Organizing the Elements Labeling the Periodic Table Properties

Organizing the Elements Labeling the Periodic Table Properties of M, NM, & Metalloids Electron Configurations in Groups

I. Labeling the Periodic Table The rows are called periods The columns are called groups or families Groups are numbered 1 -8 1 8 2 3 4 5 6 7 Skip these All Elements greater than uranium did not exist on Earth until people produced them in nuclear reactors
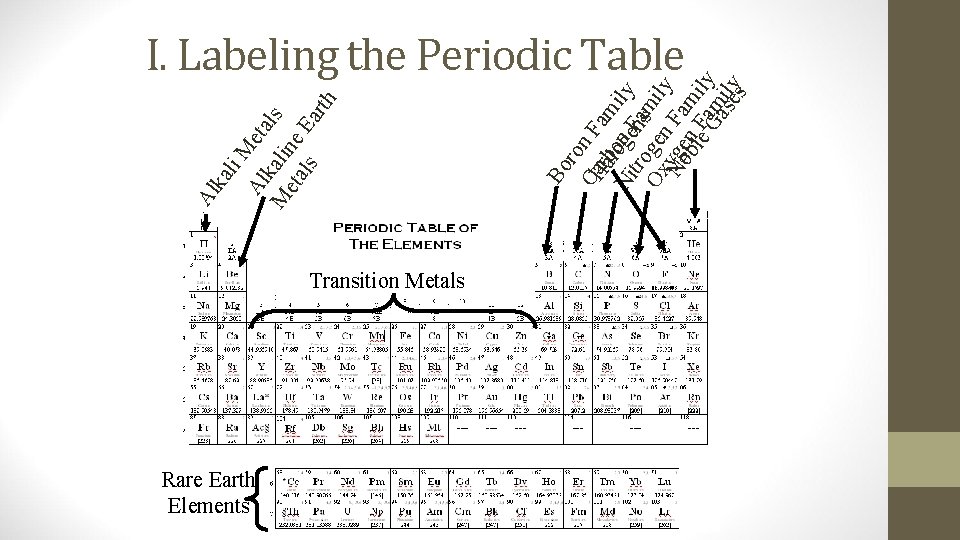
Transition Metals Rare Earth Elements th Bo ro CHa n F arbloo ami Ni gne. F ly tro nsam Ox ge ily n Nyoge Fa blen F mi Gaam ly seily s M Al eta k M alin ls eta e ls Ea r li ka Al I. Labeling the Periodic Table

I. Labeling the Periodic Table First, identify all the elements that are normally diatomic Next, let’s identify allthe thestair elements that are separates normally Now, let’s metals. Finally, color the non-metals. Elements that border step line liquids by coloring bycoloring the lower theupper left upper hand right corner hand with corner different with gases by the right hand corner with Remember that metals are found to themetalloids left of the metals from non-metals are known as one color. of your five. Color colors. different color. stair step line. the metals one of your colors.

II. Properties of Metals A. Conduct heat and electricity well 1. Called “conductors” B. Malleable – can be hammered into this sheets C. Ductile – can be pulled into long thin wires D. Metals have a metallic luster – they shine when polished

III. Properties of Non-Metals A. Poor conductors of heat and electricity 1. Called “insulators” B. Tend to be much more brittle than metal 1. When you hit a non-metal with a hammer it tends to shatter and not flatten out C. Not ductile or malleable

