ORGANIC SYNTHESIS KNOCKHARDY PUBLISHING 2015 SPECIFICATIONS KNOCKHARDY PUBLISHING
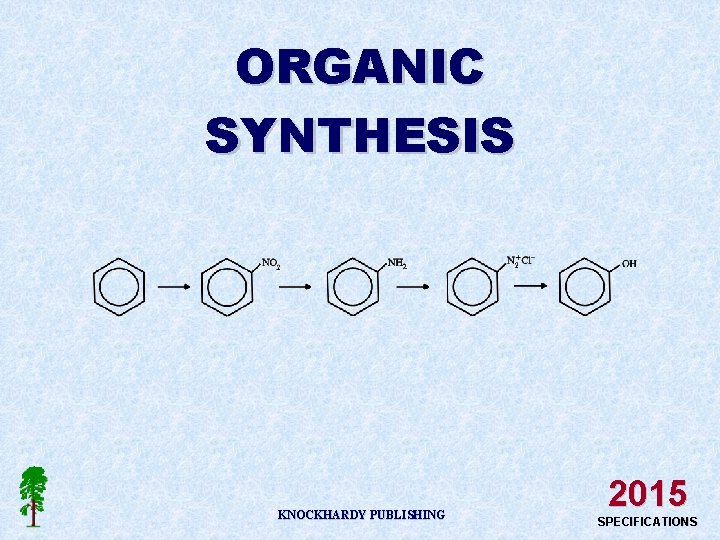
ORGANIC SYNTHESIS KNOCKHARDY PUBLISHING 2015 SPECIFICATIONS

KNOCKHARDY PUBLISHING ORGANIC SYNTHESIS INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A 2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A 2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. knockhardy. org. uk/sci. htm Navigation is achieved by. . . either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard

ORGANIC SYNTHESIS CONTENTS • Introduction • Functional groups • Extending a carbon chain • Chiral synthesis - introduction • Nucleophilic addition • Nucleophilic substitution • Synthetic methods

ORGANIC SYNTHESIS Involves the preparation of new compounds from others.

ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups.

ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . .

ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • the reagents required to convert one functional group into another • the presence of other functional groups - in case also they react
ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • • • the reagents required to convert one functional group into another the presence of other functional groups - in case also they react the conditions required - temperature, pressure, catalyst the rate of the reaction the yield - especially important for equilibrium reactions atom economy
ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • • • the reagents required to convert one functional group into another the presence of other functional groups - in case also they react the conditions required - temperature, pressure, catalyst the rate of the reaction the yield - especially important for equilibrium reactions atom economy safety - toxicity and flammability of reactants and products financial economy - cost of chemicals, demand for product problems of purification possibility of optically active products
ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • • • the reagents required to convert one functional group into another the presence of other functional groups - in case also they react the conditions required - temperature, pressure, catalyst the rate of the reaction the yield - especially important for equilibrium reactions atom economy safety - toxicity and flammability of reactants and products financial economy - cost of chemicals, demand for product problems of purification possibility of optically active products
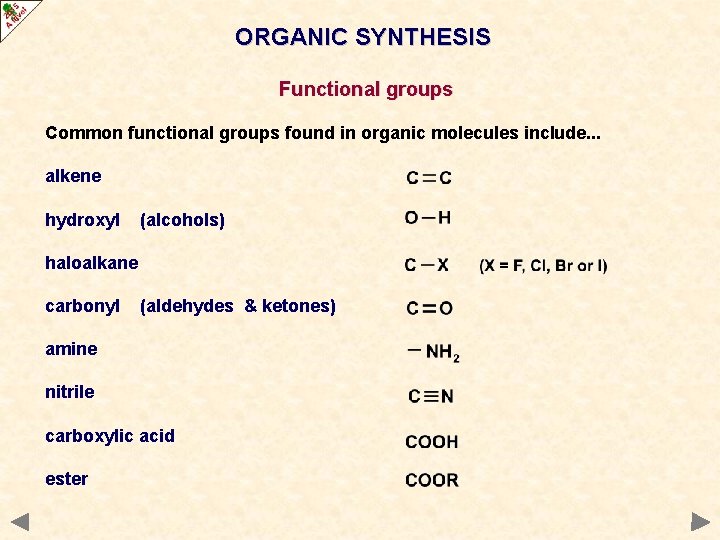
ORGANIC SYNTHESIS Functional groups Common functional groups found in organic molecules include. . . alkene hydroxyl (alcohols) haloalkane carbonyl (aldehydes & ketones) amine nitrile carboxylic acid ester
