Organization of internal pharmacological quality control of medical






















- Slides: 25

Organization of internal pharmacological quality control of medical products

The organization of the State System of quality control of drugs A main task of the National drug policy in accordance with WHO recommendations is providing of population with quality and effective medicines. Solving of the global problem of the providing of population with quality medicines in different countries placed on the State quality control system (hereinafter - System) with specific organs and structures.

The quality control system - a series of organizational measures taken to ensure that the quality of medicines to their appointment from creation to usage and include implementation of these actions in accordance with Good Laboratory Practice, Good Clinical Practice, Good Manufacturing Practice and Good Distribution Practice. The primary purpose of a quality system is to ensure that adequate quality standards are maintained. The purpose of adopting a common standard for quality system requirements is to achieve consistency in inspection standards between GMP National Pharmaceutical Inspectorates and thus to facilitate mutual recognition of and mutual confidence between those Inspectorates.

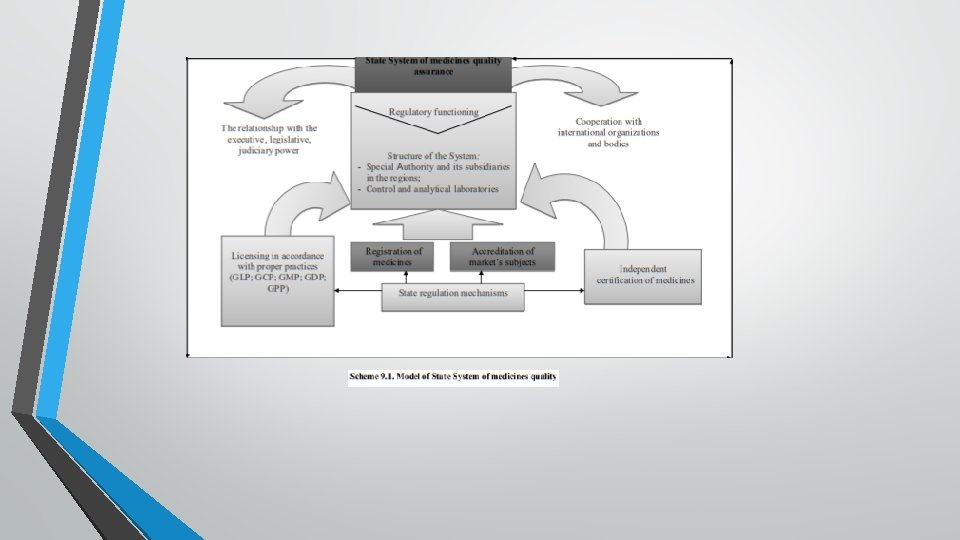
Under the System understand the complex of administrative tools that requires from each subject of business supplying of population with pharmaceutical care, compliance with applicable regulations and statutory requirements. PIC/І gives following definition - Quality system: The sum of all that is necessary to implement an organization’s quality policy and meet quality objectives. It includes organization structure, responsibilities, procedures, systems, processes and resources. Typically these features will be addressed in different kinds of documents as the quality manual and documented procedures, modus operandi etc. General Model of System is presented at scheme 9. 1.


World experience of System’s functioning shows that the most effective and economically rational way to ensure quality of Medicines is a complex of regular inspections and methods of analytical control in strict compliance by manufacturers with GMP standards. Therefore, efforts Systems in countries such as USA, Germany, United Kingdom focused mainly on the inspection of medicines, and focuses on companies that are exporting countries. For example, FDA U. S. has about 7, 000 inspectors and only about 300 employees of analytical laboratories.

In countries with economies in transition Systems are under development and reorganization. They work in a legal and regulatory uncertainty, duplication and inconsistency of actions of structures and organs, deficit of financial and human resources, absence of state policy in sphere of medicines quality assuring etc. According to international experience and the requirements of WHO, National System must function to assure a quality of medicines at all stages of their life cycle.

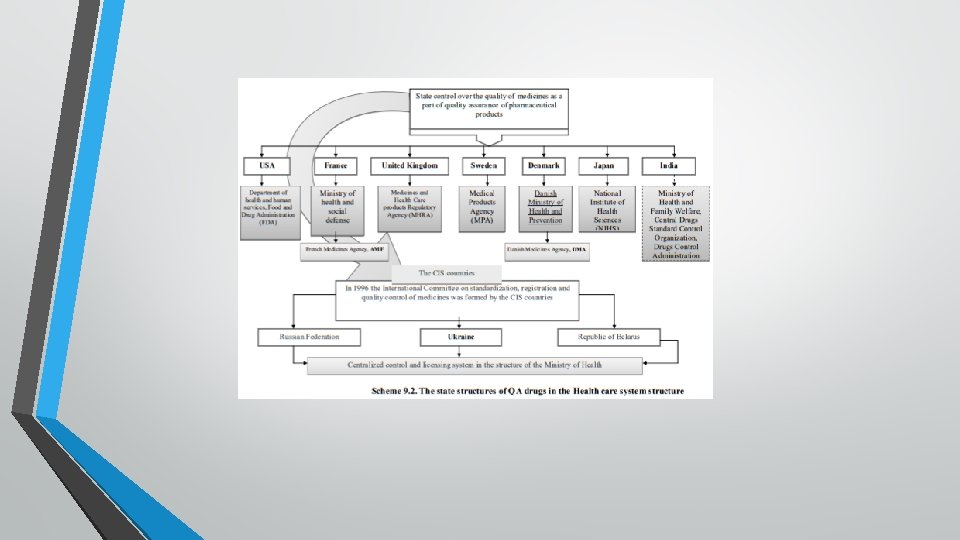
Quality assurance of medicines at these stages should be ensured in accordance with licensing rules and standards of good practices (GCP → GLP → GMP → GDP → GPP). Additionally, input proper procurement practice ("Good Pharmaceutical Procurement Practice" - GPPR) and Good Storage Practices (GSP), which are recommended. Central place in the structure of the National System has the authority empowered to exercise state control of medicines, which typically functioning in the structure of the Ministry of Health, in its direct subordination and has wide powers (scheme 9. 2).


