Organization of Autonomic Nervous System Neurotransmitters in ANS






















- Slides: 43

Organization of Autonomic Nervous System Neurotransmitters in ANS, their synthesis, release and fate By Dr. Sairah Hafeez Kamran

HISTORY

In 1921, in Germany, Otto Loewi, German born pharmacologist showed that stimulation of the vagosympathetic trunk connected to an isolated and cannulated frog’s heart could cause the release into the cannula of a substance (‘Vagusstoff’) that, if the cannula fluid was transferred from the first heart to a second, would inhibit the second heart. This is a classic and much-quoted experiment that proved extremely difficult for even Loewi to perform reproducibly. In an autobiographical sketch, Loewi tells us that the idea of chemical transmission arose in a discussion that he had in 1903, but no way of testing it experimentally occurred to him until he dreamed of the appropriate experiment one night in 1920. He wrote some notes of this very important dream in the middle of the night, but in the morning could not read them. The dream obligingly returned the next night and, taking no chances, he went to the laboratory at 3 a. m. and carried out the experiment successfully. Loewi’s experiment may be, and was, criticised on numerous grounds (it could, for example, have been potassium rather than a neurotransmitter that was acting on the recipient heart), but a series of further experiments proved him to be right. For his discovery he was awarded the Nobel Prize in Physiology or Medicine in 1936, which he shared with Sir Henry Dale, who was a lifelong friend who helped to inspire the neurotransmitter experiment.

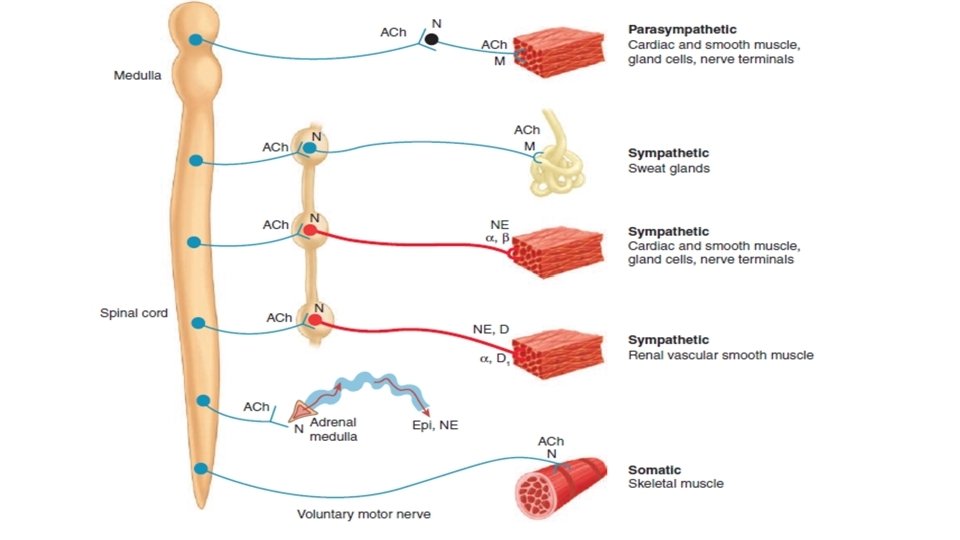
Organization of ANS • The autonomic nervous system comprises three divisions: Sympathetic (thoracolumbar) Parasympathetic (craniosacral) Enteric (Myenteric and submyenteric plexsus) • The basic (two-neuron) pattern of the sympathetic and parasympathetic systems consists of a • preganglionic neuron with a cell body in the central nervous system (CNS) • postganglionic neuron with a cell body in an autonomic ganglion.

