Organic Reactions Combustion hydrocarbons readily react with O

Organic Reactions

Combustion • hydrocarbons readily react with O 2

combustion complete combustion: incomplete combustion: • sufficient O 2 leads to only 2 products: CO 2 & H 2 O • insufficient O 2 leads to various products formed: C, CO 2 & H 2 O C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) C 3 H 8(g) + 2 O 2(g) 3 C(s) + 4 H 2 O(g)

Substitution • replace one or more H’s in hydrocarbon with something else
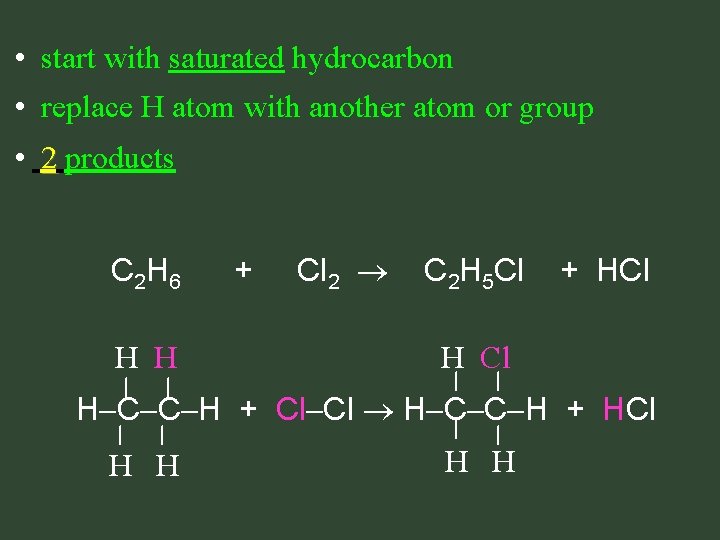
• start with saturated hydrocarbon • replace H atom with another atom or group • 2 products C 2 H 6 H H + Cl 2 C 2 H 5 Cl + HCl H C C H + Cl Cl H C C H + HCl H H

Addition • open up multiple bond(s) to add new atoms (ex: halogens) Subtraction • creating multiple bond(s) by losing atoms remove halogens from halocarbon) (ex:
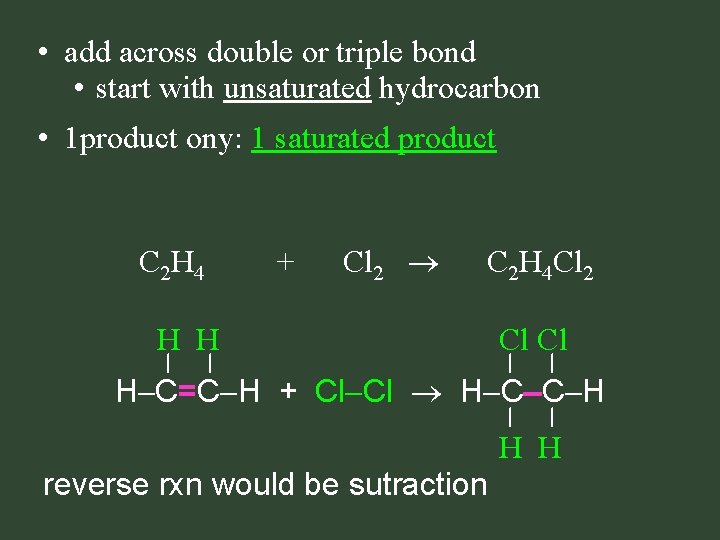
• add across double or triple bond • start with unsaturated hydrocarbon • 1 product ony: 1 saturated product C 2 H 4 + Cl 2 C 2 H 4 Cl 2 H H Cl Cl H C=C H + Cl Cl H C C H H H reverse rxn would be sutraction

