Organisms are Composed of Elements in Combinations called
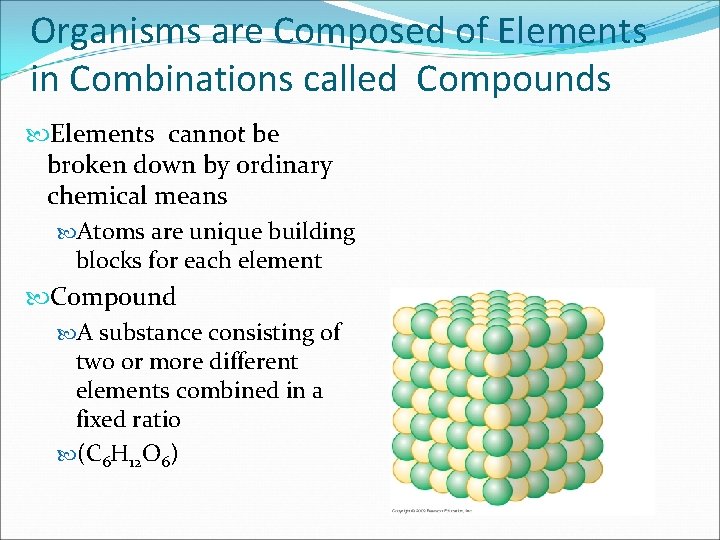
Organisms are Composed of Elements in Combinations called Compounds Elements cannot be broken down by ordinary chemical means Atoms are unique building blocks for each element Compound A substance consisting of two or more different elements combined in a fixed ratio (C 6 H 12 O 6)

Atoms Consist of Protons, Neutrons and Electrons Three important subatomic particles: Neutrons No charge in the nucleus 1 atomic mass unit (amu) Protons Positive charge, in the nucleus 1 amu Electrons Negative charge , orbit nucleus Zero amu Equal in number to protons in atom Atoms of different elements contain different numbers of subatomic particles Compare hydrogen, helium and lithium

Proton Neutron Electron Hydrogen (1 H) Deuterium (2 H) Tritium (3 H)

Atomic Number and Atomic Mass Atomic number = number of protons in nucleus Mass number = mass of protons and neutrons Mass numbers of atoms of an element: are not all identical Isotopes: Structural variations of elements; differ in the # of neutrons they contain

Isotopes Proton Neutron Electron Hydrogen (H) Helium (He) Lithium (Li) Figure 2. 2

Radioactive Isotopes can Help or Harm Us

The Distribution of Electrons Determines an Atoms Chemical Properties Electrons occupy up to seven electron shells (energy levels) around nucleus Octet rule: Except for the first shell which is full with two electrons, atoms interact in order to have eight electrons in their outermost energy level (valence shell) Stable and unreactive elements have their valence shell fully occupied Reactive elements lack a full valence shell and tend to gain, lose, or share electrons (form bonds) with other atoms to achieve stability
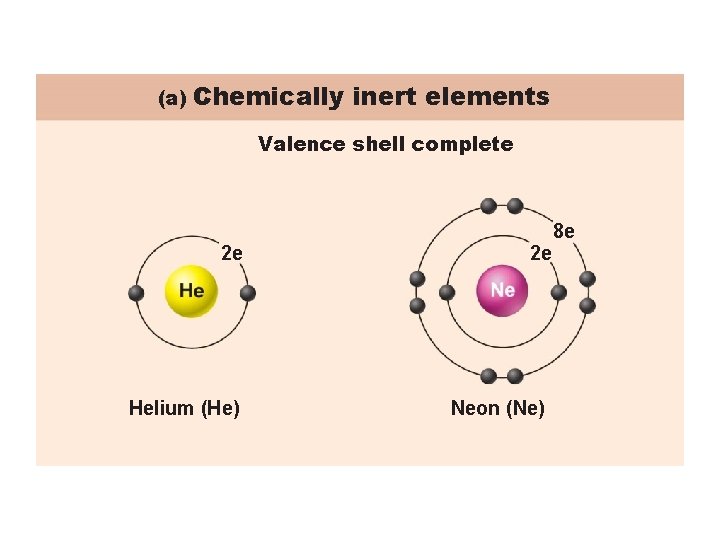
(a) Chemically inert elements Valence shell complete 2 e Helium (He) 2 e Neon (Ne) 8 e

(b) Chemically reactive elements Valence shell incomplete 1 e Hydrogen (H) 6 e 2 e Oxygen (O) 4 e 2 e Carbon © 1 e 8 e 2 e Sodium (Na)