• The parasympathetic system is connected to the CNS via: – cranial nerve outflow (III, VII, IX, X) – sacral outflow. • Parasympathetic ganglia usually lie close to or within the target organ. • Some preganglionic parasympathetic fibers terminate in parasympathetic ganglia located outside the organs innervated: the ciliary, pterygopalatine, submandibular, and otic ganglia. • However, the majority of parasympathetic preganglionic fibers terminate on ganglion cells distributed diffusely or in networks in the walls of the innervated organs. • Several pelvic ganglia are innervated by sacral preganglionic nerves that are similar to sympathetic preganglionic fiber in there origination and development

• Sympathetic outflow leaves the CNS in thoracic and lumbar spinal roots. Sympathetic ganglia form two paravertebral chains, plus some midline ganglia. • Most thoracic and lumbar sympathetic preganglionic fibers are short and terminate in ganglia located in the paravertebral chains that lie on either side of the spinal column. • Most of the remaining sympathetic preganglionic fibers are somewhat longer and terminate in prevertebral ganglia, which lie in front of the vertebrae, usually on the ventral surface of the aorta. • From the ganglia, postganglionic sympathetic fibers run to the tissues innervated

• The enteric nervous system is a large and highly organized collection neurons lying in the intramural plexuses of the gastrointestinal tract. It receives inputs from sympathetic and parasympathetic systems, but can act on its own to control the motor and secretory functions of the intestine.


Physiology of ANS • The autonomic system controls smooth muscle (visceral and vascular), exocrine (and some endocrine) secretions, rate and force of contraction of the heart, and certain metabolic processes (e. g. glucose utilisation). • Sympathetic and parasympathetic systems have opposing actions in some situations (e. g. control of heart rate, gastrointestinal smooth muscle), but not in others (e. g. salivary glands, ciliary muscle). • Sympathetic activity increases in stress (‘fight or flight’ response), whereas parasympathetic activity predominates during satiation and repose. Both systems exert a continuous physiological control of specific organs under normal conditions, when the body is at neither extreme.

Transmitters of the autonomic nervous system • The principal transmitters are Acetylcholine (ACh) and Noradrenaline. • Preganglionic neurons are cholinergic, and ganglionic transmission occurs via nicotinic ACh receptors (although excitatory muscarinic ACh receptors are also present on postganglionic cells). • Postganglionic parasympathetic neurons are cholinergic, acting on muscarinic receptors in target organs. • Postganglionic sympathetic neurons are mainly noradrenergic, although a few are cholinergic (e. g. sweat glands).

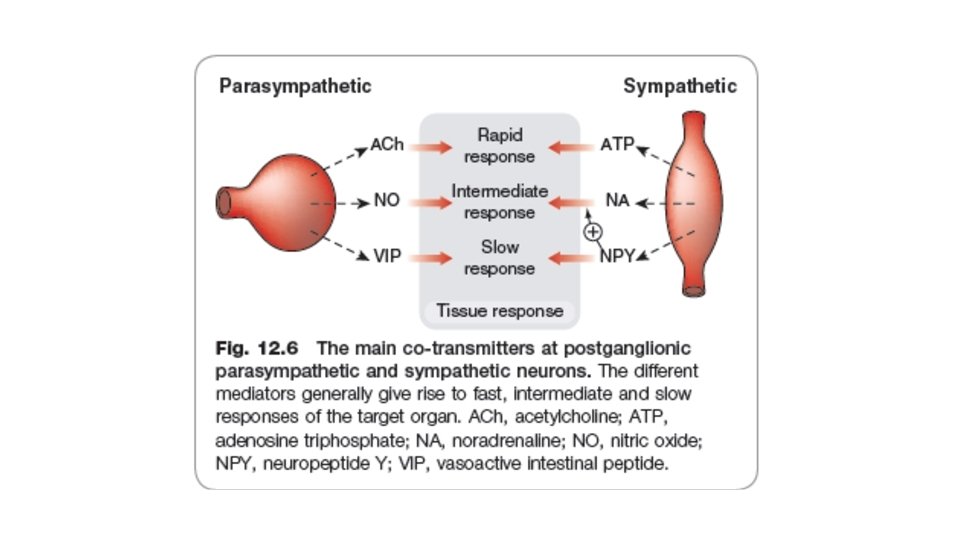
• Transmitters other than Noradrenaline and Noncholinergic (NANC transmitters) are also abundant in the autonomic nervous system. • The main ones are • • • nitric oxide and vasoactive intestinal peptide (parasympathetic), ATP neuropeptide Y (sympathetic). Others, such as 5 -hydroxytryptamine, GABA and dopamine, also play a role. Co-transmission is a general phenomenon.