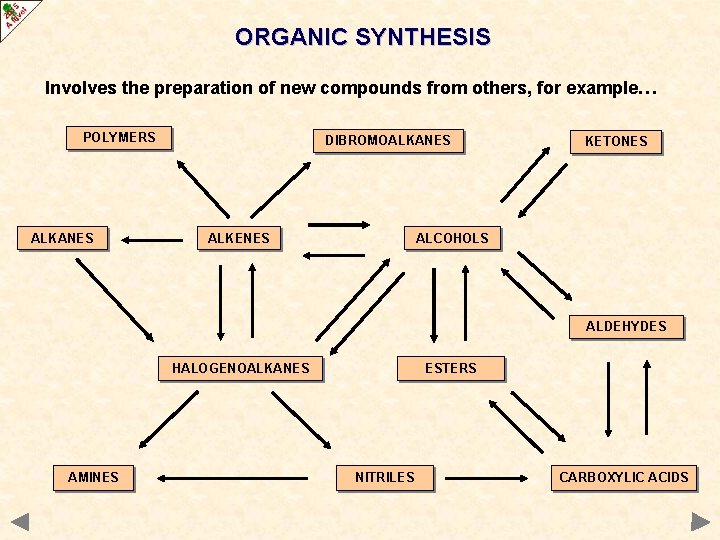
ORGANIC SYNTHESIS Involves the preparation of new compounds from others, for example… POLYMERS ALKANES DIBROMOALKANES KETONES ALCOHOLS ALKENES ALDEHYDES HALOGENOALKANES AMINES ESTERS NITRILES CARBOXYLIC ACIDS

EXTENDING A CARBON CHAIN Rationale Methods Haloalkanes Carbonyl compounds (aldehydes and ketones) Aromatic (benzene) rings
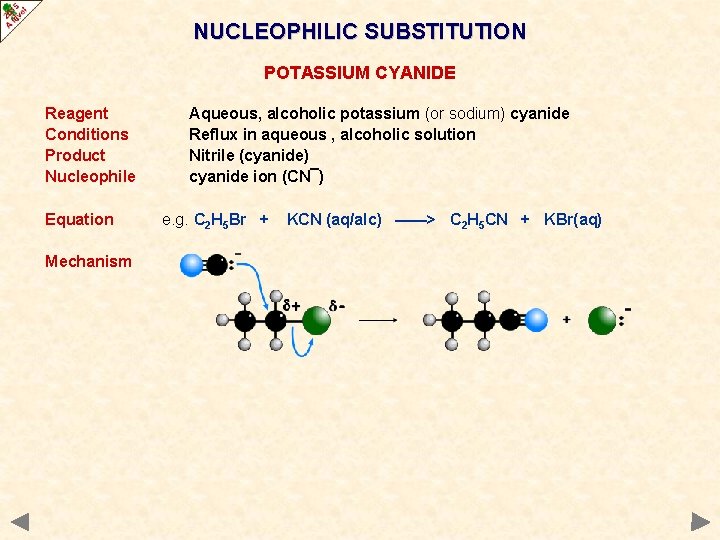
NUCLEOPHILIC SUBSTITUTION POTASSIUM CYANIDE Reagent Conditions Product Nucleophile Equation Mechanism Aqueous, alcoholic potassium (or sodium) cyanide Reflux in aqueous , alcoholic solution Nitrile (cyanide) cyanide ion (CN¯) e. g. C 2 H 5 Br + KCN (aq/alc) ——> C 2 H 5 CN + KBr(aq)
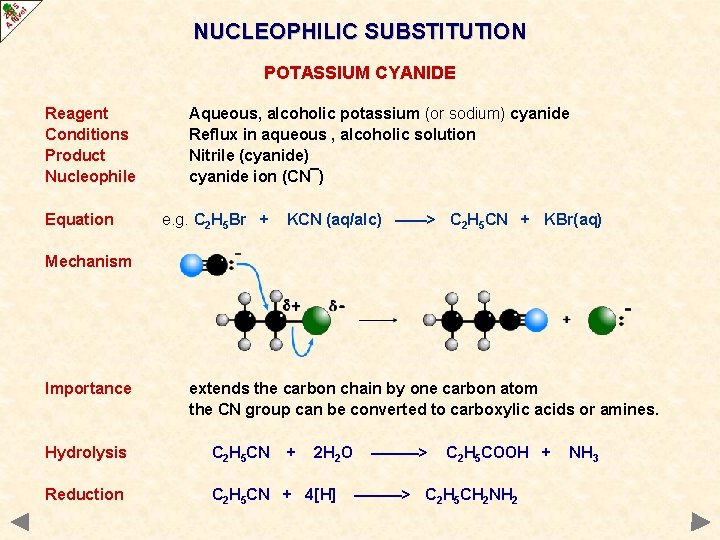
NUCLEOPHILIC SUBSTITUTION POTASSIUM CYANIDE Reagent Conditions Product Nucleophile Equation Aqueous, alcoholic potassium (or sodium) cyanide Reflux in aqueous , alcoholic solution Nitrile (cyanide) cyanide ion (CN¯) e. g. C 2 H 5 Br + KCN (aq/alc) ——> C 2 H 5 CN + KBr(aq) Mechanism Importance extends the carbon chain by one carbon atom the CN group can be converted to carboxylic acids or amines. Hydrolysis C 2 H 5 CN + 2 H 2 O Reduction C 2 H 5 CN + 4[H] ———> C 2 H 5 COOH + ———> C 2 H 5 CH 2 NH 2 NH 3
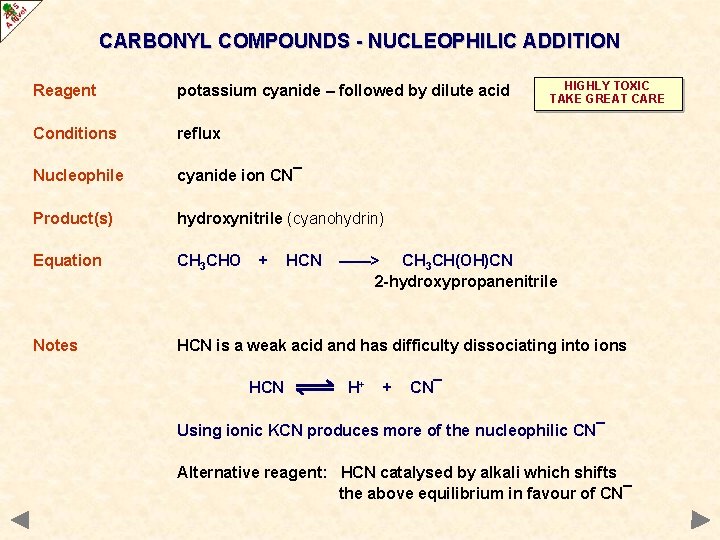
