ORGANIC SUBSTITUTION REACTIONS Generalized Polar Reactions An electrophile

ORGANIC SUBSTITUTION REACTIONS
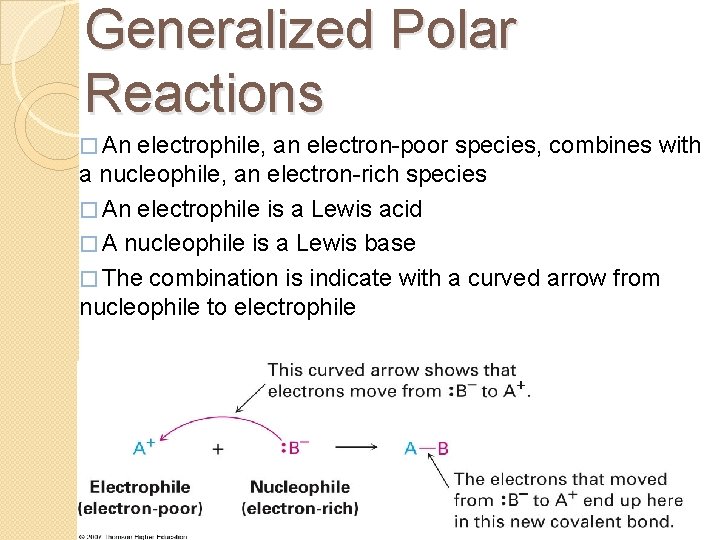
Generalized Polar Reactions � An electrophile, an electron-poor species, combines with a nucleophile, an electron-rich species � An electrophile is a Lewis acid � A nucleophile is a Lewis base � The combination is indicate with a curved arrow from nucleophile to electrophile

Nucleophilic Substitution �If the nucleophile and substrate are neutral, the product will be positively charged. �If the nucleophile is a negative ion and substrate is neutral, the product will be neutral. �The unshared pair of electron in the nucleophile can be used to make new covalent bond.
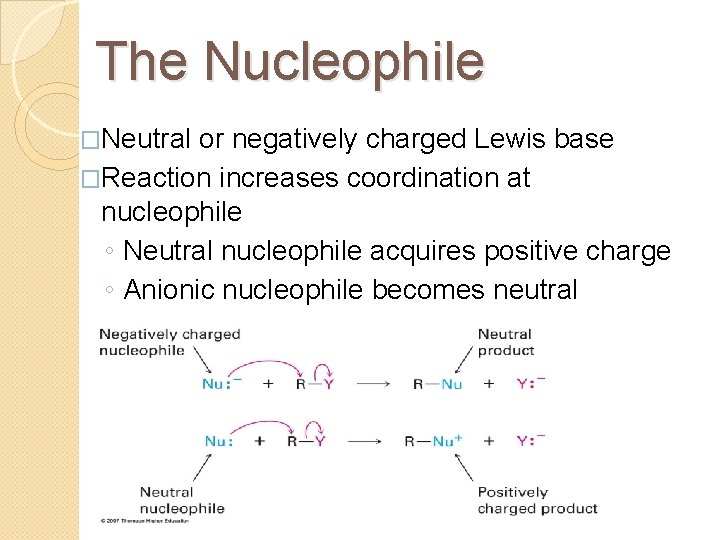
The Nucleophile �Neutral or negatively charged Lewis base �Reaction increases coordination at nucleophile ◦ Neutral nucleophile acquires positive charge ◦ Anionic nucleophile becomes neutral
