Organizing the Elements Chapter 9 Lesson 1 Page


- Slides: 15

Organizing the Elements Chapter 9 Lesson 1 Page 314

Mendeleev • Created the first version of the periodic table in 1869 • Arranged them according to their atomic masses • Total of 63 elements in 1869 • He discovered a set of patterns that applied to the elements

Mendeleev • He knew there were certain similarities and patterns to the elements, he also knew there was more than he had already discovered about them so he did more research • He found: melting point, density, color, and atomic mass • Atomic mass- the average mass of all the isotopes of the elements • Isotopes- different forms of the same element

Mendeleev • Mendeleev noticed that a pattern of properties appeared when he arranged the elements in order of increasing atomic mass. He found that the properties repeated regularly. • He put the elements with similar properties in groups • Read last paragraph page 315

Periodic Table • Periodic table- an arrangement of elements showing the repeating pattern of their properties • Mendeleev left blanks in his periodic table knowing that elements with the properties he predicted would later be discovered. He was right! • Do we still do that?


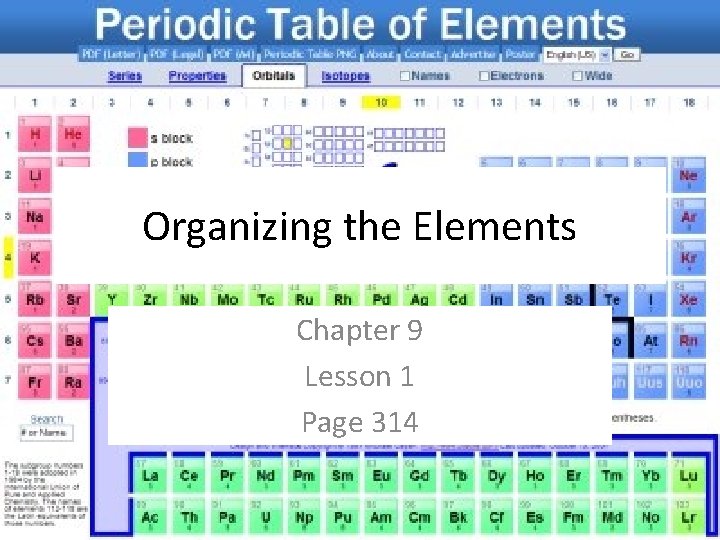
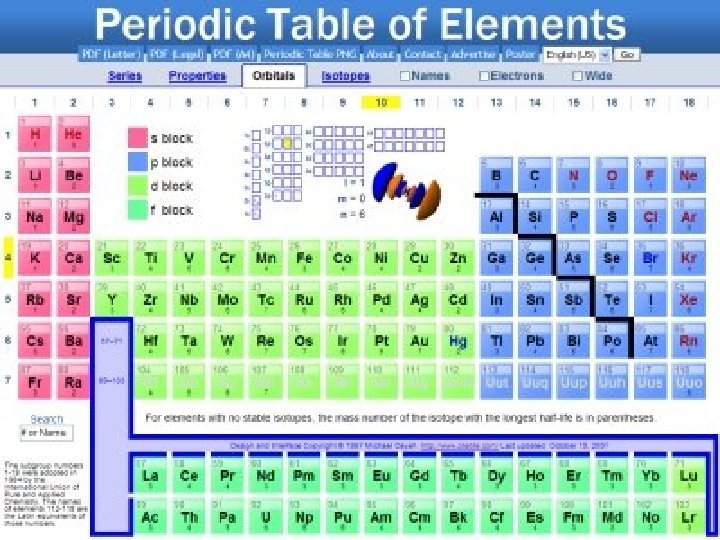
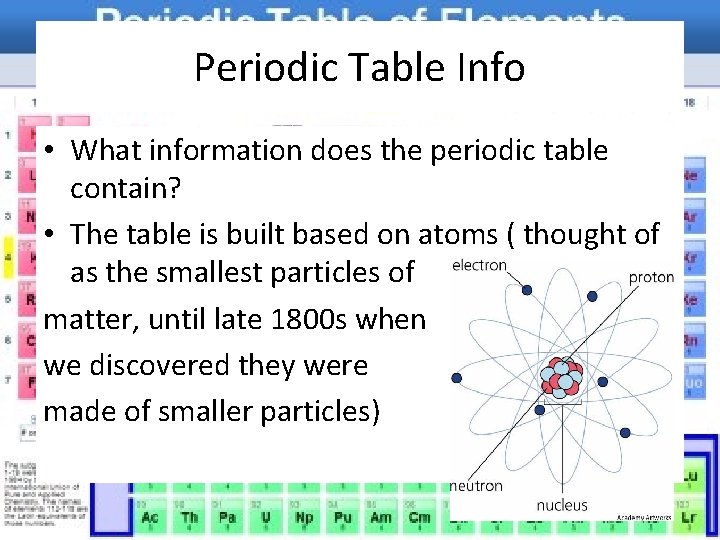
Periodic Table Info • What information does the periodic table contain? • The table is built based on atoms ( thought of as the smallest particles of matter, until late 1800 s when we discovered they were made of smaller particles)