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION HIGHLY TOXIC TAKE GREAT CARE Reagent potassium cyanide – followed by dilute acid Conditions reflux Nucleophile cyanide ion CN¯ Product(s) hydroxynitrile (cyanohydrin) Equation CH 3 CHO Notes HCN is a weak acid and has difficulty dissociating into ions + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile H+ + CN¯ Using ionic KCN produces more of the nucleophilic CN¯ Alternative reagent: HCN catalysed by alkali which shifts the above equilibrium in favour of CN¯
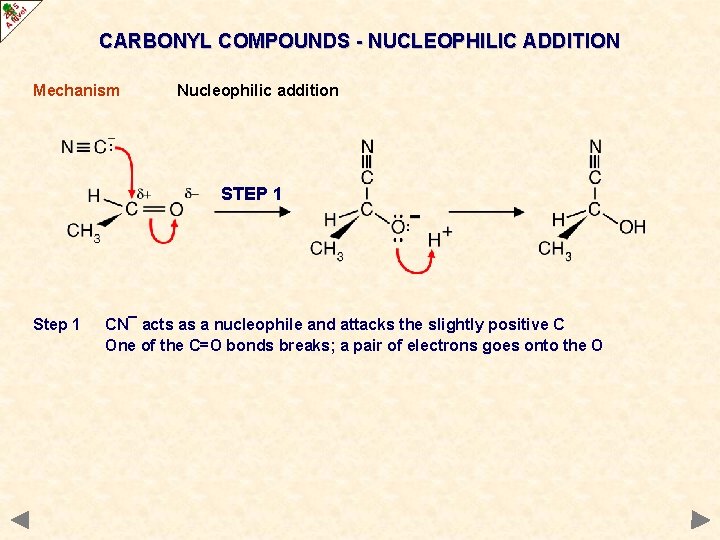
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O
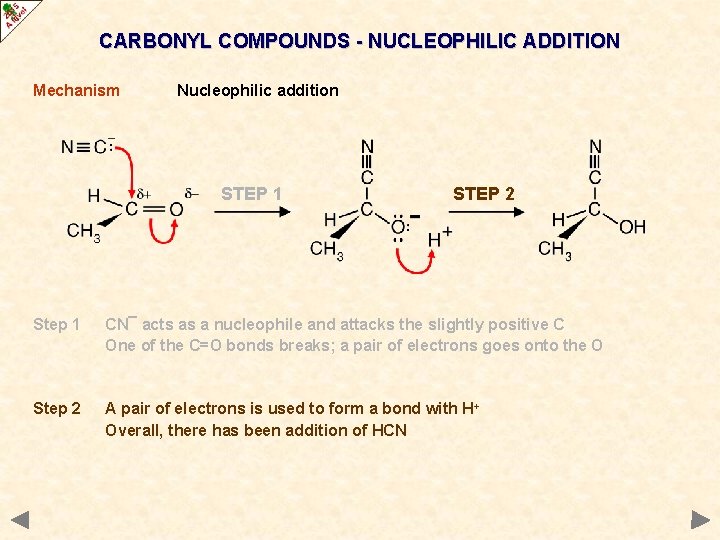
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 STEP 2 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O Step 2 A pair of electrons is used to form a bond with H+ Overall, there has been addition of HCN
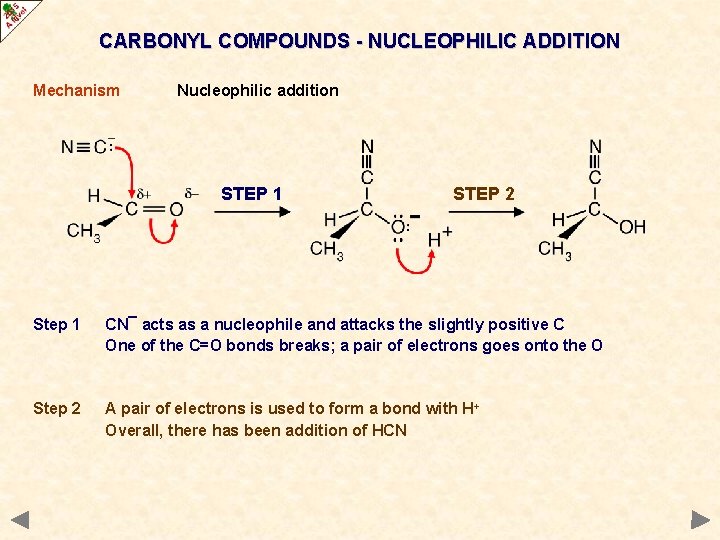
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 STEP 2 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O Step 2 A pair of electrons is used to form a bond with H+ Overall, there has been addition of HCN
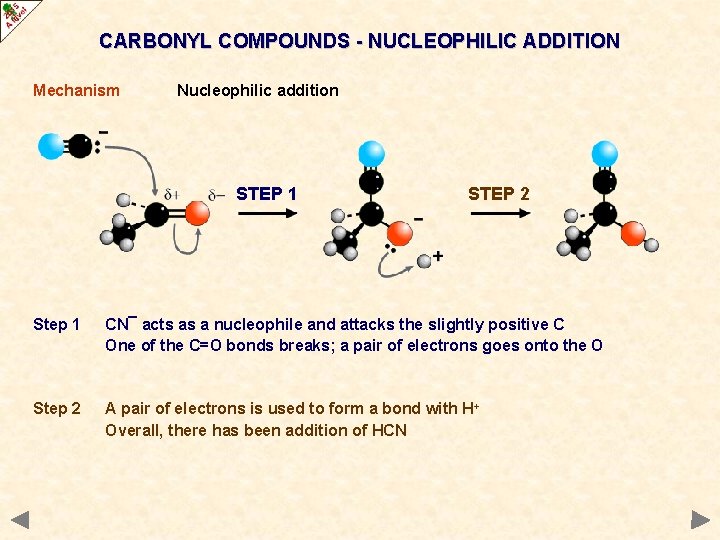
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 STEP 2 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O Step 2 A pair of electrons is used to form a bond with H+ Overall, there has been addition of HCN