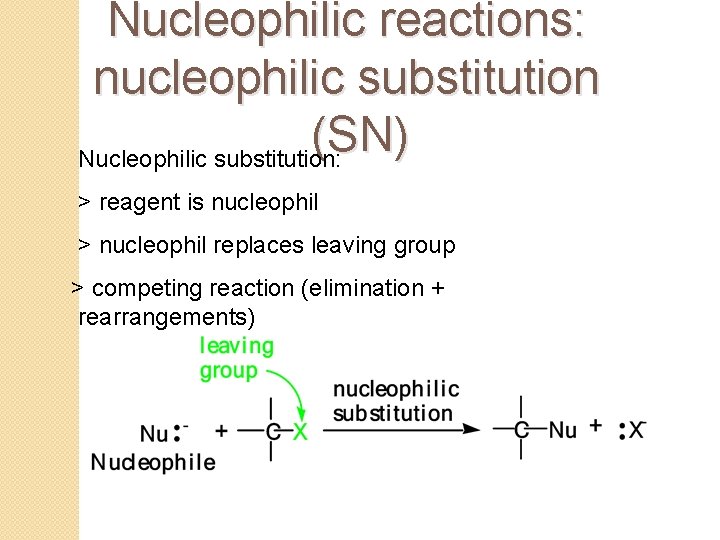
Nucleophilic reactions: nucleophilic substitution (SN) Nucleophilic substitution: > reagent is nucleophil > nucleophil replaces leaving group > competing reaction (elimination + rearrangements)
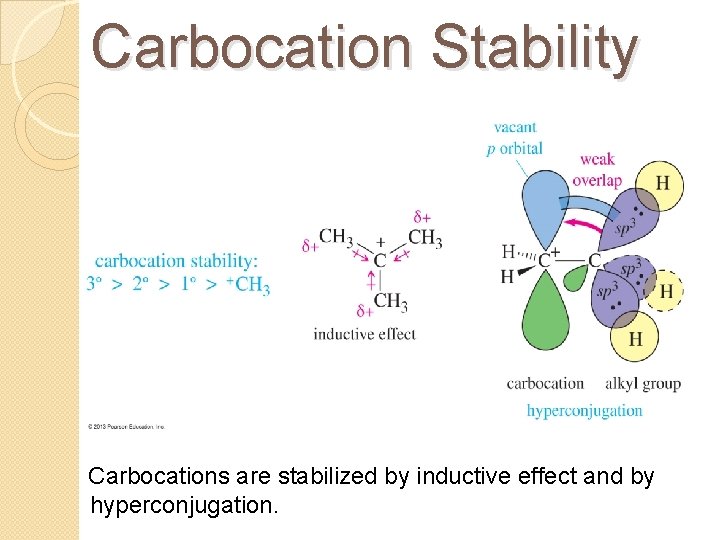
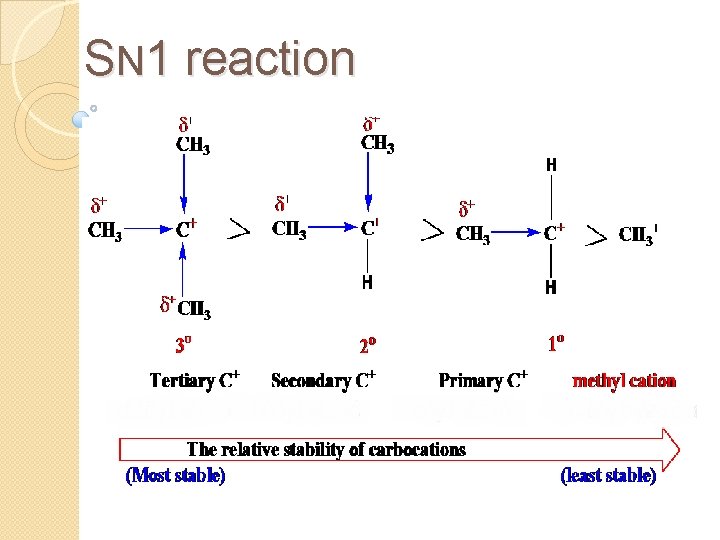
Carbocation Stability Carbocations are stabilized by inductive effect and by hyperconjugation.
Effect of variables on SN Reactio § the nature of substituents bonded to the atom attacked by nucleophile § the nature of the leaving group § the solvent effect
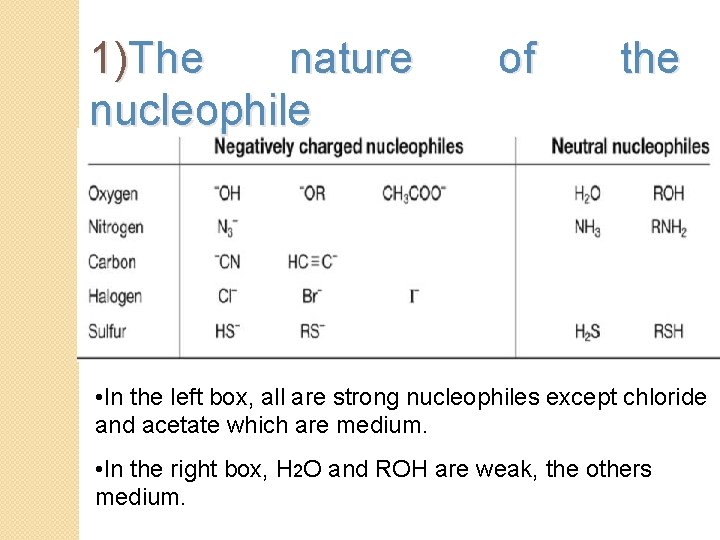
1)The nature nucleophile of the • In the left box, all are strong nucleophiles except chloride and acetate which are medium. • In the right box, H 2 O and ROH are weak, the others medium.

