Organics Organic Structures Many materials that forensic scientists


- Slides: 13

Organics

Organic Structures • Many materials that forensic scientists collect at a crime scene are organic in nature. • Carbon atoms make the backbone of all organic structures. • This is because carbon is able to make four stable bonds which provides a lot of variety in bonding possibilities. • The carbon chains can change their behavior depending on the type of bond as well. • How they are shaped determines not only how they interact with the body or environment, but also in the method of detection and identification.

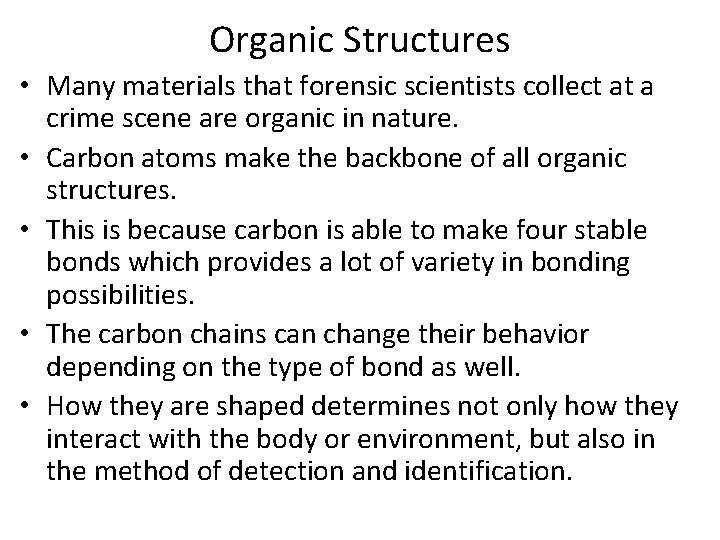
Carbon – Carbon bonds • Carbon can make single bonds (a sharing of one pair of electrons) with other carbons or other elements. • Carbon can also make double bonds (a sharing of two pairs of electrons) or triple bonds (3 pairs of electrons)

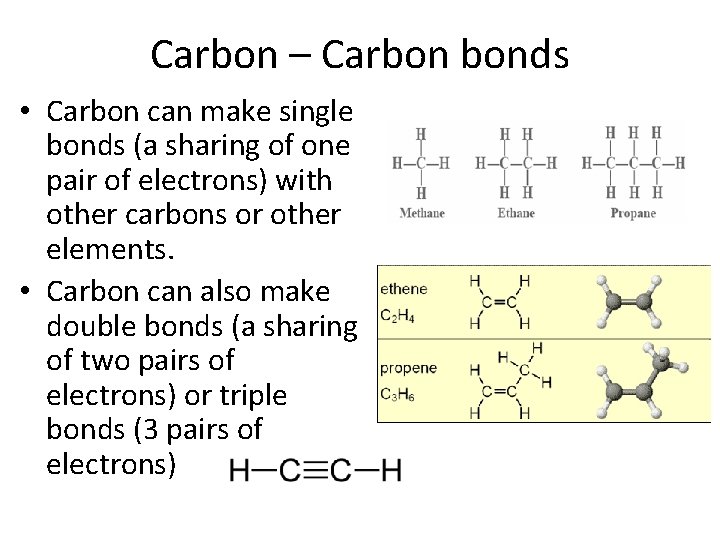
More Carbon – Carbon bonds • Carbon chains can also make a ring structure or single bonds. • The ring can also contain double bonds. • Alternating single and double bonded rings are called aromatics. Benzene or a phenyl group

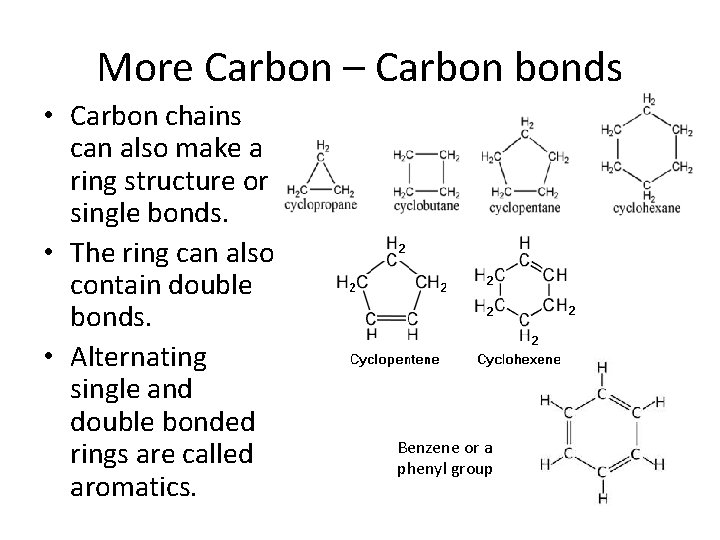
Branches • Off of the main chains or rings can be carbon branches. • In addition, rings may connect and link up.