Functional organization of Autonomic activity • At the highest level—midbrain and medulla—the two divisions of the ANS and the endocrine system are integrated with each other, with sensory input, and with information from higher CNS centers, including the cerebral cortex. These interactions are such that early investigators called • The parasympathetic system a trophotropic one (ie, leading to growth) used to “rest and digest” • The sympathetic system an ergotropic one (ie, leading to energy expenditure), which is activated for “fight or flight. ”

• At level of interactions in the brain stem, medulla, and spinal cord, there are important cooperative interactions between the parasympathetic and sympathetic systems. • For some organs, sensory fibers associated with the parasympathetic system exert reflex control over motor outflow in the sympathetic system. Thus, the sensory carotid sinus baroreceptor fibers in the glossopharyngeal nerve have a major influence on sympathetic outflow from the vasomotor center.

Presynaptic Regulation • Presynaptic receptors that respond to the primary transmitter substance released by the nerve ending are called autoreceptors. • Autoreceptors are usually inhibitory, but in addition to the excitatory β receptors on noradrenergic fibers, many cholinergic fibers, especially somatic motor fibers, have excitatory nicotinic autoreceptors • Control of transmitter release is not limited to modulation by the transmitter itself. Nerve terminals also carry regulatory receptors that respond to many other substances. Such heteroreceptors, may be activated by substances released from other nerve terminals that synapse with the nerve ending.

Post synaptic Regulation Postsynaptic regulation can be considered from two perspectives • The first mechanism involves up-regulation and down-regulation of the receptors. An extreme form of up-regulation occurs after denervation of some tissues, resulting in denervation supersensitivity of the tissue to activators of that receptor type. • In skeletal muscle, for example, nicotinic receptors are normally restricted to the end plate regions underlying somatic motor nerve terminals. Surgical or traumatic denervation results in marked proliferation of nicotinic cholinoceptors over all parts of the fiber, including areas not previously associated with any motor nerve junctions.

• The second mechanism involves modulation of the primary transmitterreceptor event by events evoked by the same or other transmitters acting on different postsynaptic receptors. Ganglionic transmission is a good example of this phenomenon • The postganglionic cells are activated (depolarized) as a result of binding of an appropriate ligand to a neuronal nicotinic (NN) acetylcholine receptor. • The resulting fast excitatory postsynaptic potential (EPSP) evokes a propagated action potential if threshold is reached. • This event is often followed by a small and slowly developing but longerlasting hyperpolarizing afterpotential—a inhibitory postsynaptic potential (IPSP).

• This hyperpolarization involves opening of potassium channels by M 2 cholinoceptors. • The IPSP is followed by a small, slow excitatory postsynaptic potential caused by closure of potassium channels linked to M 1 cholinoceptors. • Finally, a late, very slow EPSP may be evoked by peptides released from other fibers. • These slow potentials serve to modulate the responsiveness of the postsynaptic cell to subsequent primary excitatory presynaptic nerve activity