2)The nature of the Leaving Group • the best leaving groups in this series are the halogens I-, Br-, and Cl • OH-, RO-, and NH 2 - are such poor leaving groups that they are rarely if ever displaced in nucleophilic substitution reactions
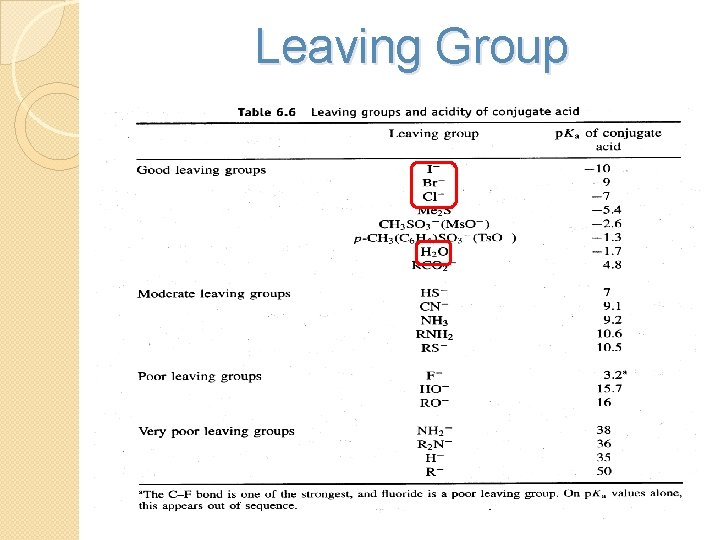
Leaving Group
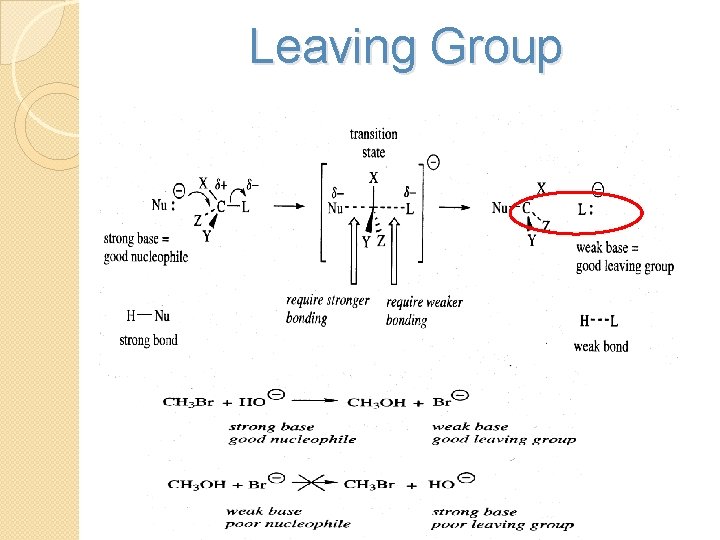
Leaving Group

3) the solvent effect �The solvent chosen for the reaction medium can have a significant effect on the products formed �Polar Protic Solvent—polar solvent that can form hydrogen bonds ◦ Ex: Water & Acetic Acid �Polar Aprotic Solvent—polar solvent that cannot hydrogen bond to the nucleophile ◦ Ex: Acetone & DMSO
S N 1

The SN 1 Reaction �The SN 1 reaction is a unimolecular nucleophilic substitution. �It has a carbocation intermediate. �Rate = k [alkyl halide]. �Racemization occurs.
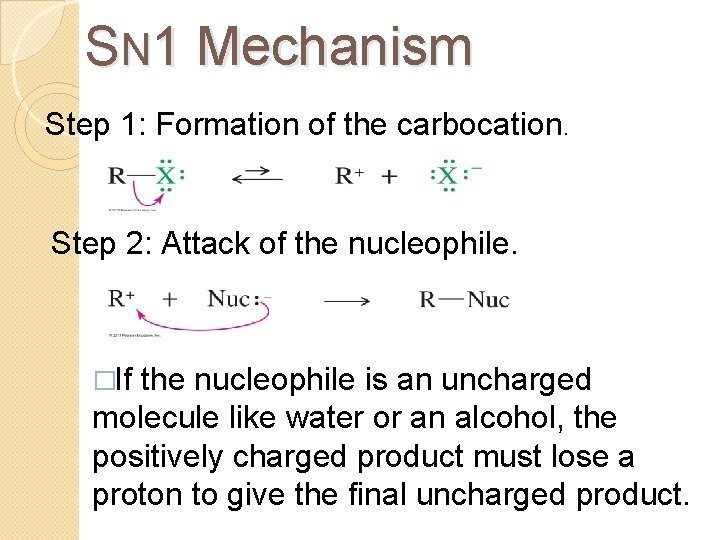
SN 1 Mechanism Step 1: Formation of the carbocation. Step 2: Attack of the nucleophile. �If the nucleophile is an uncharged molecule like water or an alcohol, the positively charged product must lose a proton to give the final uncharged product.
![SN 1 Energy Diagram and Mechanism �Rate-determining is formation of carbocation �rate = k[RX] SN 1 Energy Diagram and Mechanism �Rate-determining is formation of carbocation �rate = k[RX]](http://slidetodoc.com/presentation_image_h/37af1eb3c0d74f82c0e8b2d260bbdfe7/image-16.jpg)