Esterification • formation of ester: organic acid + alcohol ester + water
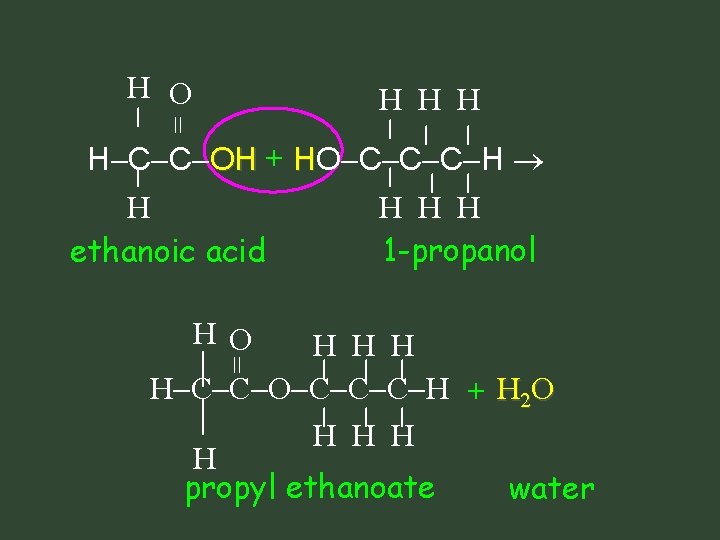
H O = H H C C OH + HO C C C H H ethanoic acid H O H H H H C C O C C C H + H 2 O H H propyl ethanoate water = H H H 1 -propanol

Saponification • make soap: fat + base glycerol + soap
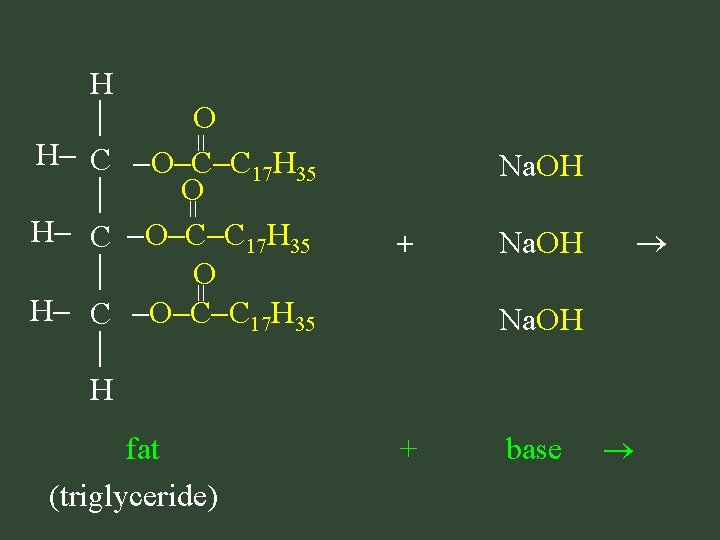
= H O H C O C C 17 H 35 H = Na. OH = + fat (triglyceride) Na. OH + base
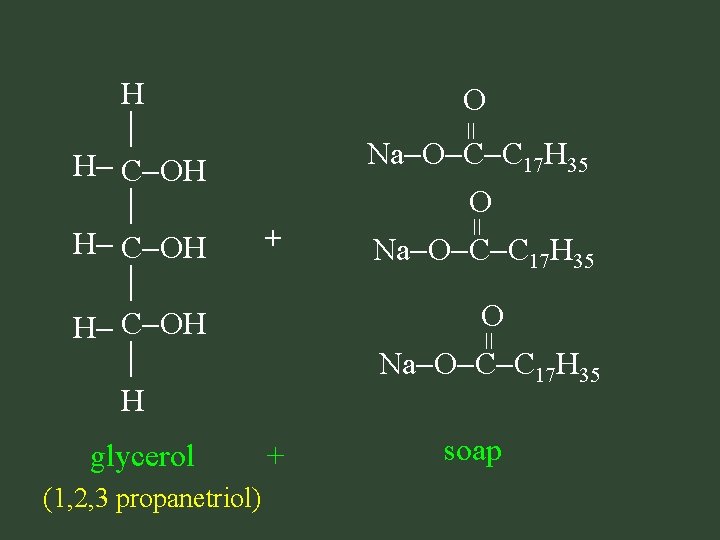
H H C OH H + glycerol + (1, 2, 3 propanetriol) = O = Na O C C 17 H 35 O Na O C C 17 H 35 = O Na O C C 17 H 35 soap