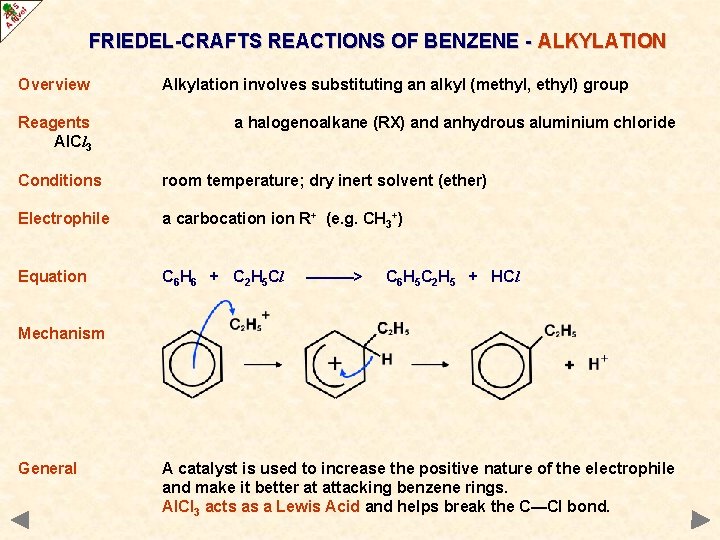
FRIEDEL-CRAFTS REACTIONS OF BENZENE - ALKYLATION Overview Reagents Al. Cl 3 Alkylation involves substituting an alkyl (methyl, ethyl) group a halogenoalkane (RX) and anhydrous aluminium chloride Conditions room temperature; dry inert solvent (ether) Electrophile a carbocation R+ (e. g. CH 3+) Equation C 6 H 6 + C 2 H 5 Cl ———> C 6 H 5 C 2 H 5 + HCl Mechanism General A catalyst is used to increase the positive nature of the electrophile and make it better at attacking benzene rings. Al. Cl 3 acts as a Lewis Acid and helps break the C—Cl bond.
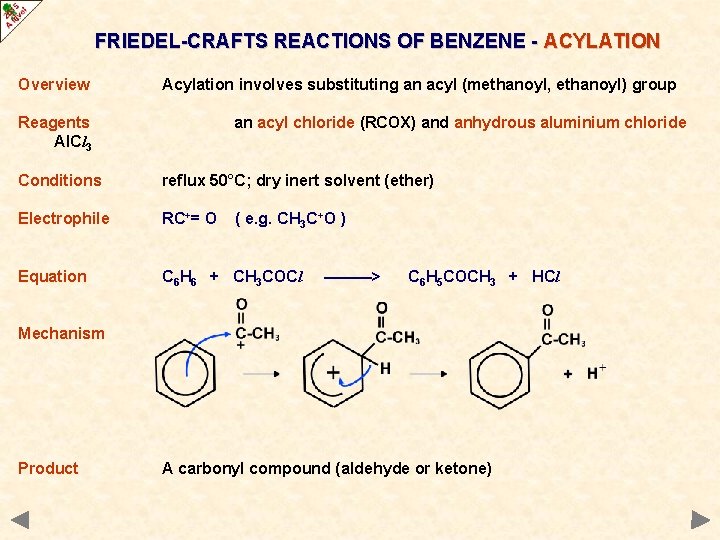
FRIEDEL-CRAFTS REACTIONS OF BENZENE - ACYLATION Overview Acylation involves substituting an acyl (methanoyl, ethanoyl) group Reagents Al. Cl 3 an acyl chloride (RCOX) and anhydrous aluminium chloride Conditions reflux 50°C; dry inert solvent (ether) Electrophile RC+= O Equation C 6 H 6 + CH 3 COCl ( e. g. CH 3 C+O ) ———> C 6 H 5 COCH 3 + HCl Mechanism Product A carbonyl compound (aldehyde or ketone)

EXTENDING A CARBON CHAIN Rationale Methods Haloalkanes Carbonyl compounds (aldehydes and ketones) Aromatic (benzene) rings

CHIRAL SYNTHESIS Rationale Pharmaceutical synthesis often requires the production of just one optical isomer. This is because. . .