Pharmaceutical Inspectorate - the National body responsible for co-ordinating and carrying out of GMP inspections, including inspections of pharmaceutical manufacturers and/or wholesale distributors. If relevant, this could include making decisions concerning the issue or withdrawal of establishment licenses or authorizations for their activities, the issue or withdrawal of GMP certificates, providing advice and handling suspected quality defects. (http: //www. picscheme. org/publication. php? id=17)

In accordance with WHO, special authority should have the following components: • administrative unit with clearly defined rights and responsibilities of employees; • Inspectorate Service • Analytical laboratory with the necessary financial, personnel, regulatory, technical and information support. The structure, membership and operation of the Pharmaceutical Inspectorate should be such as to enable it to meet the objectives of quality management and to ensure that impartiality is safeguarded.

The personnel of the inspection service, including sub-contracted personnel and experts, should be free from any commercial, financial and other pressures which might affect their judgment and freedom to act. The Pharmaceutical Inspectorate should ensure that persons or organizations external to the inspection organization cannot influence the result of inspections. The system for obtaining fees should not improperly influence the inspection procedure. Rules for deontology, ethics, conflict of interest and improper influence should be clearly defined. The relationship of the Pharmaceutical Inspectorate to other agencies and to other organizations within and outside the Inspectorate should be described where relevant.

The Pharmaceutical Inspectorate should have sufficient resources at all levels to enable it to meet its objectives effectively and efficiently. Senior management should ensure that all personnel are competent and qualified to carry out their assigned duties and that they receive appropriate training. Such training should be documented and its effectiveness assessed periodically. The Pharmaceutical Inspectorate should conduct repeated inspections of manufacturers and/ or wholesale distributors and should issue inspection reports in accordance with National or European Community requirements as appropriate.

The Pharmaceutical Inspectorate should have the documented procedures and resources to enable inspection of manufacturing and wholesale distribution operations to be carried out in accordance with the official guidelines and National legislation and in accordance with a formal inspection plan. All instructions, standards or written procedures, worksheets, check lists and reference data relevant to the work of the Pharmaceutical Inspectorate should be maintained up-to-date and be readily available to staff. When more than one inspector is involved in an inspection, a lead inspector should be appointed to co-ordinate inspection activities. The inspection report should normally be prepared by the lead inspector and should be agreed by all participating inspectors.

The Pharmaceutical Inspectorate should establish and maintain a system for the issue and withdrawal of licenses and GMP certificates, or for advising about the issue and withdrawal of licenses and GMP certificates, as appropriate. The Pharmaceutical Inspectorate should establish and maintain a system for handling of reports of suspected quality defects in medicinal products as defined in a related Standard Operating Procedure or the related Community procedure. The Pharmaceutical Inspectorate should establish and maintain a system for issuing Rapid Alert as defined in a related Standard Operating Procedure or the related Community procedure and an updated list of all performed recalls.

The next element of the System is the availability of the Inspectorate (inspection services) and the national Pharmacopoeia - a legal act that is legislative in nature and contains general requirements for drugs, pharmacopoeial articles (monographs), and methods of quality control of drugs. An important mechanism of integration of national systems of different countries is the European Pharmacopoeia (http: //www. edqm. eu ).

The texts of the European Pharmacopoeia (Ph. Eur. ) concern the qualitative and quantitative composition of medicines, the tests to be carried out on medicines, on the raw materials used in the production of medicines and on the intermediates of synthesis. It contains texts covering substances, excipients and preparations for pharmaceutical use of chemical, animal, human or herbal origin, homoeopathic preparations and homoeopathic stocks, antibiotics, as well as dosage forms and containers. The texts also cover biologicals, blood and plasma derivatives, vaccines and radiopharmaceutical preparations. They are legally binding.