Fermentation • sugars broken down into alcohol + CO 2
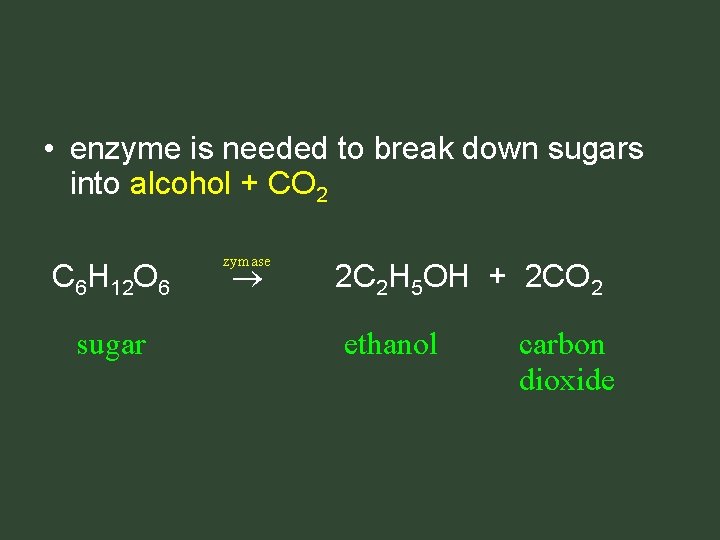
• enzyme is needed to break down sugars into alcohol + CO 2 C 6 H 12 O 6 sugar zymase 2 C 2 H 5 OH + 2 CO 2 ethanol carbon dioxide

What do protein in eggs, plastic in soda bottles, rubber in tires and teflon coating cookware have in common? • all are made up of giant molecules called polymers – poly means many – mers means parts
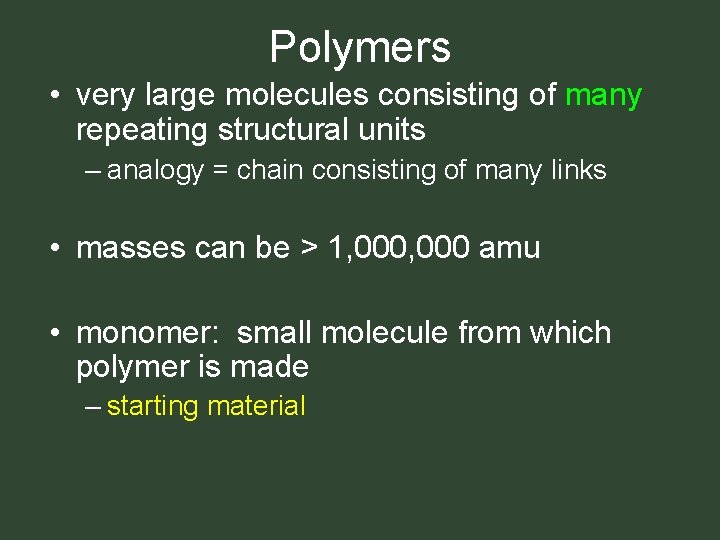
Polymers • very large molecules consisting of many repeating structural units – analogy = chain consisting of many links • masses can be > 1, 000 amu • monomer: small molecule from which polymer is made – starting material

Natural Polymers • wool

silk

rubber

starch found in root plants corn starch

proteins

nucleic acids Nucleic acids are polymers formed by attaching monomers called nucleotides • two nucleic acids: DNA & RNA • nucleotides fall into two classes: purines & pyrimidines – purines: A & G – pyrimidines: T & C • RNA has U instead of T

Synthetic Polymers • plastics – polyethylene, PVC • synthetic fibers – nylon, rayon, polyester • rubber substitutes – polyurethane

Polymerization Reactions: • chemical reactions that produce polymers • revolutionized our everyday lives two reaction types: addition & condensation

