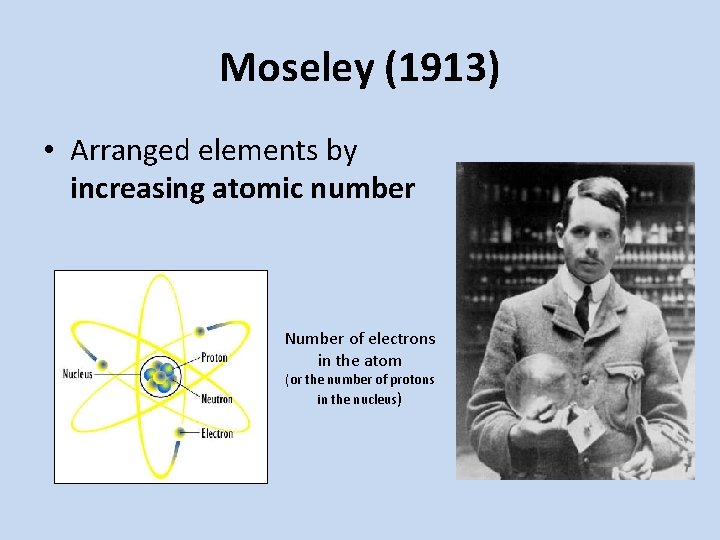
Moseley (1913) • Arranged elements by increasing atomic number Number of electrons in the atom (or the number of protons in the nucleus)
Moseley (1913) • Discovered a clear periodic pattern of properties
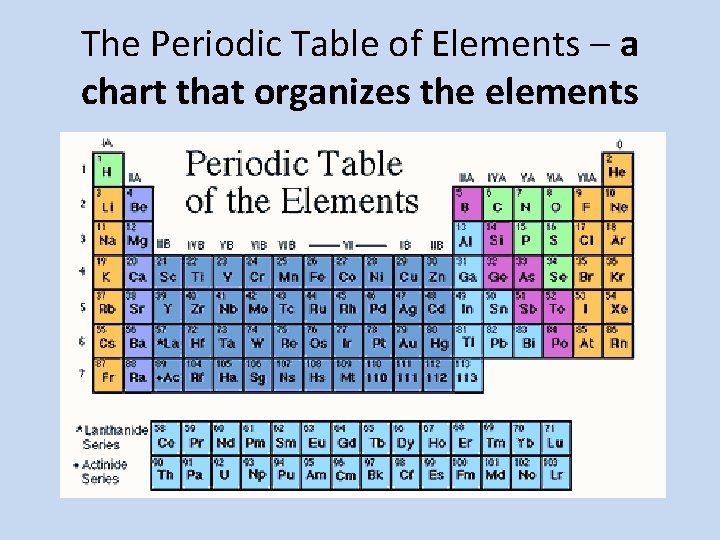
The Periodic Table of Elements – a chart that organizes the elements
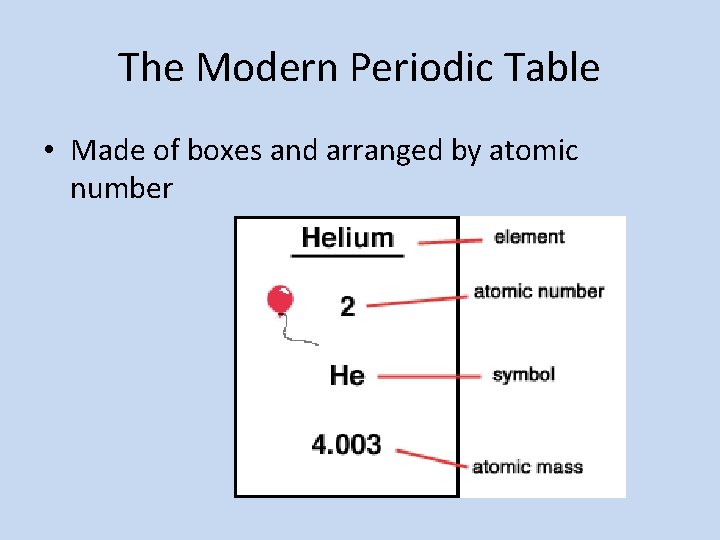
The Modern Periodic Table • Made of boxes and arranged by atomic number
Modern Periodic Table
Groups • Vertical columns (families)
Groups • Elements in same group have very similar properties
Periods • Horizontal rows
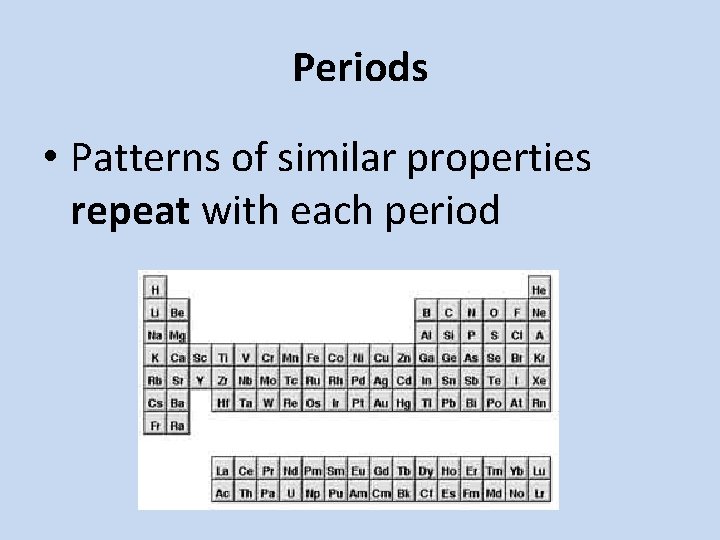
Periods • Patterns of similar properties repeat with each period
- Slides: 19