The European Pharmacopoeia is a single reference work for the quality control of medicines in the signatory states of the Convention on its elaboration. The official standards published within provide a legal and scientific basis for quality control during the development, production and marketing processes. They concern the qualitative and quantitative composition and the tests to be carried out on medicines, on the raw materials used in production of medicines and on the intermediates of synthesis. All producers of medicines and/or substances for pharmaceutical use must therefore apply these quality standards in order to market their products in the signatory states of the Convention.

Several legal texts make the European Pharmacopoeia mandatory. These are as follows: • the Convention developed by the Council of Europe on the Elaboration of a European Pharmacopoeia; • a Protocol adopted in 1994 and amending the Convention to prepare for the accession of the European Union and defining the respective powers of the European Union and its member states within the European Pharmacopoeia Commission; • European Union Directives 2001/82/EC, 2001/83/EC, and 2003/63/EC, as amended, on medicines for human and veterinary use. These maintain the mandatory character of European Pharmacopoeia monographs when requesting marketing authorisation (MA).

The contracting parties of the Convention undertake to: • progressively elaborate a Pharmacopoeia which shall become common to the countries concerned and which shall be entitled "European Pharmacopoeia"; • take the necessary measures to ensure that the monographs shall become the official standards applicable within their country by direct implementation in the national legislation or by indirect implementation through national translation.

Organization of entrance quality control of drugs and medicinal goods entered to pharmacies, pharmaceutical companies Pharmacy bases, stores, wholesalers, pharmacies various forms of ownership should provide entrance control in full. To do this, by the manager should be appointed Inspector (Authorized Persons), the proper performance of duties which guarantees the quality of drugs and medicinal goods coming to the entities. An authorized person must have higher pharmaceutical education and experience not less than 2 years. Its jurisdiction shall prepare and issue an opinion on the results of the entrance series of quality control of drugs received by the institution, with a note of the possibility of transmission to their implementation.

An authorized person performing the following duties: 1. incoming quality control of drugs, including test: • appearance of drugs and its visual inspection; • accompanying documents-invoices, which must contain the name, dosage form, batch number, quantity, name of the manufacturer of each drug; • сertificate as manufacturer certified by the seal of the last supplier; • information about state registration of drugs; • a report incoming quality control of drugs; 2. keeping a register of drugs that are received by the business entity that will allows you to track the source of any unsafe or counterfeit drugs party;

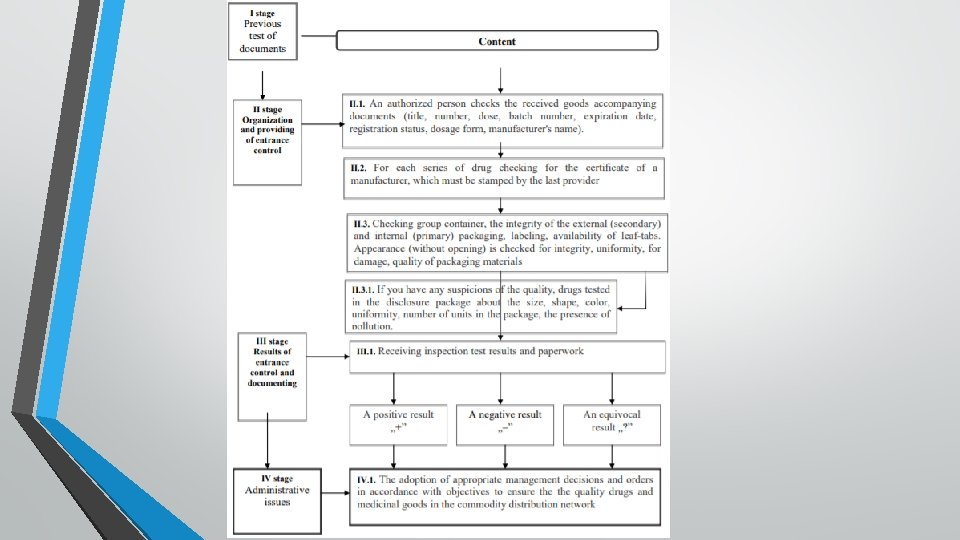
3. keeping a register of drugs that are sold business entity, allowing, if necessary, to withdraw identified batch of counterfeit or substandard drugs (for wholesale organizations); 4. check for substandard and counterfeit medicines series reportedly State Administrations and territorial services; 5. granting territorial authority public service information identified substandard and counterfeit drugs or who is suspected of nonconformity to quality requirements; 6. suspension of trading and placing in quarantine the area of drugs; 7. coordination of internal order drugs trafficking. The algorithm of the entrance control is shown at scheme 9. 17. Before receiving the written opinion of the authorized person obtained drugs are prohibited.


More must be accompanied by a conclusion about the quality: • substances used in pharmacies for the manufacture of parenteral dosage forms and drugs used in ophthalmic practice; • narcotic drugs, psychotropic substances and precursors, which belong to the respective categories according to the list of narcotic drugs, psychotropic substances and precursors, and subject to special scrutiny under the law; • drugs used for anesthesia, including inhalation (except for oxygen and nitrous oxide); • radiopaque medicines; • medicines (including combined) containing rifampicin, isoniazid, ethambutol, pirzynamid