IV. Metalloids A. Elements that border the stair step line that separates metals from non-metals are known as metalloids 1. Their properties are a mixture of metallic and non-metallic
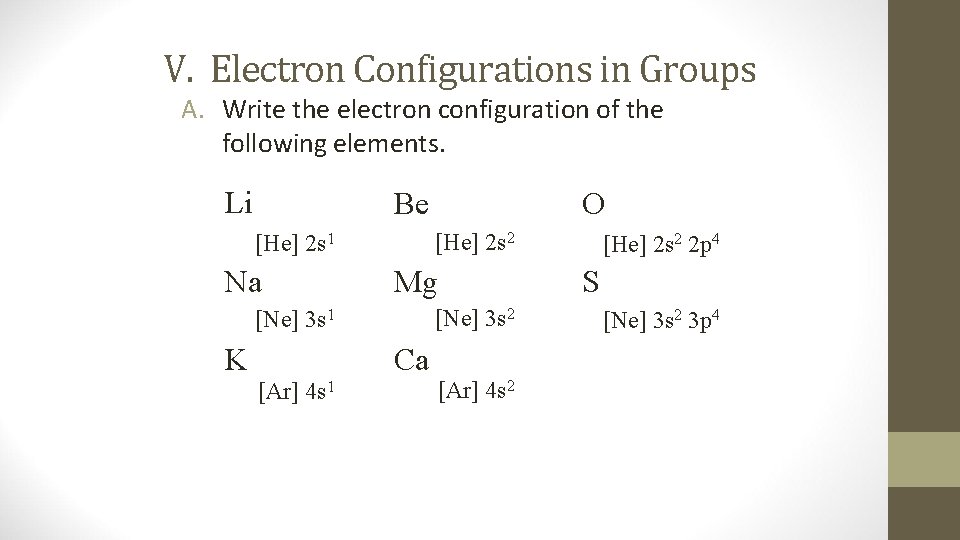
V. Electron Configurations in Groups A. Write the electron configuration of the following elements. Li Be [He] 2 s 2 [He] 2 s 1 Na Mg [Ne] 3 s 2 [Ne] 3 s 1 K Ca [Ar] 4 s 1 O [Ar] 4 s 2 [He] 2 s 2 2 p 4 S [Ne] 3 s 2 3 p 4
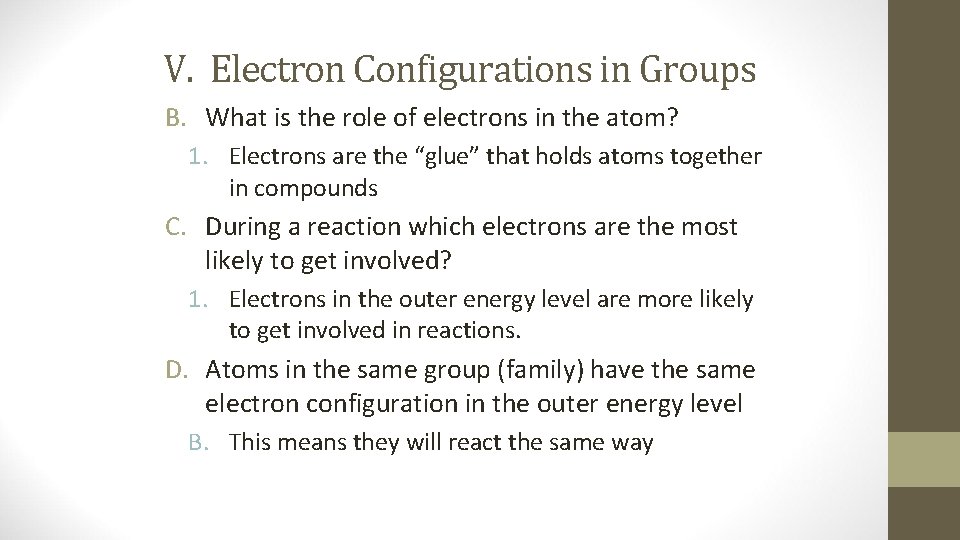
V. Electron Configurations in Groups B. What is the role of electrons in the atom? 1. Electrons are the “glue” that holds atoms together in compounds C. During a reaction which electrons are the most likely to get involved? 1. Electrons in the outer energy level are more likely to get involved in reactions. D. Atoms in the same group (family) have the same electron configuration in the outer energy level B. This means they will react the same way
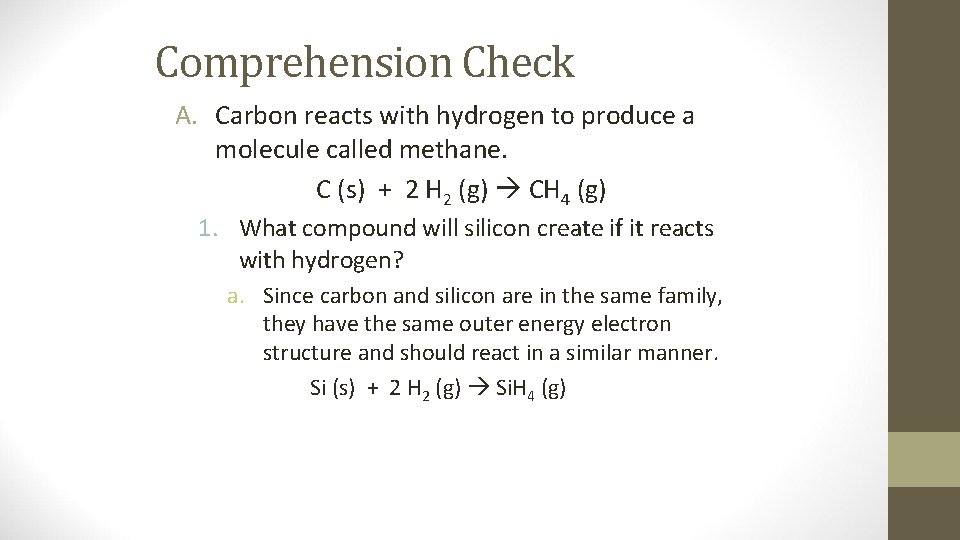
Comprehension Check A. Carbon reacts with hydrogen to produce a molecule called methane. C (s) + 2 H 2 (g) CH 4 (g) 1. What compound will silicon create if it reacts with hydrogen? a. Since carbon and silicon are in the same family, they have the same outer energy electron structure and should react in a similar manner. Si (s) + 2 H 2 (g) Si. H 4 (g)