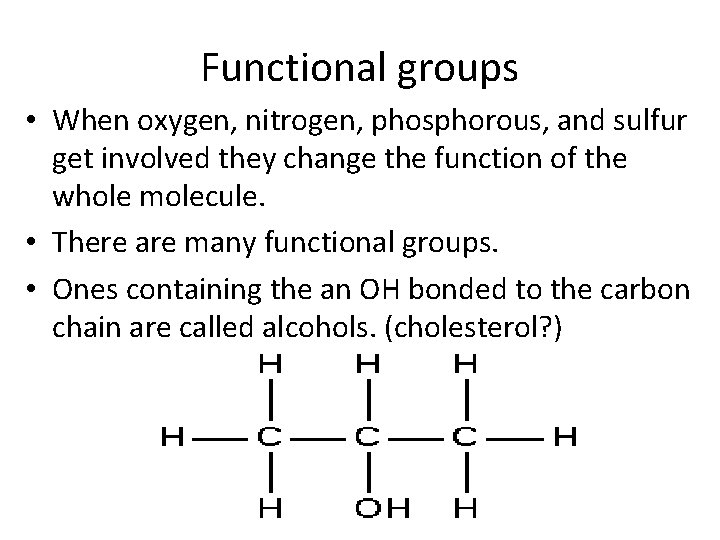
Functional groups • When oxygen, nitrogen, phosphorous, and sulfur get involved they change the function of the whole molecule. • There are many functional groups. • Ones containing the an OH bonded to the carbon chain are called alcohols. (cholesterol? )

Carbonyl group • The carbonyl group is when a carbon is double bonded to and oxygen. • These are called aldehydes or ketones if the carbonyl group is not attached to other oxygen, nitrogen, or other functional atoms.

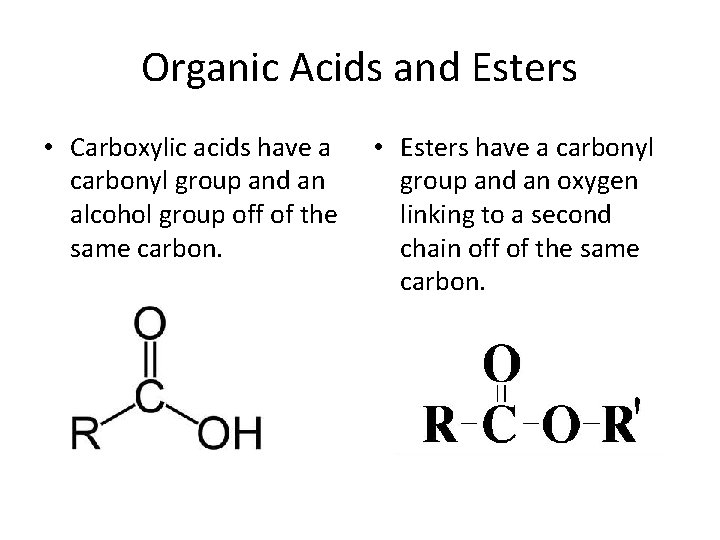
Organic Acids and Esters • Carboxylic acids have a carbonyl group and an alcohol group off of the same carbon. • Esters have a carbonyl group and an oxygen linking to a second chain off of the same carbon.

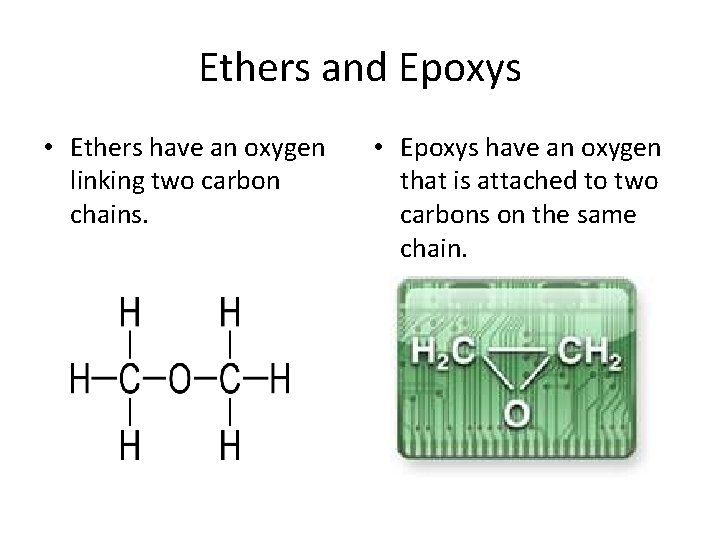
Ethers and Epoxys • Ethers have an oxygen linking two carbon chains. • Epoxys have an oxygen that is attached to two carbons on the same chain.