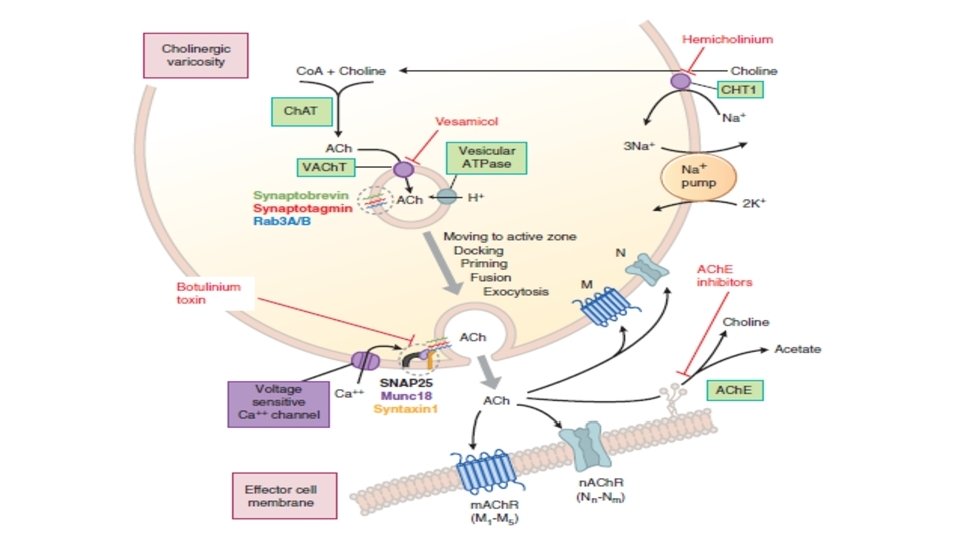
Neurotransmitter Chemistry • Cholinergic Transmission 1. Acetylcholine (ACh) is synthesized in the cytoplasm from acetyl-Co. A and choline through the catalytic action of the enzyme choline acetyltransferase (Ch. AT). 2. Acetyl-Co. A is synthesized in mitochondria, which are present in large numbers in the nerve ending. 3. Choline is transported from the extracellular fluid into the neuron terminal by a sodium-dependent membrane choline transporter CHT 1 4. Once synthesized, acetylcholine is transported from the cytoplasm into the vesicles by a vesicle-associated transporter (VAT) that is driven by proton efflux 5. Storage of acetylcholine is accomplished by the packaging of “quanta” of acetylcholine molecules

6. Vesicles are concentrated on the inner surface of the nerve terminal facing the synapse through the interaction of so-called SNARE proteins on the vesicle and on the inside of the terminal cell membrane 7. Physiologic release of transmitter from the vesicles is dependent on extracellular calcium and occurs when an action potential reaches the terminal and triggers sufficient influx of calcium ions via N-type calcium channels. 8. Calcium interacts with the vesicle-associated membrane proteins on the vesicle membrane and triggers fusion of the vesicle membrane with the terminal membrane and opening of a pore into the synapse 9. The opening of the pore and inrush of cations results in release of the acetylcholine from the proteoglycan and exocytotic expulsion into the synaptic cleft.


Acetylcholinesterase (ACh. E) • ACh. E is found in cholinergic neurons and is highly concentrated at the postsynaptic end plate of the NMJ. Bu. Ch. E (butyrylcholinesterase, also called pseudocholinesterase) is virtually absent in neuronal elements of the central and peripheral nervous systems. Bu. Ch. E is synthesized primarily in the liver and is found in liver and plasma; its likely physiological function is the hydrolysis of ingested esters from plant source

• At the neuromuscular junction in skeletal muscle, immediate hydrolysis of ACh by ACh. E reduces lateral diffusion of the transmitter and activation of adjacent receptors. • Rapid release of ACh onto the n. ACh. Rs of the motor end plate, followed by rapid hydrolysis of the neurotransmitter, limits receptor activation and facilitates rapid control of responses. • The time required for hydrolysis of ACh at the NMJ is less than a millisecond. • ACh. E also terminate impulse transmission at cholinergic synapses by causing hydrolysis of acetylcholine.

Adrenergic Transmission • Synthesis: Adrenergic neurons transport the precursor amino acid tyrosine into the nerve ending, convert it to dopa, and then synthesize a catecholamine transmitter (dopamine, norepinephrine, or epinephrine and store it in membrane-bound vesicles. In most sympathetic postganglionic neurons, norepinephrine is the final product. In the adrenal medulla and certain areas of the brain, some norepinephrine is further converted to epinephrine. • In dopaminergic neurons, synthesis terminates with dopamine.