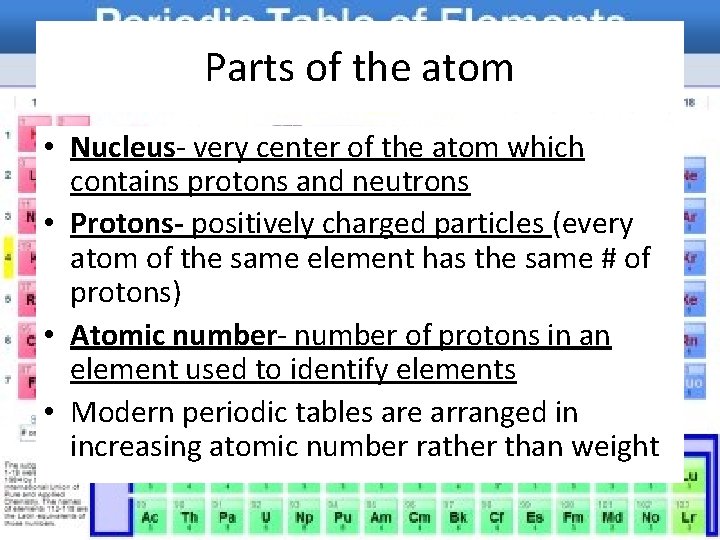
Parts of the atom • Nucleus- very center of the atom which contains protons and neutrons • Protons- positively charged particles (every atom of the same element has the same # of protons) • Atomic number- number of protons in an element used to identify elements • Modern periodic tables are arranged in increasing atomic number rather than weight

Parts of the atom • Neutrons- particles that have no charge found in the nucleus (neutral) • Protons and neutrons have about the same mass • Electrons- negatively charged particles that move around the outside of the nucleus • Neutral atoms has the same number of electrons and protons

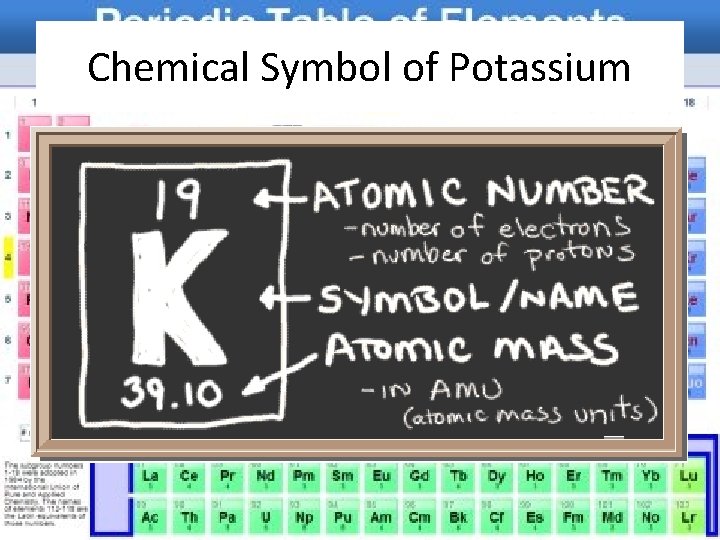
Reading the Periodic Table • The periodic table includes, the atomic number, chemical symbol, name, and atomic mass for each element • Look at page 319 to see the information that is found for each element in the book’s periodic table

Chemical Symbol of Potassium

How is the Periodic Table used? • As you look across the table, the elements’ properties change in predictable ways • An element’s properties can be predicted from its location in the periodic table

Periods • Period- the rows of the periodic table • Metals are located on the left • Non-metals on the right • Metalloids are found in between • This patter is repeated in each period

Groups • Modern table has 7 periods which forms 18 columns • Groups- columns (aka families) • Patter repeats in each period • Characteristics are similar in each group • Example: except for H, all elements in group 1 react violently with water

Homework • Figure out why the two rows at the bottom are separate from the rest of the table.