ORGANIC SYNTHESIS KNOCKHARDY PUBLISHING 2008 SPECIFICATIONS KNOCKHARDY PUBLISHING

ORGANIC SYNTHESIS KNOCKHARDY PUBLISHING 2008 SPECIFICATIONS

KNOCKHARDY PUBLISHING ORGANIC SYNTHESIS INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A 2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A 2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. knockhardy. org. uk/sci. htm Navigation is achieved by. . . either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard

ORGANIC SYNTHESIS CONTENTS • Introduction • Functional groups • Chiral synthesis - introduction • Nucleophilic addition • Nucleophilic substitution • Synthetic methods

ORGANIC SYNTHESIS Involves the preparation of new compounds from others.

ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups.

ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . .

ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • the reagents required to convert one functional group into another • the presence of other functional groups - in case also they react
ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • • • the reagents required to convert one functional group into another the presence of other functional groups - in case also they react the conditions required - temperature, pressure, catalyst the rate of the reaction the yield - especially important for equilibrium reactions atom economy
ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • • • the reagents required to convert one functional group into another the presence of other functional groups - in case also they react the conditions required - temperature, pressure, catalyst the rate of the reaction the yield - especially important for equilibrium reactions atom economy safety - toxicity and flammability of reactants and products financial economy - cost of chemicals, demand for product problems of purification possibility of optically active products
ORGANIC SYNTHESIS Involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider. . . • • • the reagents required to convert one functional group into another the presence of other functional groups - in case also they react the conditions required - temperature, pressure, catalyst the rate of the reaction the yield - especially important for equilibrium reactions atom economy safety - toxicity and flammability of reactants and products financial economy - cost of chemicals, demand for product problems of purification possibility of optically active products
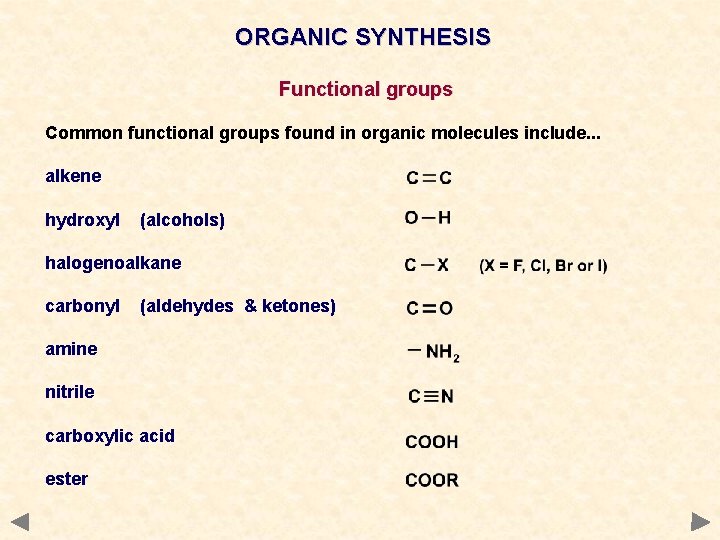
ORGANIC SYNTHESIS Functional groups Common functional groups found in organic molecules include. . . alkene hydroxyl (alcohols) halogenoalkane carbonyl (aldehydes & ketones) amine nitrile carboxylic acid ester