SN 1 Energy Diagram and Mechanism �Rate-determining is formation of carbocation �rate = k[RX] step
SN 1 reaction
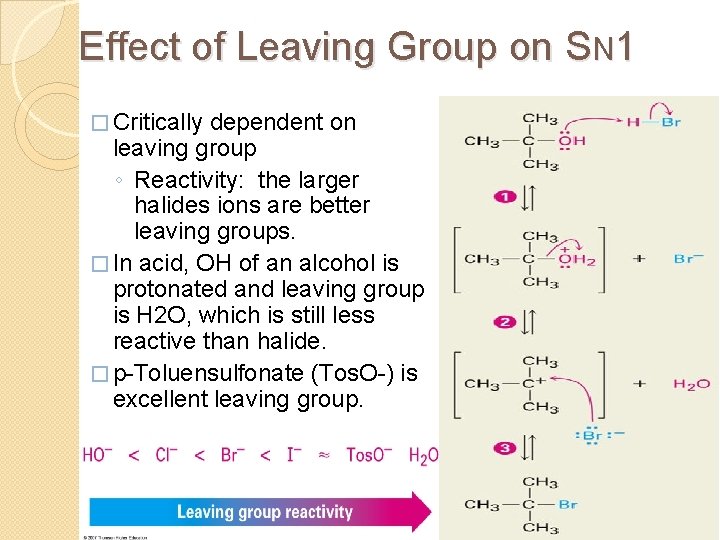
Effect of Leaving Group on SN 1 � Critically dependent on leaving group ◦ Reactivity: the larger halides ions are better leaving groups. � In acid, OH of an alcohol is protonated and leaving group is H 2 O, which is still less reactive than halide. � p-Toluensulfonate (Tos. O-) is excellent leaving group. 18
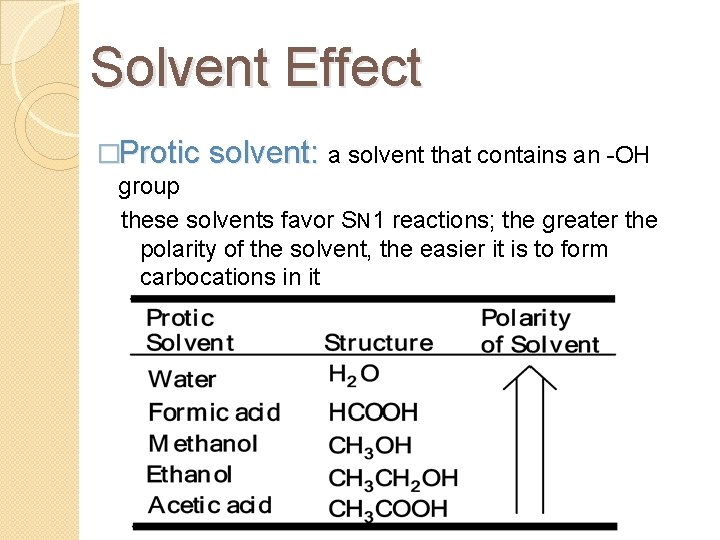
Solvent Effect �Protic solvent: a solvent that contains an -OH group these solvents favor SN 1 reactions; the greater the polarity of the solvent, the easier it is to form carbocations in it

Characteristics of SN 1 reactions
S N 2
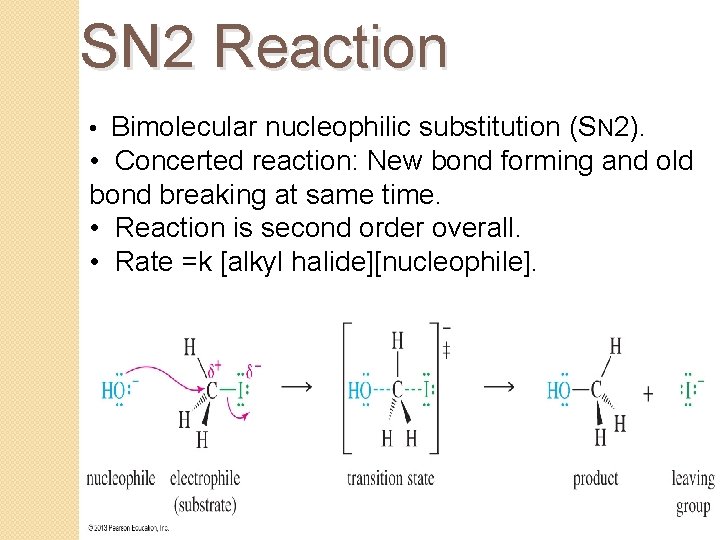
SN 2 Reaction • Bimolecular nucleophilic substitution (SN 2). • Concerted reaction: New bond forming and old bond breaking at same time. • Reaction is second order overall. • Rate =k [alkyl halide][nucleophile].
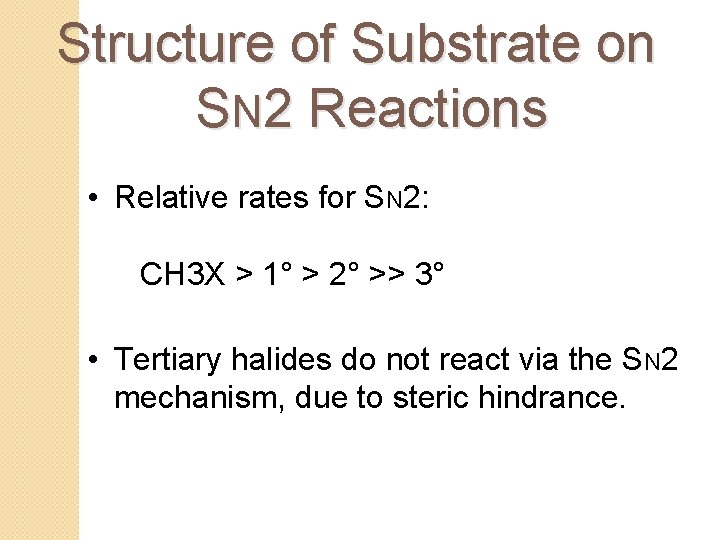
Structure of Substrate on SN 2 Reactions • Relative rates for SN 2: CH 3 X > 1° > 2° >> 3° • Tertiary halides do not react via the SN 2 mechanism, due to steric hindrance.