CHIRAL SYNTHESIS Rationale Pharmaceutical synthesis often requires the production of just one optical isomer. This is because. . . • one optical isomer usually works better than the other • the other optical isomer may cause dangerous side effects • laboratory reactions usually produce both optical isomers • naturally occurring reactions usually produce just one optical isomer
CHIRAL SYNTHESIS Rationale Pharmaceutical synthesis often requires the production of just one optical isomer. This is because. . . • one optical isomer usually works better than the other • the other optical isomer may cause dangerous side effects • laboratory reactions usually produce both optical isomers • naturally occurring reactions usually produce just one optical isomer Example Aldehydes and ketones undergo nucleophilic addition with cyanide (nitrile) ions; CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal Problem - + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile the C=O bond is planar the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the C=O
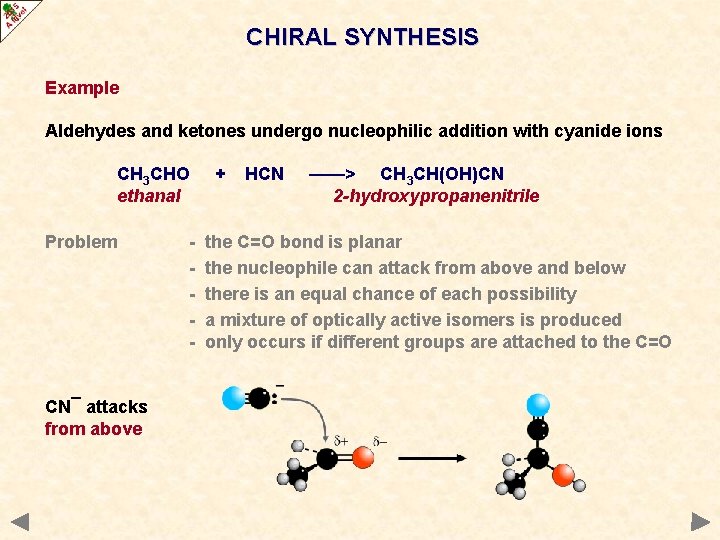
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal Problem CN¯ attacks from above - + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile the C=O bond is planar the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the C=O
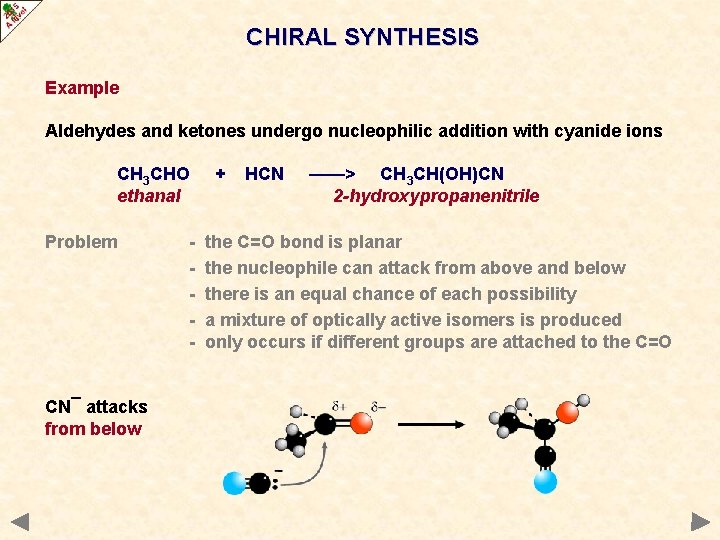
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal Problem CN¯ attacks from below - + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile the C=O bond is planar the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the C=O