CHIRAL SYNTHESIS Rationale Pharmaceutical synthesis often requires the production of just one optical isomer. This is because. . .
CHIRAL SYNTHESIS Rationale Pharmaceutical synthesis often requires the production of just one optical isomer. This is because. . . • one optical isomer usually works better than the other • the other optical isomer may cause dangerous side effects • laboratory reactions usually produce both optical isomers • naturally occurring reactions usually produce just one optical isomer
CHIRAL SYNTHESIS Rationale Pharmaceutical synthesis often requires the production of just one optical isomer. This is because. . . • one optical isomer usually works better than the other • the other optical isomer may cause dangerous side effects • laboratory reactions usually produce both optical isomers • naturally occurring reactions usually produce just one optical isomer Example Aldehydes and ketones undergo nucleophilic addition with cyanide (nitrile) ions; CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal Problem - + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile the C=O bond is planar the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the C=O
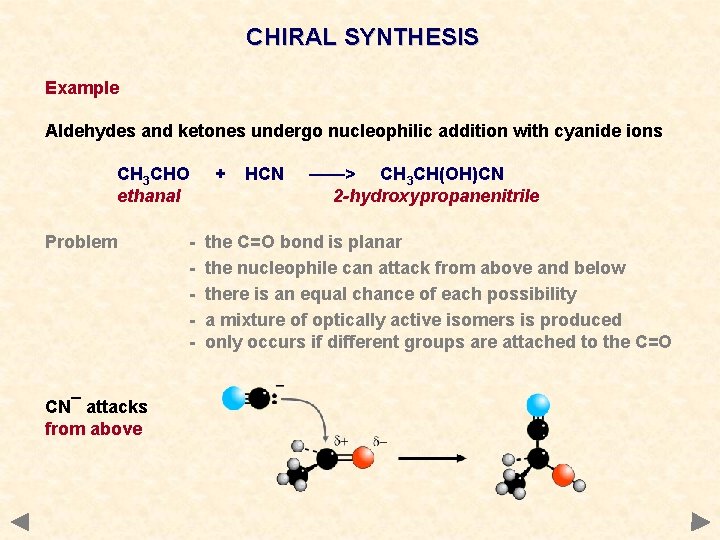
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal Problem CN¯ attacks from above - + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile the C=O bond is planar the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the C=O
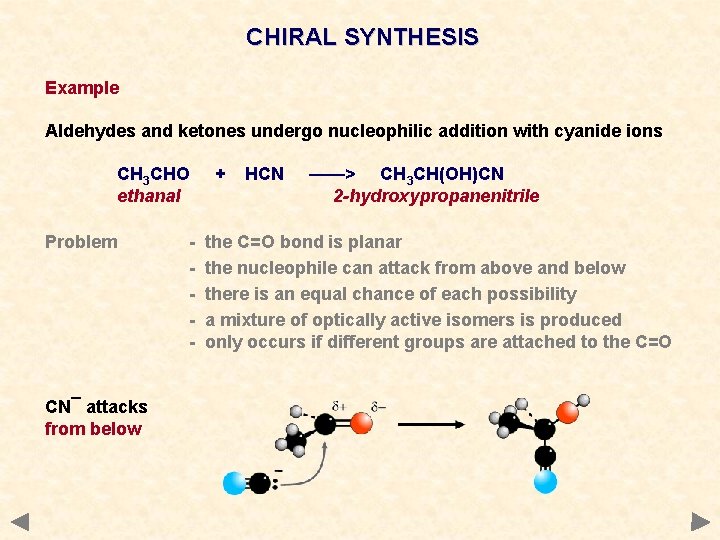