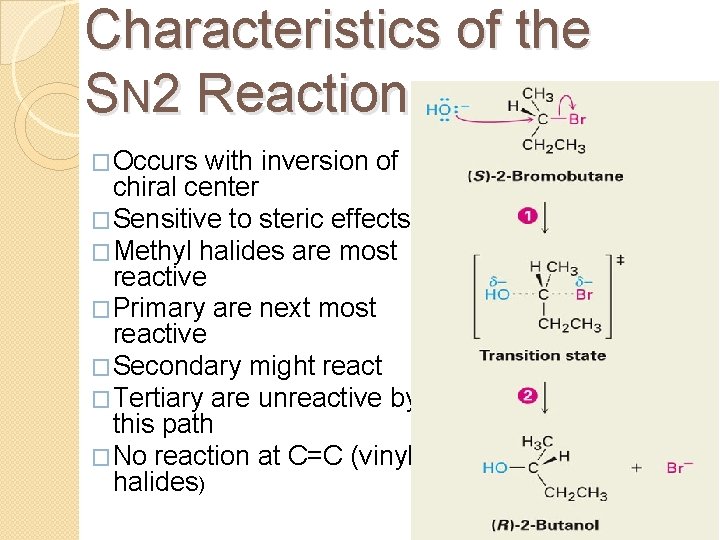
Characteristics of the SN 2 Reaction �Occurs with inversion of chiral center �Sensitive to steric effects �Methyl halides are most reactive �Primary are next most reactive �Secondary might react �Tertiary are unreactive by this path �No reaction at C=C (vinyl halides)
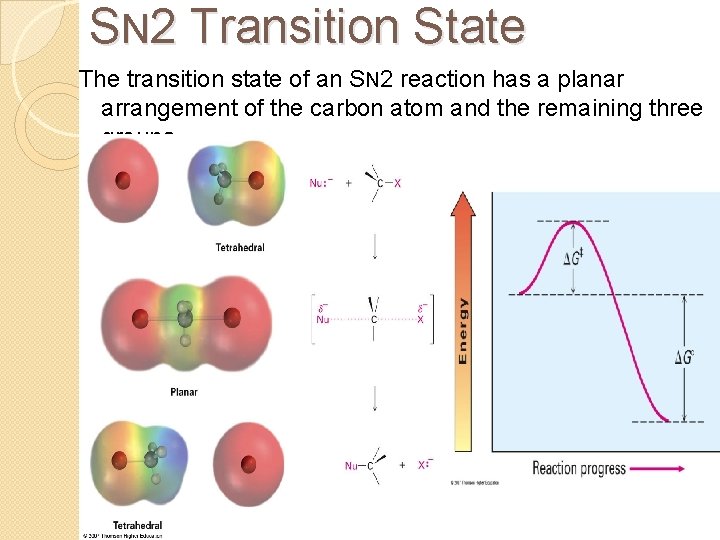
SN 2 Transition State The transition state of an SN 2 reaction has a planar arrangement of the carbon atom and the remaining three groups
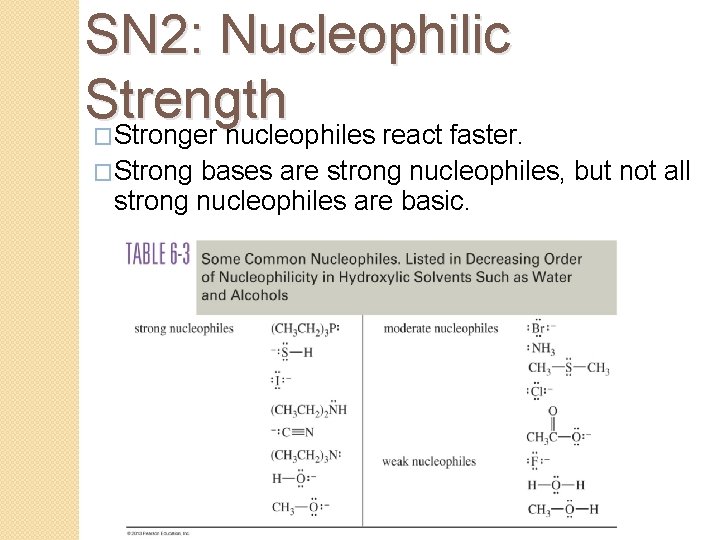
SN 2: Nucleophilic Strength �Stronger nucleophiles react faster. �Strong bases are strong nucleophiles, but not all strong nucleophiles are basic.

Solvent Effect �Aprotic solvent: does not contain an -OH group ◦ it is more difficult to form carbocations in aprotic solvents ◦ aprotic solvents favor SN 2 reactions 27
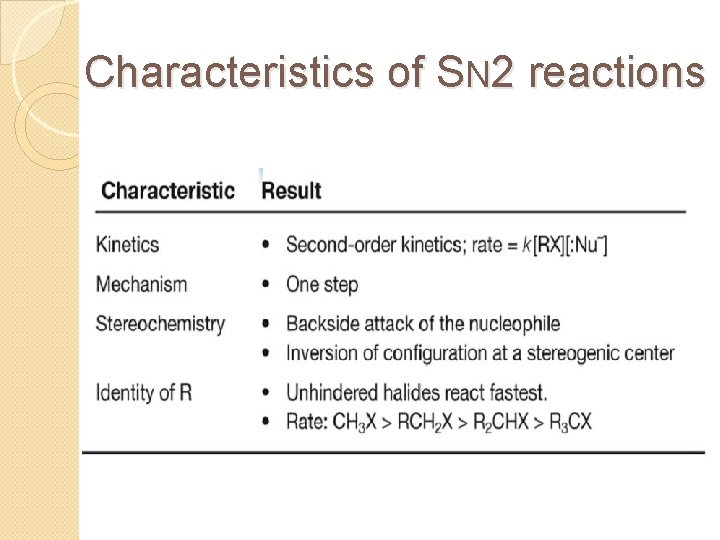
Characteristics of SN 2 reactions

A QUICK SUMMARY OF TWO SUBSTITUTION REACTIONS S N 1 / S N 2