Steps in the enzymatic synthesis of dopamine, norepinephrine and epinephrine. The enzymes involved are shown in red; essential cofactors in italics. The final step occurs only in the adrenal medulla and in a few epinephrine-containing neuronal pathways in the brainstem.

• Storage: Catecholamines are stored in vesicles, thereby ensuring their regulated release, protecting them from metabolism by cellular enzymes, and preventing their leakage out of the neuron. The vesicular monoamine transporter VMAT 2, a vesicular membrane protein, moves NE and other catecholamines from the cytosol into neuronal storage vesicle. • Reserpine inhibits monoamine transport into storage vesicles and ultimately leads to depletion of catecholamine from sympathetic nerve endings and in the brain.

• Release: The release of the transmitters occurs via exocytosis, a process activated by depolarization of the varicosity, which allows entry of Ca 2+ through voltage-dependent Ca 2+ channels and the interaction of numerous docking and fusion proteins located in the vesicle and the neuronal cell membrane

• Termination of action: Uptake of released catecholamine terminates the neurotransmitter’s effects at the synaptic junction. • Following uptake, catecholamines can be metabolized (in neuronal and nonneuronal cells) or re-stored in vesicles (in neurons). • Two enzymes are important in the initial steps of metabolic transformation of catecholamines—MAO (monoamine oxidase) and COMT (catechol-O-methyltransferease).

Autonomic Receptors CHOLINOCEPTORS Nicotinic receptors: Cholinoceptors that are activated by the alkaloid nicotine 1. Nicotinic receptors are localized at myoneural junctions of somatic nerves and skeletal muscle (NM), autonomic ganglia (NG), including the adrenal medulla, and certain areas in the brain. 2. Nicotinic receptors are a component of postjunctional transmembrane polypeptide that forms a ligand-gated (i. e. , regulated) cation-selective ion channel. Binding of ACh to the receptor site causes opening of the ion channel and an influx of positively charged ions (sodium and potassium) and across the cellular membrane. This influx of positive charge depolarizes the postsynaptic membrane.

3. In skeletal muscle, ACh interacts with nicotinic receptors to produce membrane depolarization and a propagated action potential through the transverse tubules of skeletal muscle. This results in the release of Ca+2 from the sarcoplasmic reticulum and, through a further series of chemical and mechanical events, muscle contraction. Hydrolysis of ACh by ACh. E results in muscle cell repolarization. (a) The continued presence of a nicotine agonist, like succinylcholine, at nicotinic receptors, or excessive cholinergic stimulation, can lead to a “depolarizing blockade” (phase I block), in which normal depolarization is followed by persistent depolarization. During phase I block, skeletal muscle is unresponsive to either neuronal stimulation or direct stimulation. (b) The selective nicotinic receptor antagonists tubocurarine and trimethaphan can block the effect of ACh at skeletal muscle and autonomic ganglia, respectively.

Muscarinic receptors: Cholinoceptors that are activated by the alkaloid muscarine 1. Muscarinic receptors are localized on numerous autonomic effector cells, including cardiac atrial muscle and cells of the sinoatrial (SA) and atrioventricular (AV) nodes, smooth muscle, exocrine glands, and vascular endothelium (mostly arterioles), although the latter does not receive parasympathetic innervation, as well as certain areas in the brain.

2. Muscarinic receptors consist of at least five receptor subtypes (M 1 –M 5). a) Muscarinic M 1 -receptors are found in sympathetic postganglionic neurons b) M 2 -receptors are found in cardiac and smooth muscles c) M 3 -receptors are found in glandular cells (e. g. , gastric parietal cells), and the vascular endothelium and vascular smooth muscle. d) M 5 -receptors are found in the vascular endothelium. All five of the receptor subtypes, including M 4 -receptors, are found in CNS neurons.