CHIRAL SYNTHESIS Example Aldehydes and ketones undergo nucleophilic addition with cyanide ions CH 3 CHO ethanal Problem CN¯ attacks from below - + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile the C=O bond is planar the nucleophile can attack from above and below there is an equal chance of each possibility a mixture of optically active isomers is produced only occurs if different groups are attached to the C=O
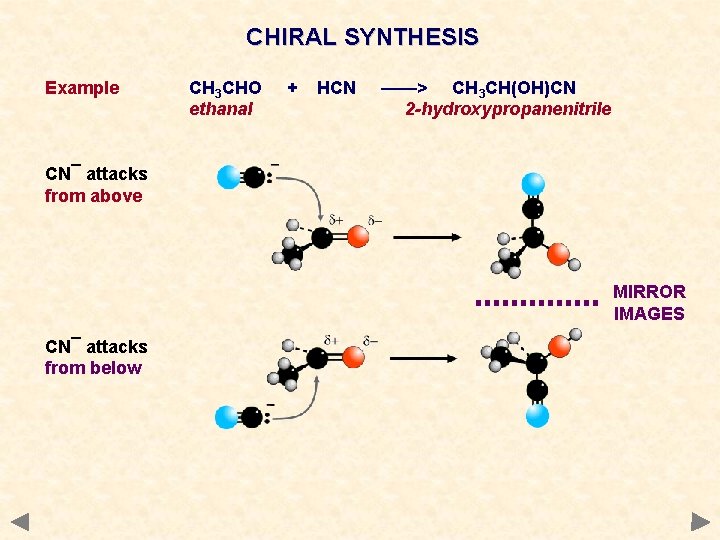
CHIRAL SYNTHESIS Example CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile CN¯ attacks from above MIRROR IMAGES CN¯ attacks from below
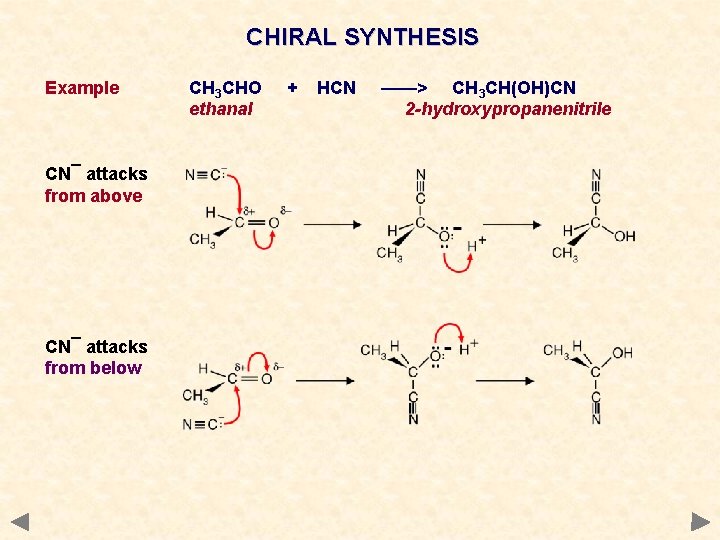
CHIRAL SYNTHESIS Example CN¯ attacks from above CN¯ attacks from below CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile
CHIRAL SYNTHESIS Example CH 3 CHO ethanal + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile ANIMATION
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to larger doses needed possible dangerous side effects possible legal action
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to larger doses needed possible dangerous side effects possible legal action Solution • use natural chiral molecules as starting materials • use stereoselective reactions which give one isomer • use catalysts which give a specific isomer • use enzymes or bacteria which are stereoselective
