Organizing the Elements Finding Data on Elements Each


- Slides: 14

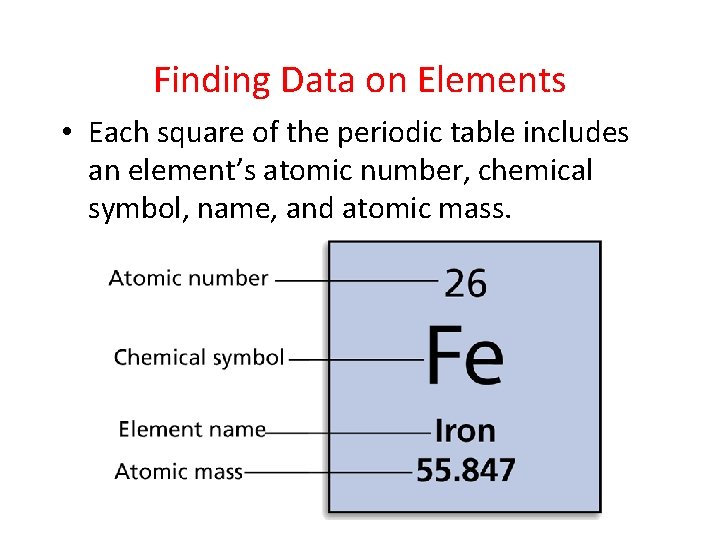
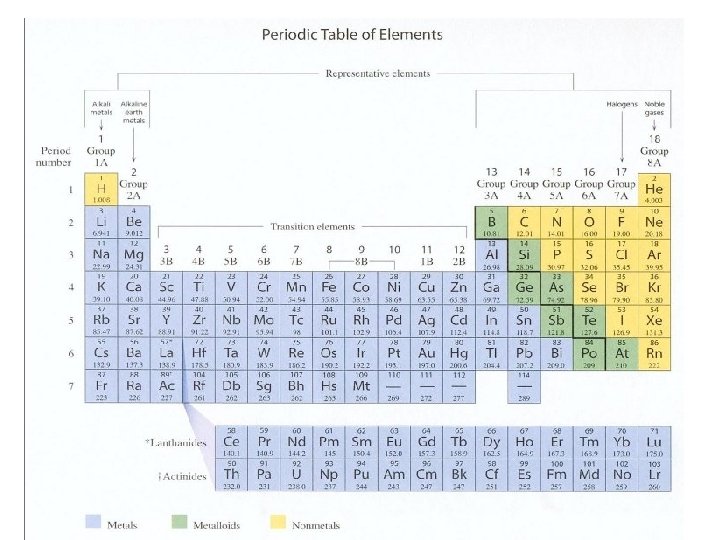
- Organizing the Elements Finding Data on Elements • Each square of the periodic table includes an element’s atomic number, chemical symbol, name, and atomic mass.

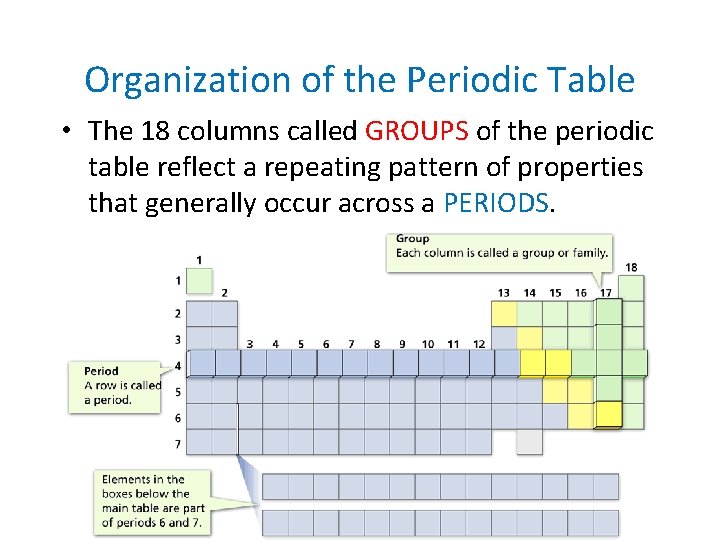
- Organizing the Elements Organization of the Periodic Table • The 18 columns called GROUPS of the periodic table reflect a repeating pattern of properties that generally occur across a PERIODS.