3. ACh interacts with M 1, M 3, and M 5 muscarinic cholinoceptors to increase phosphatidylinositol (PI) turnover and Ca+2 mobilization a) By activation of G protein (Gq), the interaction of ACh with M 1 and M 3 muscarinic cholinoceptors stimulates polyphosphatidylinositol phosphodiesterase (phospholipase C), which hydrolyzes PI to inositol trisphosphate (IP 3) and diacylglycerol (DAG). b) IP 3 mobilizes intracellular Ca+2 from the endoplasmic and sarcoplasmic reticula, and activates Ca-regulated enzymes and cell processes. c) DAG activates protein kinase C, which results in phosphorylation of cellular enzymes and other protein substrates and the influx of extracellular calcium that results in activation of contractile elements in smooth muscle.

4. ACh also interacts with M 2 and M 4 muscarinic cholinoceptors to activate G proteins (G 1), which leads to inhibition of adenylyl cyclase activity with decreased levels of cyclic AMP (c. AMP) and to increased K 1 conductance with effector cell hyperpolarization. 5. Cholinergic agonists act on M 3 muscarinic receptors of endothelial cells to promote the release of nitric oxide (NO), which diffuses to the vascular smooth muscle to activate guanylyl cyclase and increase cyclic GMP (c. GMP) and to produce relaxation

Adrenoceptors α-Adrenoceptors 1. α-Adrenoceptors are classified into two major receptor subgroups (there are subtypes of each group). a) α 1 -Receptors are located in postjunctional effector cells, notably vascular smooth muscle, where responses are mainly excitatory; b) α 2 -receptors are located primarily in prejunctional adrenergic nerve terminals, and also in fat cells and in the β cells of the pancreas. 2. α-Adrenoceptors mediate vasoconstriction (α 1), GI relaxation (α 1), mydriasis (α 1), prejunctional inhibition of release of norepinephrine and other neurotransmitters (α 2), inhibition of insulin release (α 2), and inhibition of lipolysis (α 2).

3. α-Adrenoceptors are distinguished from β-adrenoceptors by their interaction (in descending order of potency), with the adrenergic agonists epinephrine = norepinephrine >> isoproterenol 4. α 1 -Receptors, like muscarinic M 1 cholinoceptors, activate guanine nucleotidebinding proteins (Gq) in many cells, which results in activation of phospholipase C and stimulation of phosphoinositide (PI) hydrolysis that leads to increased formation of IP 3 and mobilization of intracellular stores of Ca+2 and to increased DAG and activation of protein kinase C.

5. α 2 -Receptors, like muscarinic M 2 -cholinoceptors, activate inhibitory guanine nucleotide- binding proteins (Gi), inhibit adenylyl cyclase activity, and decrease intracellular c. AMP levels and the activity of c. AMP-dependent protein kinases β-Adrenoceptors 1. β-Adrenoceptors, located mostly in postjunctional effector cells, are classified into two major receptor subtypes a) β 1 -receptors (primarily excitatory) b) β 2 -receptors (primarily inhibitory) c) Β 3 -receptors

2. β 1 -Receptor subtype a) β 1 -Receptors mediate increased contractility and conduction velocity, and renin secretion in the kidney. b) The β 1 -receptor subtype is defined by its interaction (in descending order of potency) with the adrenergic agonists isoproterenol > epinephrine = norepinephrine. 3. β 2 -Receptor subtype a) β 2 -Receptors mediate vasodilation and intestinal, bronchial, and uterine smooth muscle relaxation. b) The β 2 -receptor subtype is defined by its interaction (in descending order of potency) with the adrenergic agonists isoproterenol = epinephrine >> norepinephrine.