CHIRAL SYNTHESIS Consequences • isomers have to be separated to obtain the effective one • separation can be expensive and complicated • non-separation leads to larger doses needed possible dangerous side effects possible legal action Solution • use natural chiral molecules as starting materials • use stereoselective reactions which give one isomer • use catalysts which give a specific isomer • use enzymes or bacteria which are stereoselective Other examples Nucleophilic substitution of halogenoalkanes
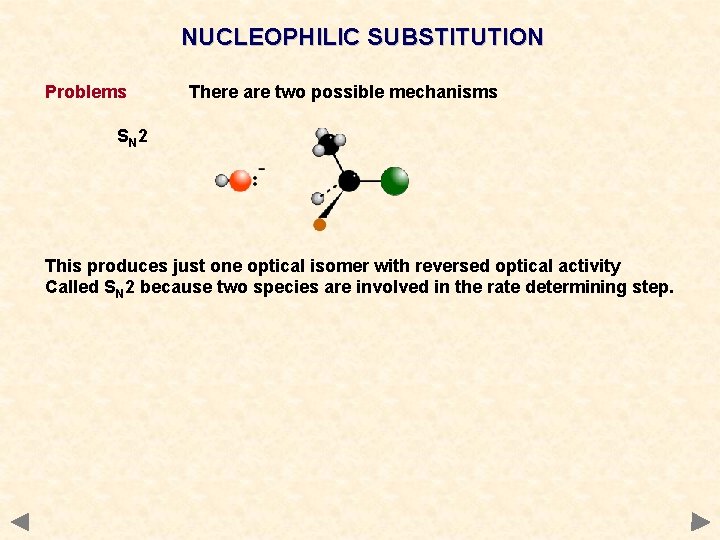
NUCLEOPHILIC SUBSTITUTION Problems There are two possible mechanisms SN 2 This produces just one optical isomer with reversed optical activity Called SN 2 because two species are involved in the rate determining step.
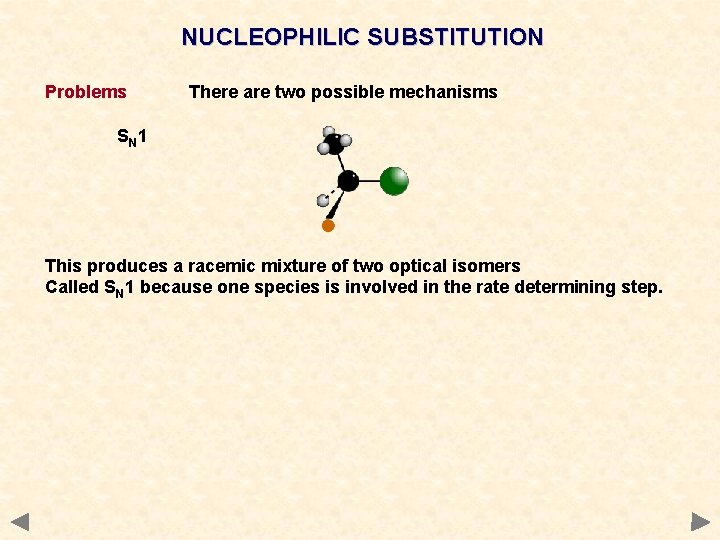
NUCLEOPHILIC SUBSTITUTION Problems There are two possible mechanisms SN 1 This produces a racemic mixture of two optical isomers Called SN 1 because one species is involved in the rate determining step.
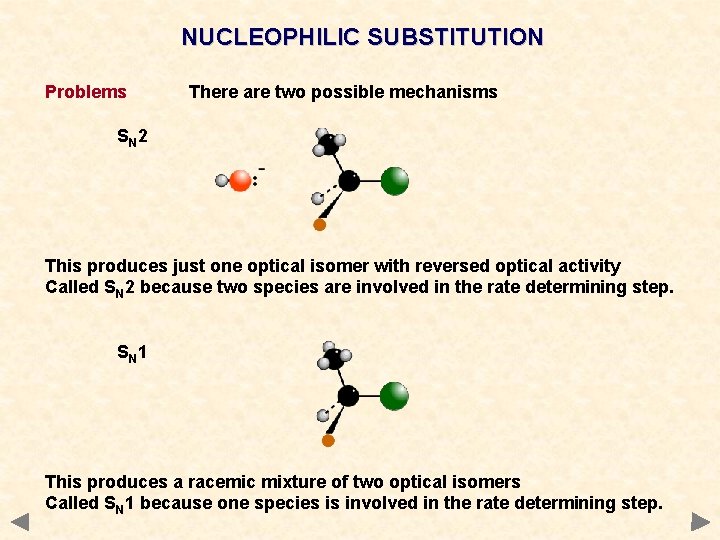
NUCLEOPHILIC SUBSTITUTION Problems There are two possible mechanisms SN 2 This produces just one optical isomer with reversed optical activity Called SN 2 because two species are involved in the rate determining step. SN 1 This produces a racemic mixture of two optical isomers Called SN 1 because one species is involved in the rate determining step.

MODERN SYNTHETIC METHODS The following methods can be used to synthesise a single optical isomer Enzymes / bacteria Chiral chemicals Chiral catalysts Natural chiral molecules

ORGANIC SYNTHESIS THE END © 2009 JONATHAN HOPTON & KNOCKHARDY PUBLISHING
- Slides: 30