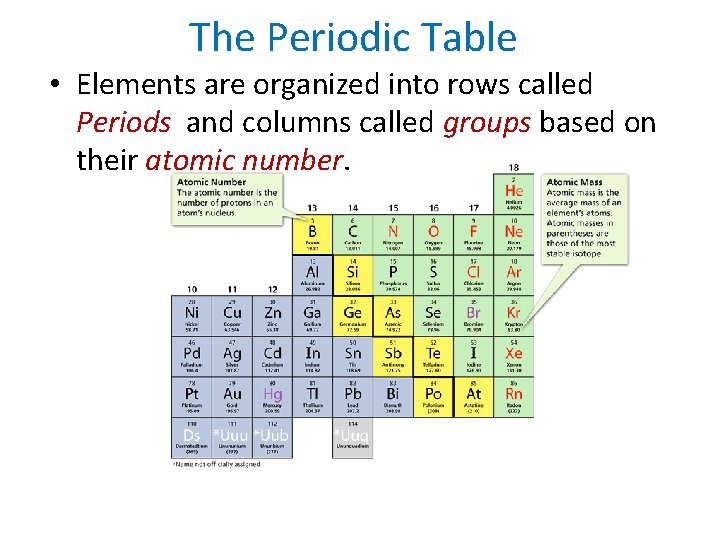
- Atoms, Bonding, and the Periodic Table The Periodic Table • Elements are organized into rows called Periods and columns called groups based on their atomic number.


Practice • A vertical column in The Periodic Table is called ________. • The elements of the same group has similar ________. • As we go down the group the number of electrons ________.

Practice • The horizontal rows in the periodic table are called _______. • Across the row the atomic number _____. • Across the row the metallic nature of elements ________.

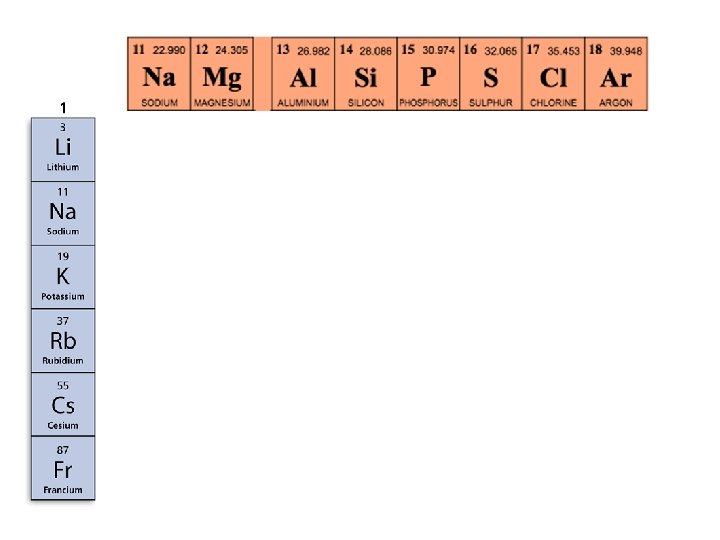
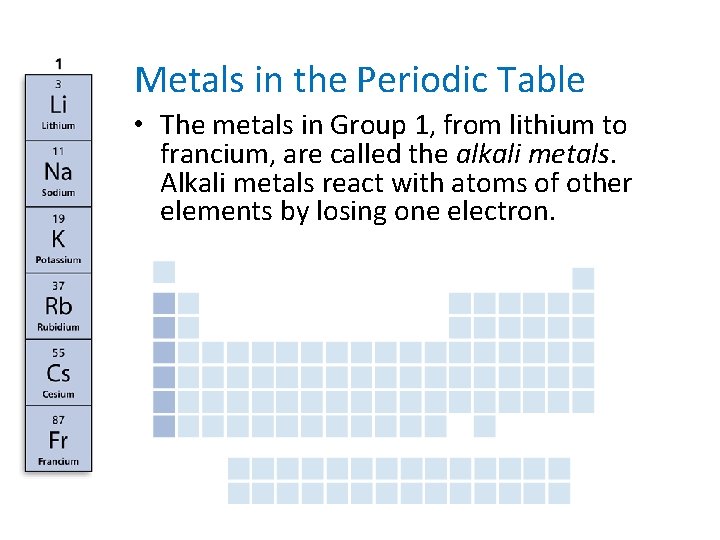
- Metals in the Periodic Table • The metals in Group 1, from lithium to francium, are called the alkali metals. Alkali metals react with atoms of other elements by losing one electron.