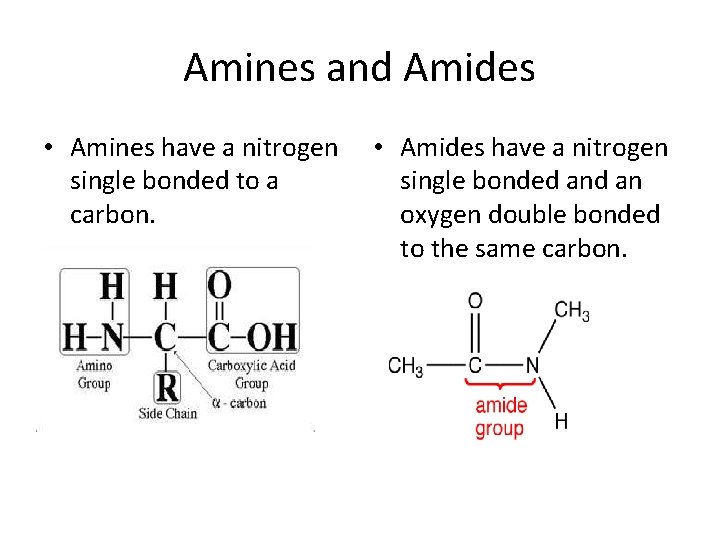
Amines and Amides • Amines have a nitrogen single bonded to a carbon. • Amides have a nitrogen single bonded an oxygen double bonded to the same carbon.

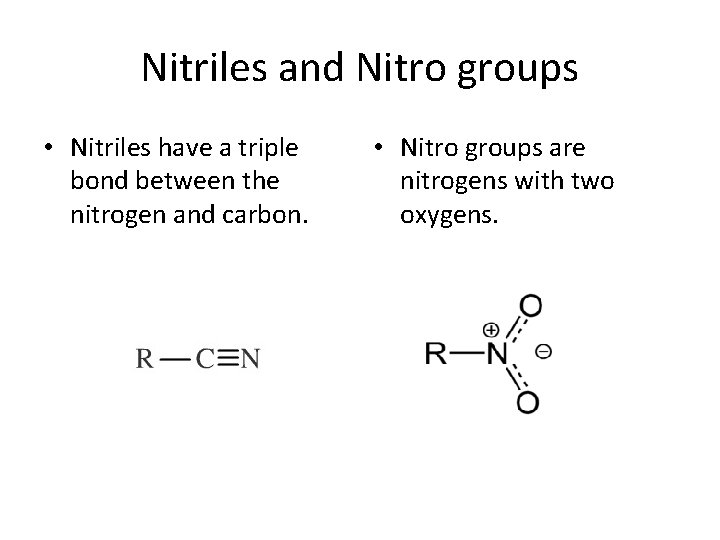
Nitriles and Nitro groups • Nitriles have a triple bond between the nitrogen and carbon. • Nitro groups are nitrogens with two oxygens.

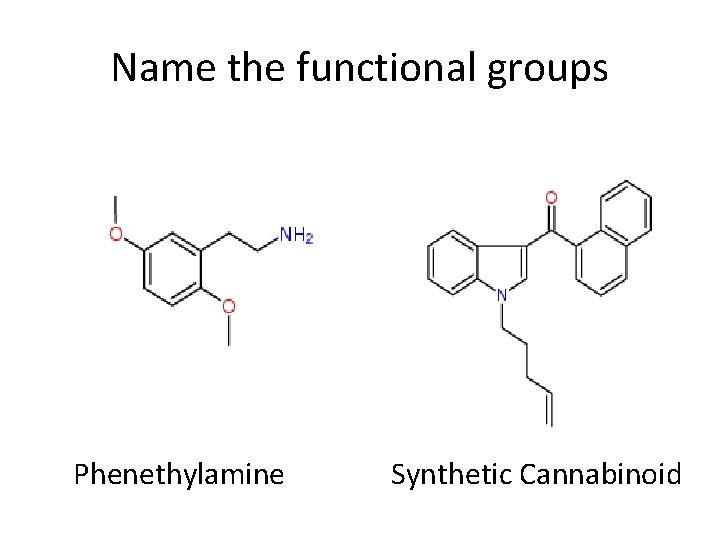
Name the functional groups Phenethylamine Synthetic Cannabinoid

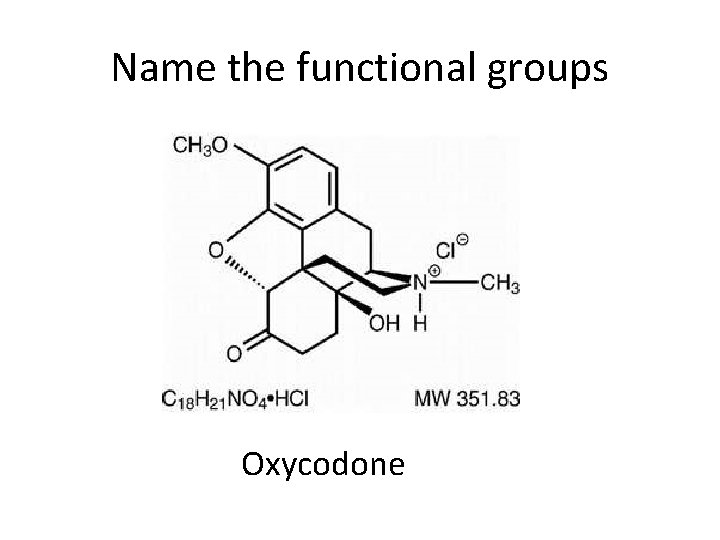
Name the functional groups Oxycodone