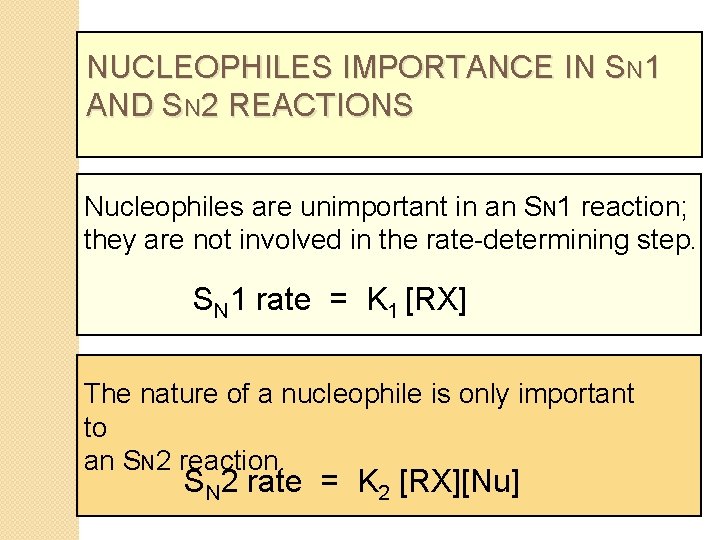
NUCLEOPHILES IMPORTANCE IN SN 1 AND SN 2 REACTIONS Nucleophiles are unimportant in an SN 1 reaction; they are not involved in the rate-determining step. SN 1 rate = K 1 [RX] The nature of a nucleophile is only important to an SN 2 reaction. SN 2 rate = K 2 [RX][Nu]
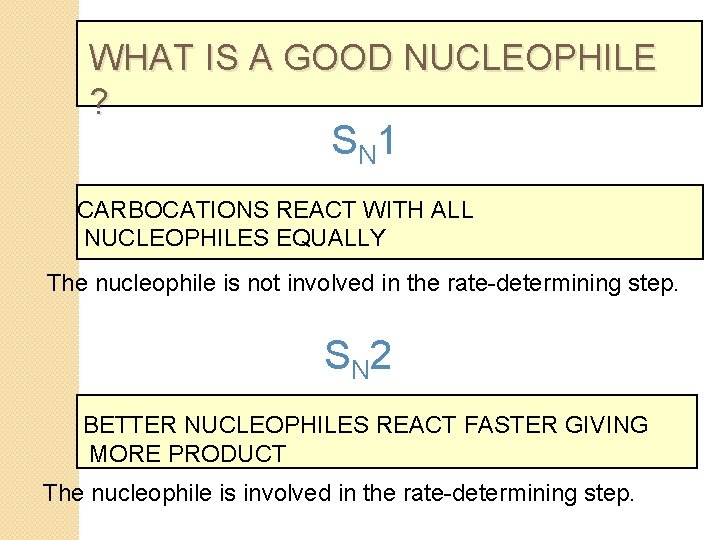
WHAT IS A GOOD NUCLEOPHILE ? S N 1 CARBOCATIONS REACT WITH ALL NUCLEOPHILES EQUALLY The nucleophile is not involved in the rate-determining step. S N 2 BETTER NUCLEOPHILES REACT FASTER GIVING MORE PRODUCT The nucleophile is involved in the rate-determining step.
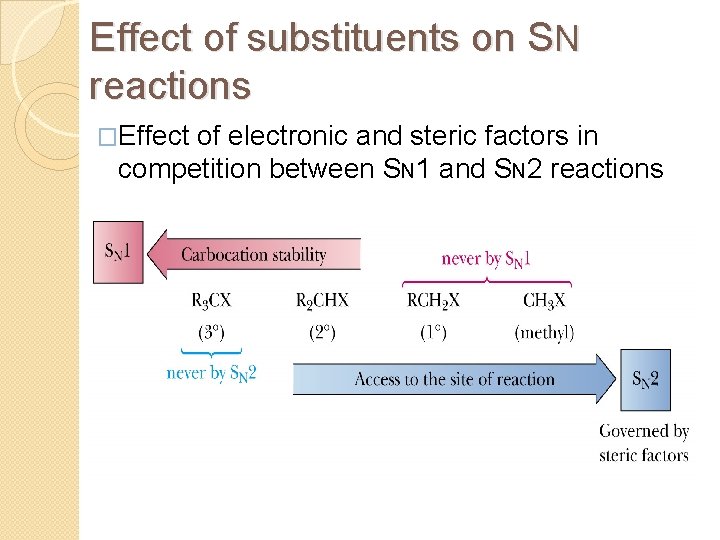
Effect of substituents on SN reactions �Effect of electronic and steric factors in competition between SN 1 and SN 2 reactions
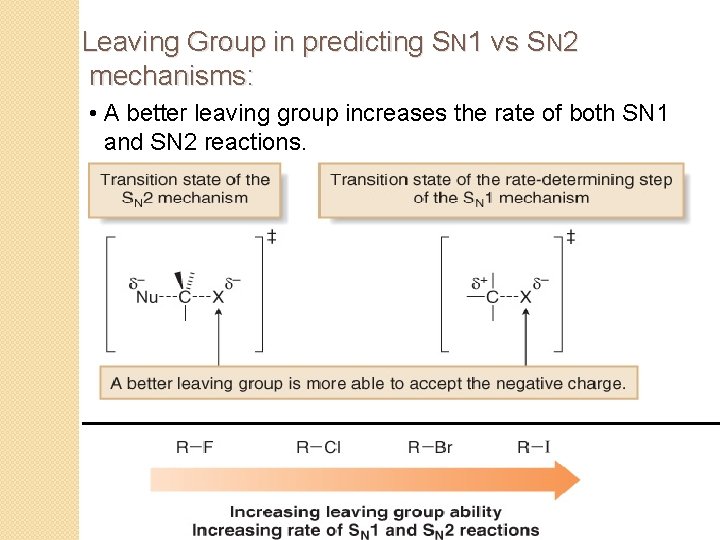
Leaving Group in predicting SN 1 vs SN 2 mechanisms: • A better leaving group increases the rate of both SN 1 and SN 2 reactions.