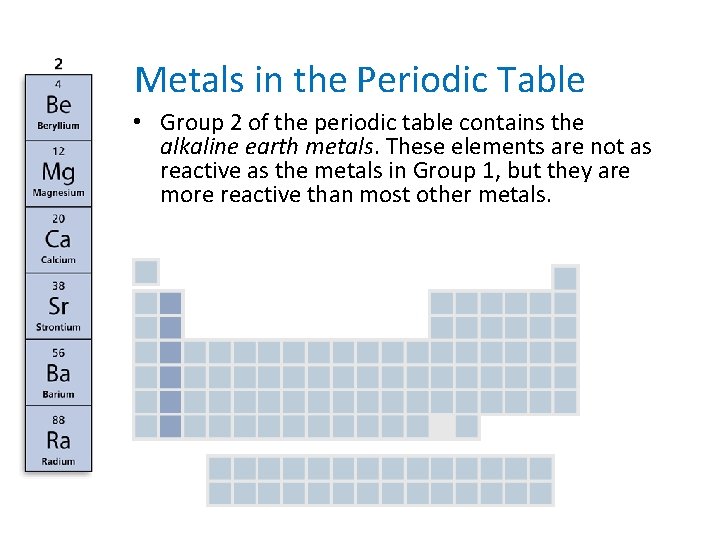
- Metals in the Periodic Table • Group 2 of the periodic table contains the alkaline earth metals. These elements are not as reactive as the metals in Group 1, but they are more reactive than most other metals.

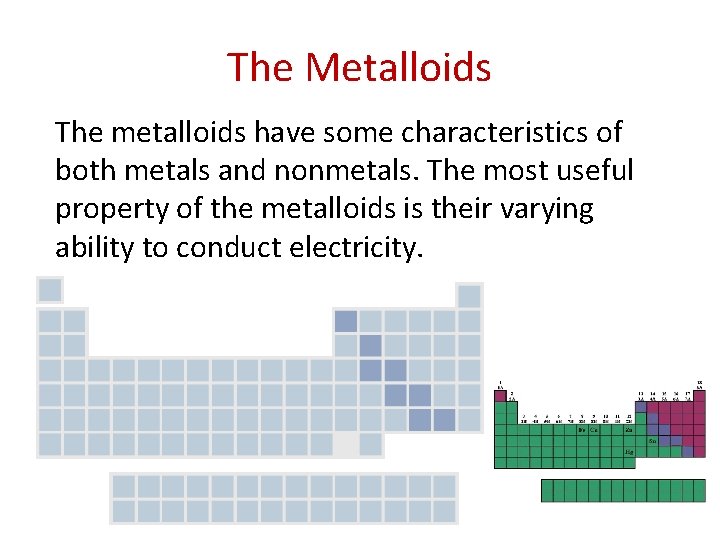
The Metalloids The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity.

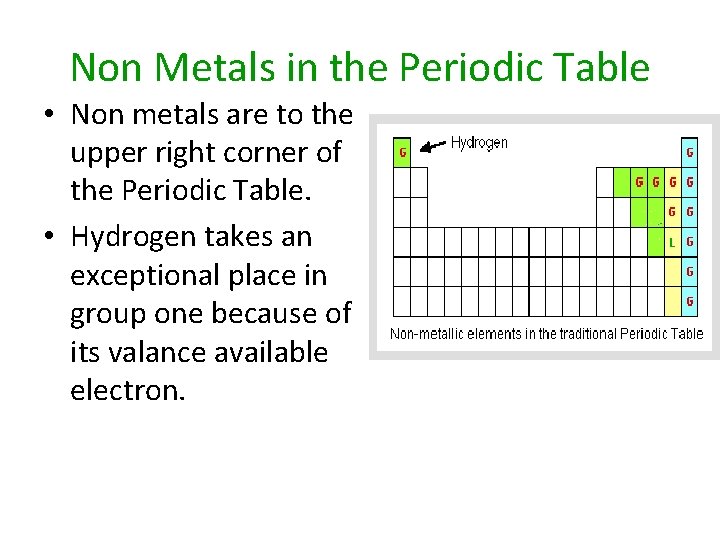
Non Metals in the Periodic Table • Non metals are to the upper right corner of the Periodic Table. • Hydrogen takes an exceptional place in group one because of its valance available electron.