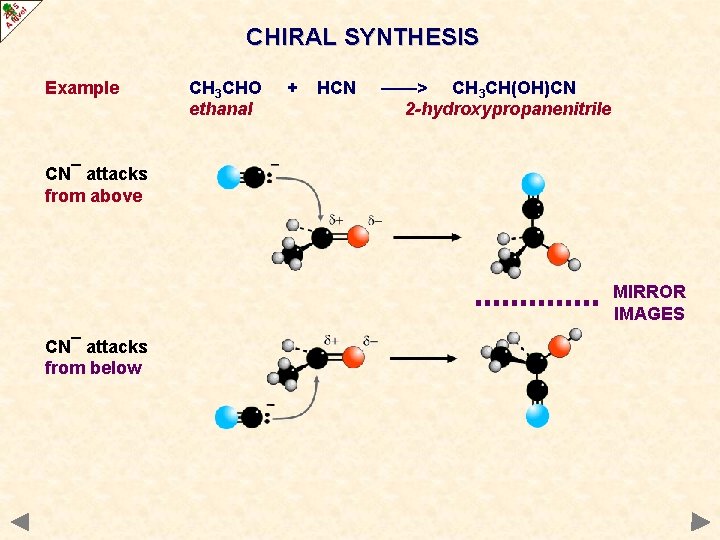
CHIRAL SYNTHESIS Example CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile CN¯ attacks from above MIRROR IMAGES CN¯ attacks from below
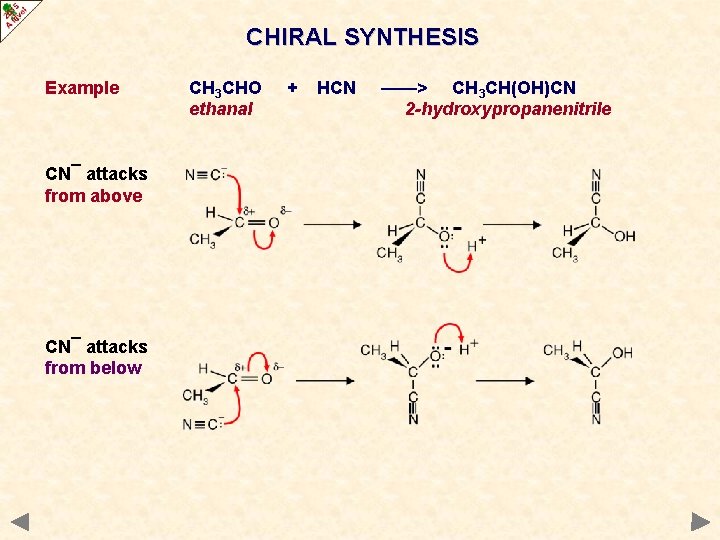
CHIRAL SYNTHESIS Example CN¯ attacks from above CN¯ attacks from below CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile
CHIRAL SYNTHESIS Example CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile ANIMATION
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to larger doses needed possible dangerous side effects possible legal action
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to larger doses needed possible dangerous side effects possible legal action Solution • use natural chiral molecules as starting materials • use stereoselective reactions which give one isomer • use catalysts which give a specific isomer • use enzymes or bacteria which are stereoselective
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to larger doses needed possible dangerous side effects possible legal action Solution • use natural chiral molecules as starting materials • use stereoselective reactions which give one isomer • use catalysts which give a specific isomer • use enzymes or bacteria which are stereoselective Other examples Nucleophilic substitution of haloalkanes
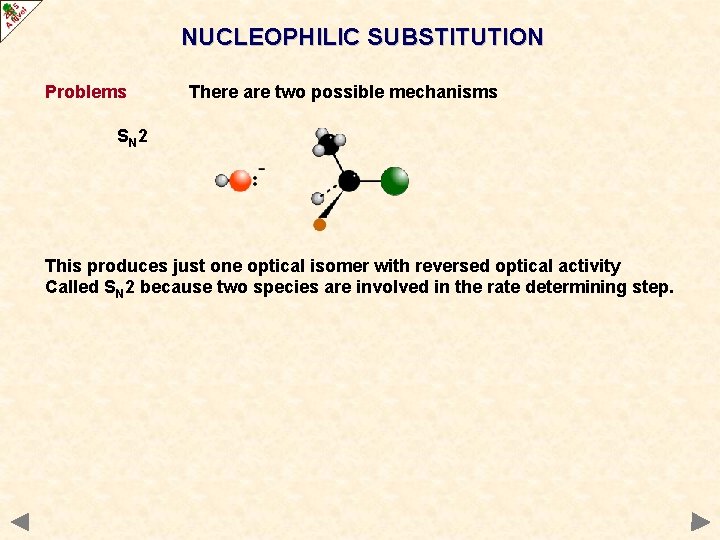
NUCLEOPHILIC SUBSTITUTION Problems There are two possible mechanisms SN 2 This produces just one optical isomer with reversed optical activity Called SN 2 because two species are involved in the rate determining step.