Covalent Bonds join atoms into molecules through electron sharing Formed by sharing of two or more valence shell electrons Allows each atom to fill its valence shell at least part of the time Sharing of electrons may be equal or unequal Equal sharing produces electrically balanced, nonpolar molecules CO 2 Unequal sharing by atoms with different electron-attracting abilities produces polar molecules H 2 O Atoms with six or seven valence shell electrons are electronegative, e. g. , oxygen

Reacting atoms Resulting molecules + Hydrogen atoms or Carbon atom Molecule of methane gas (CH 4) (a) Formation of four single covalent bonds:

Reacting atoms Resulting molecules + Oxygen atom or Oxygen atom Molecule of oxygen gas (O 2) (b) Formation of a double covalent bond: Figure 2. 7 b
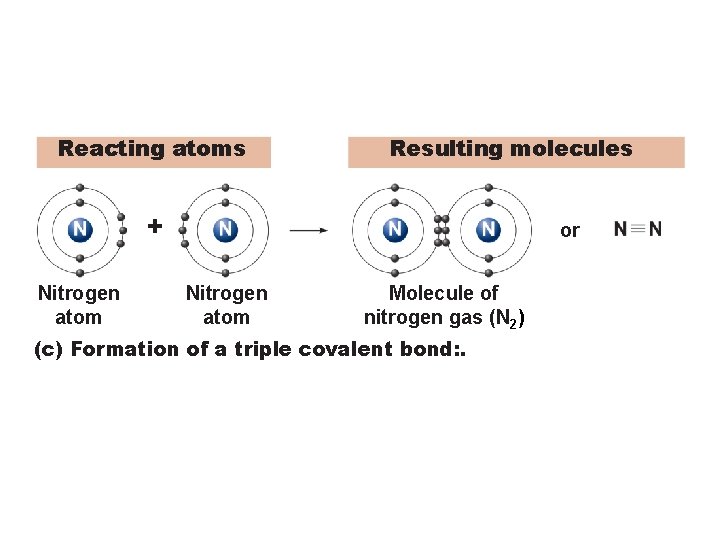
Reacting atoms Resulting molecules + Nitrogen atom or Nitrogen atom Molecule of nitrogen gas (N 2) (c) Formation of a triple covalent bond: .

Ionic bonds are attractions between ions of opposite charge Ions are formed by: transfer of valence shell electrons between atoms Anions (– charge): have gained one or more electrons Cations (+ charge): have lost one or more electrons Attraction of opposite charges: results in an ionic bond + Sodium atom (Na) Chlorine atom (Cl) Sodium ion (Na+) – Chloride ion (Cl–) Sodium chloride (Na. Cl)

Attractive force between electropositive hydrogen of one molecule and an electronegative atom of another molecule + – Hydrogen bond + + – – – + + + – (a) The slightly positive ends ( +) of the water molecules become aligned with the slightly negative ends ( –) of other water molecules.

Properties of Water Charged Exists as Gas, Liquid and Solid Great solvent Adhesion and Cohesion (via H-bonds) Surface tension Ionizes into acid and base
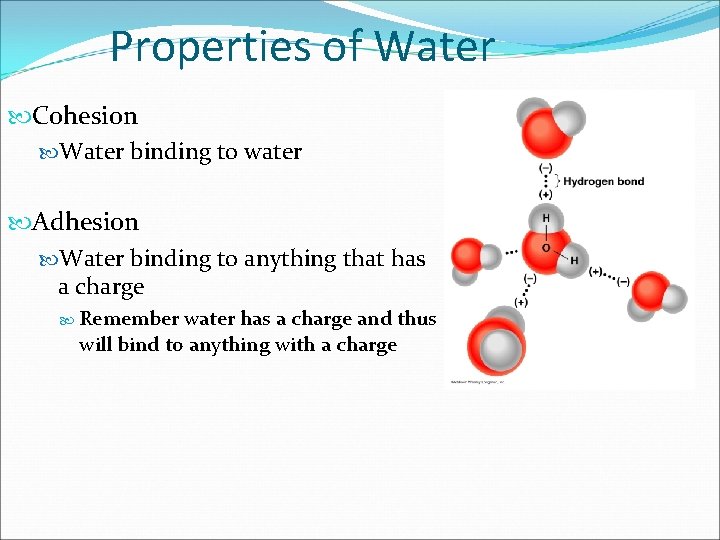
Properties of Water Cohesion Water binding to water Adhesion Water binding to anything that has a charge Remember water has a charge and thus will bind to anything with a charge

Capillary Action
- Slides: 18