4. β 3 -receptors mediate activation of fat cell lipolysis 5. β-Receptor activation a) β-Receptors activate guanine nucleotide-binding proteins (Gs ) b) Activation stimulates adenylate cyclase activity and increases intracellular CAMP levels and the activity of c. AMP-dependent protein kinases. Adrenoceptor-mediated changes in the activity of protein kinases (and also levels of intracellular Ca+2) bring about changes in the activity of specific enzymes and structural and regulatory proteins, resulting in modification of cell and organ activity.

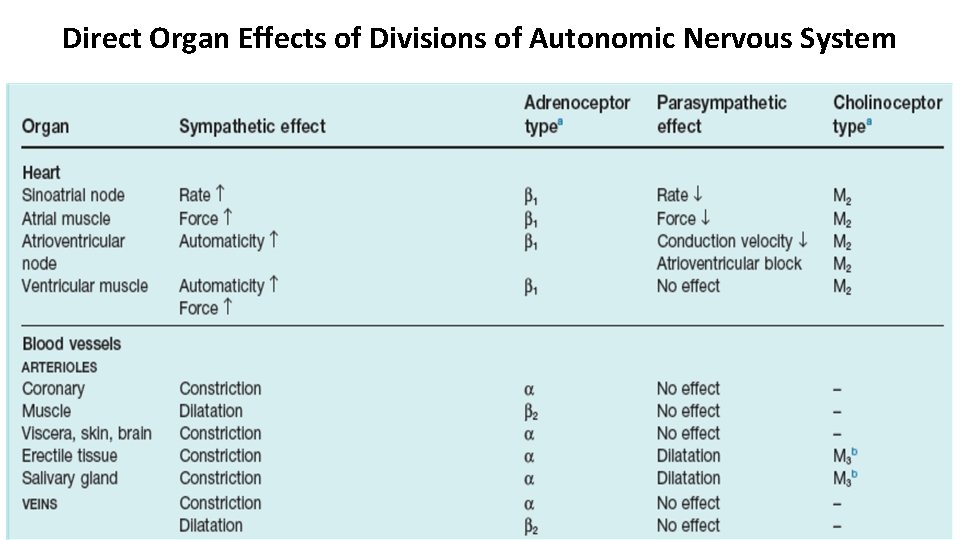
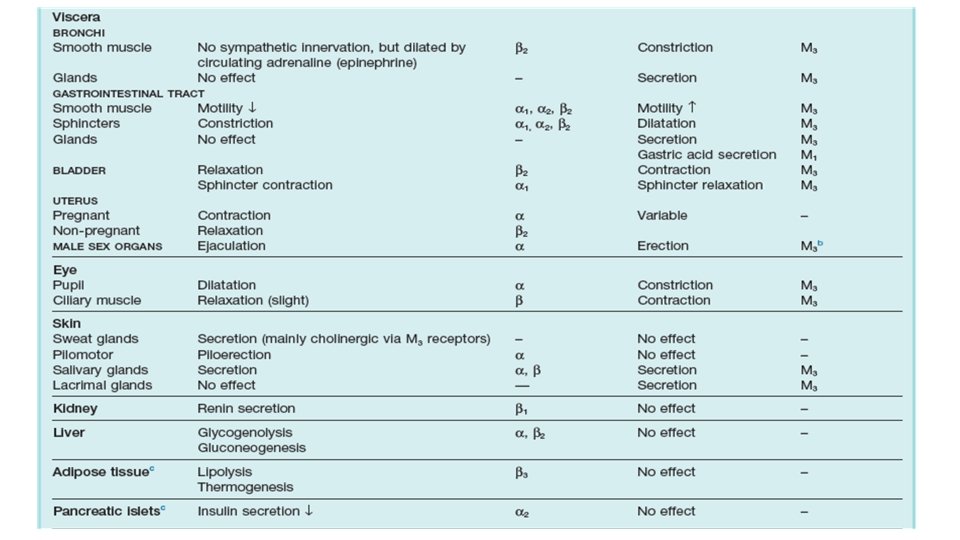
Direct Organ Effects of Divisions of Autonomic Nervous System


Thank you