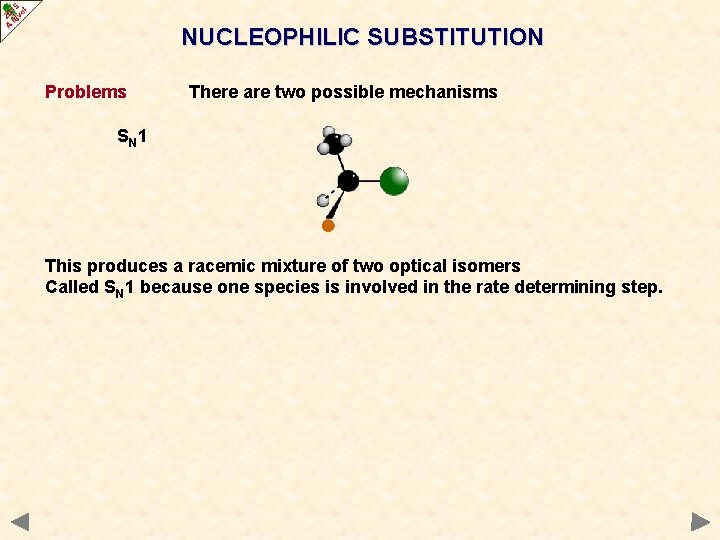
NUCLEOPHILIC SUBSTITUTION Problems There are two possible mechanisms SN 1 This produces a racemic mixture of two optical isomers Called SN 1 because one species is involved in the rate determining step.
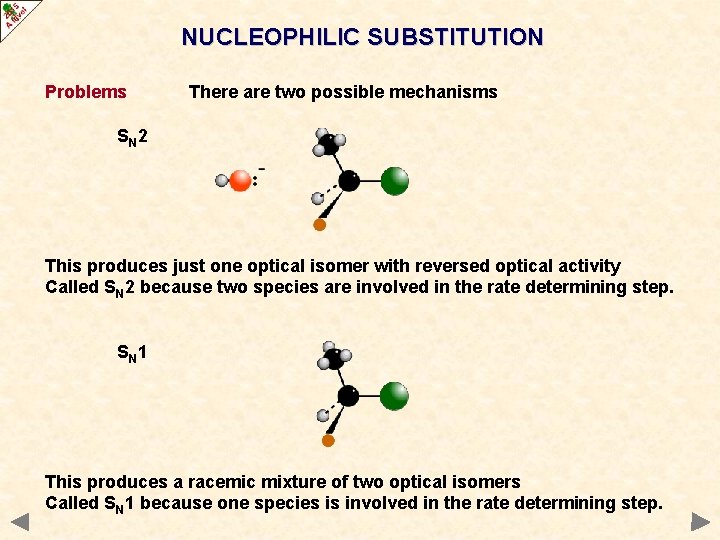
NUCLEOPHILIC SUBSTITUTION Problems There are two possible mechanisms SN 2 This produces just one optical isomer with reversed optical activity Called SN 2 because two species are involved in the rate determining step. SN 1 This produces a racemic mixture of two optical isomers Called SN 1 because one species is involved in the rate determining step.

MODERN SYNTHETIC METHODS The following methods can be used to synthesise a single optical isomer Enzymes / bacteria Chiral chemicals Chiral catalysts Natural chiral molecules

ORGANIC SYNTHESIS THE END © 2015 JONATHAN HOPTON & KNOCKHARDY PUBLISHING
- Slides: 42