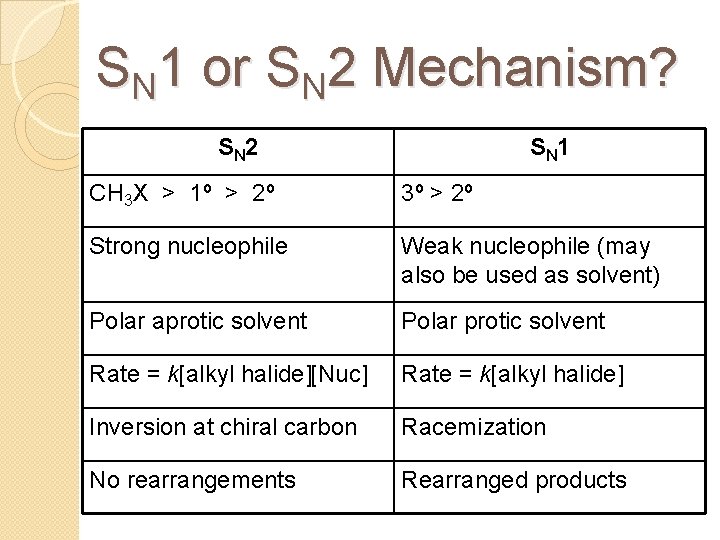
SN 1 or SN 2 Mechanism? S N 2 S N 1 CH 3 X > 1º > 2º 3º > 2º Strong nucleophile Weak nucleophile (may also be used as solvent) Polar aprotic solvent Polar protic solvent Rate = k[alkyl halide][Nuc] Rate = k[alkyl halide] Inversion at chiral carbon Racemization No rearrangements Rearranged products
HYDROLYSIS OF ESTER • The hydrolyses of ordinary esters are catalysed by a number of agents, such as acids, bases and enzymes. • The acid and base catalysis in aqueous solution is largely of the specific type, being brought about by the H₃O⁺ and OH⁻ ions. • Molecule for molecule the enzymes are very much mor effective catalysts than the H₃O⁺ and OH⁻ ions , as is shown by the compariso in Table.
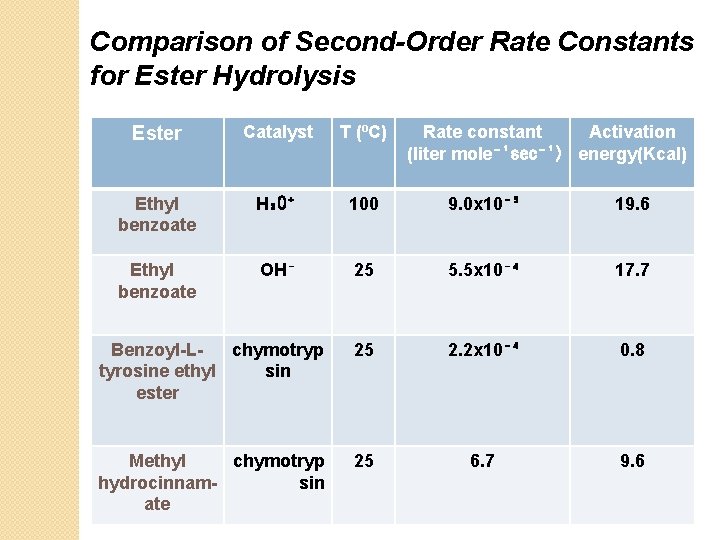
Comparison of Second-Order Rate Constants for Ester Hydrolysis Ester Catalyst T (⁰C) Rate constant Activation (liter mole⁻¹sec⁻¹) energy(Kcal) Ethyl benzoate H₃O⁺ 100 9. 0 x 10⁻⁵ 19. 6 Ethyl benzoate OH⁻ 25 5. 5 x 10⁻⁴ 17. 7 Benzoyl-Lchymotryp tyrosine ethyl sin ester 25 2. 2 x 10⁻⁴ 0. 8 Methyl chymotryp hydrocinnamsin ate 25 6. 7 9. 6