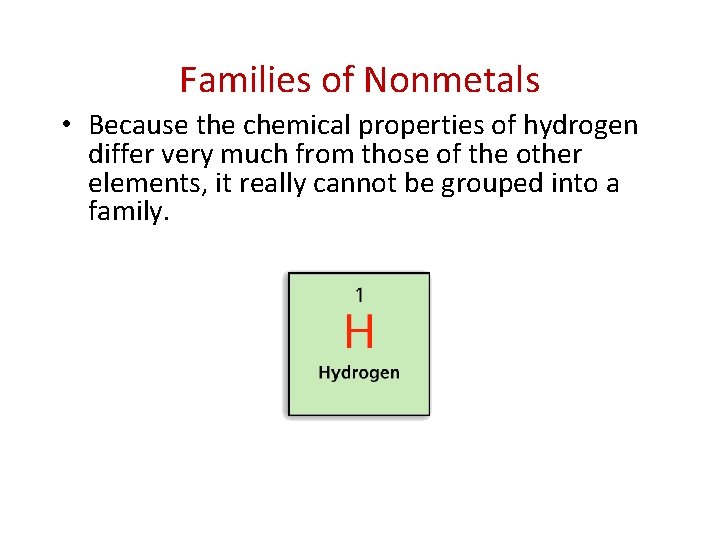
- Nonmetals and Metalloids Families of Nonmetals • Because the chemical properties of hydrogen differ very much from those of the other elements, it really cannot be grouped into a family.


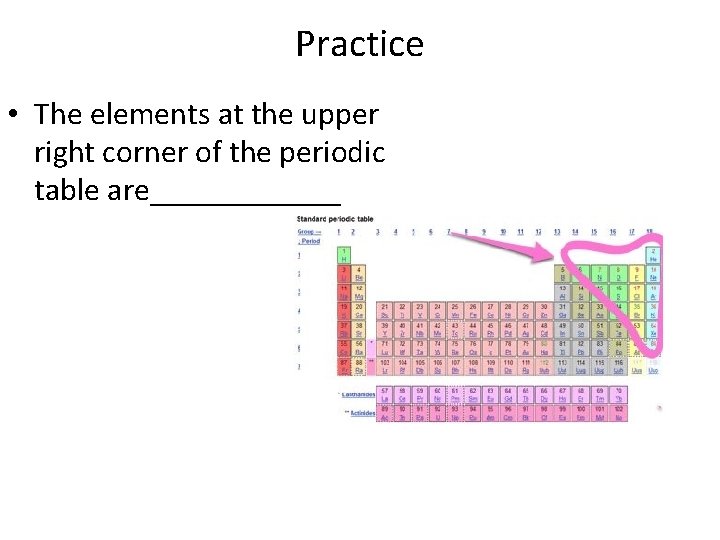
Practice • The elements at the upper right corner of the periodic table are______

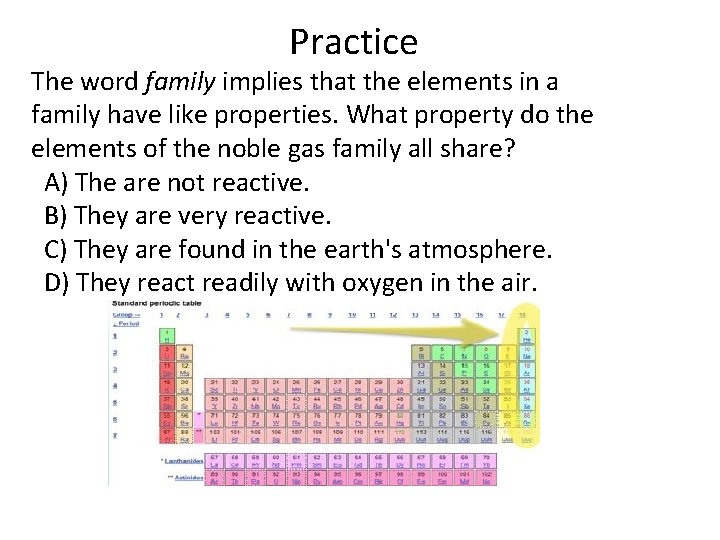
Practice The word family implies that the elements in a family have like properties. What property do the elements of the noble gas family all share? A) The are not reactive. B) They are very reactive. C) They are found in the earth's atmosphere. D) They react readily with oxygen in the air.