VI. Reactivity of Metals Demonstration A. Did sodium and potassium react in a similar manner when dropped in water? 1. Yes, they both produced a gas and some flames. B. From what we know about electrons structure, why should we expect sodium and potassium to react in a similar manner? 1. Since they have similar electron structures in the outer energy level, they have the same number of electrons to use for bonding and will therefore react the same.
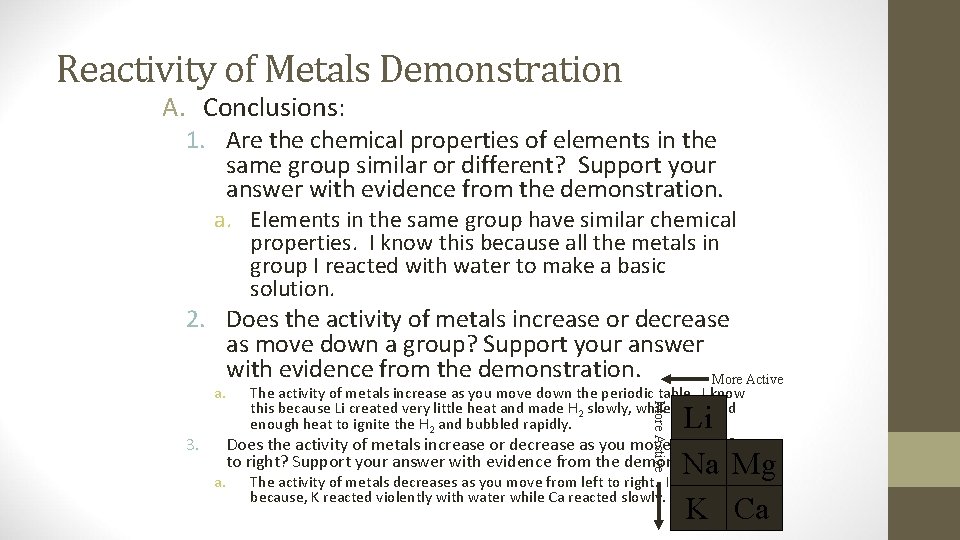
Reactivity of Metals Demonstration A. Conclusions: 1. Are the chemical properties of elements in the same group similar or different? Support your answer with evidence from the demonstration. a. Elements in the same group have similar chemical properties. I know this because all the metals in group I reacted with water to make a basic solution. 2. Does the activity of metals increase or decrease as move down a group? Support your answer with evidence from the demonstration. More Active a. Li Does the activity of metals increase or decrease as you move from left to right? Support your answer with evidence from the demonstration. Na Mg a. The activity of metals decreases as you move from left to right. I know this because, K reacted violently with water while Ca reacted slowly. K Ca More Active 3. The activity of metals increase as you move down the periodic table. I know this because Li created very little heat and made H 2 slowly, while K created enough heat to ignite the H 2 and bubbled rapidly.
- Slides: 12