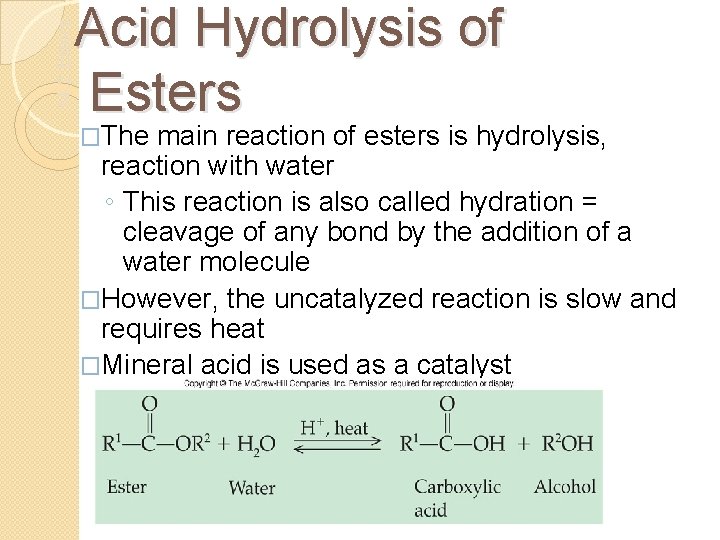
14. 2 Esters Acid Hydrolysis of Esters �The main reaction of esters is hydrolysis, reaction with water ◦ This reaction is also called hydration = cleavage of any bond by the addition of a water molecule �However, the uncatalyzed reaction is slow and requires heat �Mineral acid is used as a catalyst
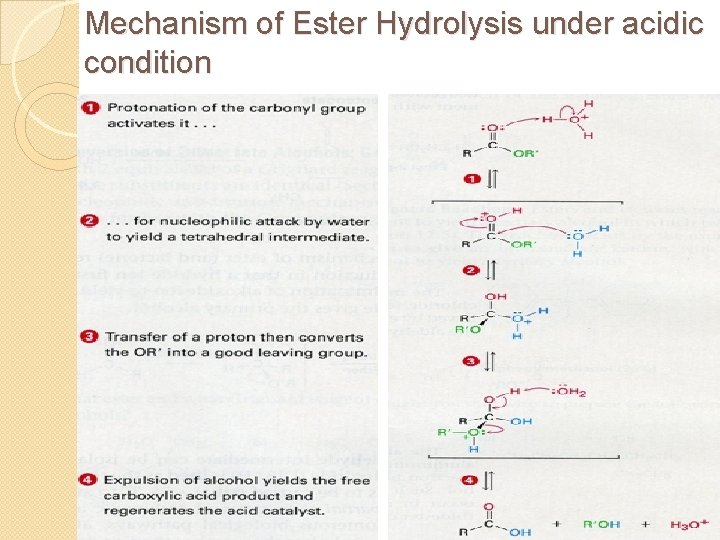
Mechanism of Ester Hydrolysis under acidic condition
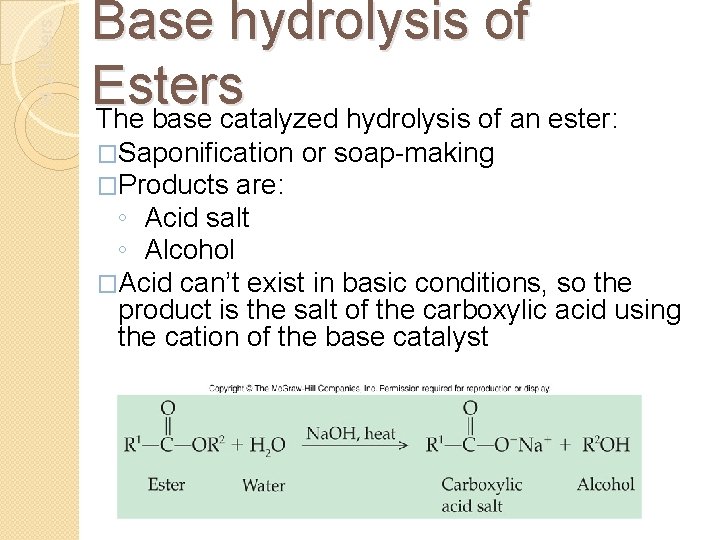
14. 2 Esters Base hydrolysis of Esters The base catalyzed hydrolysis of an ester: �Saponification �Products are: or soap-making ◦ Acid salt ◦ Alcohol �Acid can’t exist in basic conditions, so the product is the salt of the carboxylic acid using the cation of the base catalyst
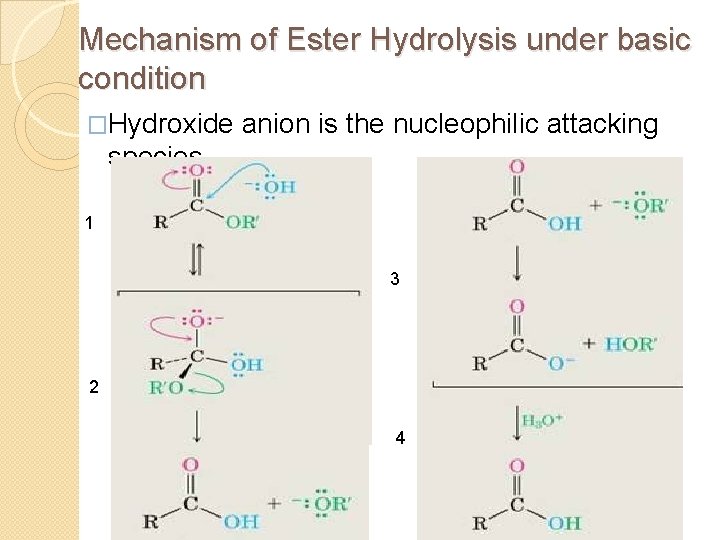
Mechanism of Ester Hydrolysis under basic condition �Hydroxide anion is the nucleophilic attacking species. 1 3 2 4
- Slides: 40