1. addition polymerization: polymerization - start with unsaturated monomers - all reactants used up 1 saturated product formed
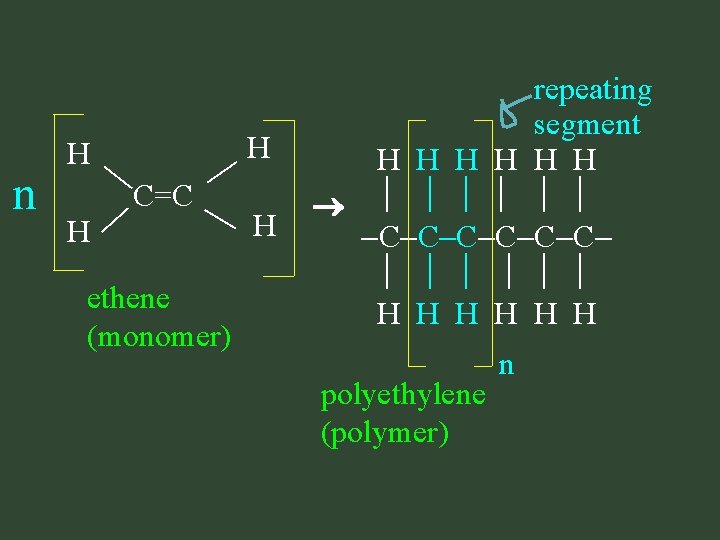
H C=C n H H ethene (monomer) H repeating segment H H H C C C H H H polyethylene (polymer) n

Polyethylene Varieties • • milk bottles detergent bottles oil bottles bottle caps • plastic grocery bags • shrink-wrap films • sandwich bags • toys

Polyethylene Products #1: PETE or PET (Polyethylene Terephthalate) • usually clear • used for: soda bottles, water bottles, beer bottles, salad dressing containers, mouthwash bottles, and peanut butter containers • known to allow bacteria to accumulate • recycled into: tote bags, furniture, carpet, paneling, fiber, and polar fleece
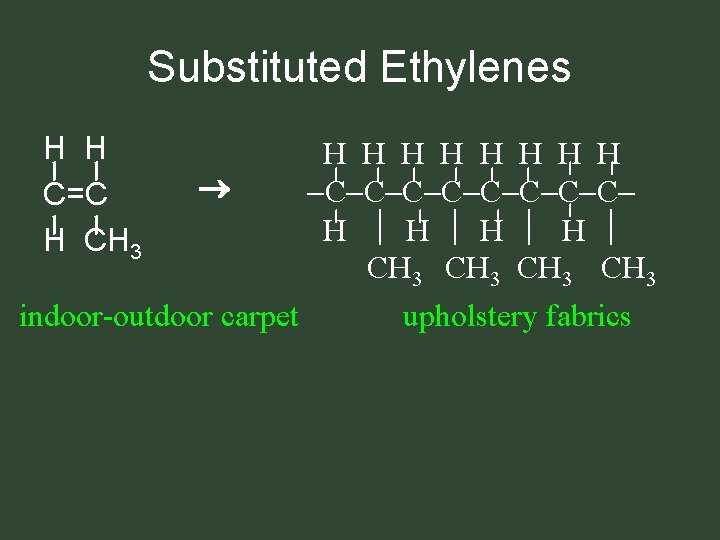
Substituted Ethylenes H H C=C H CH 3 indoor-outdoor carpet H H H H C C C C H H CH 3 upholstery fabrics
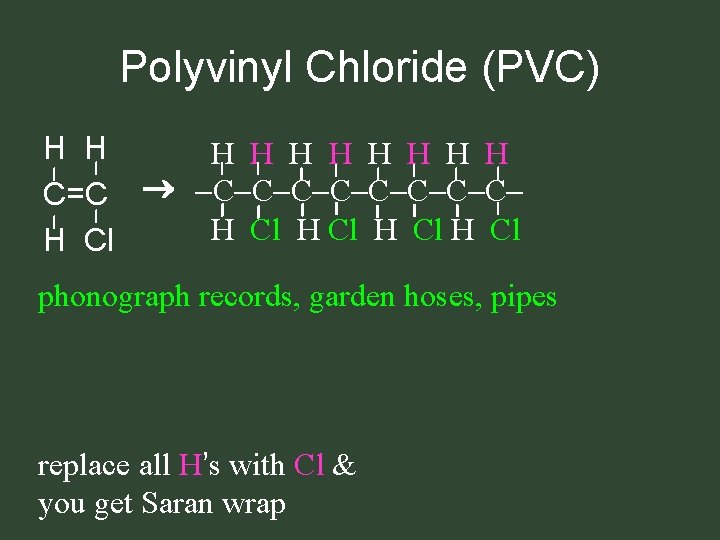
Polyvinyl Chloride (PVC) H H H H H C=C C C C C H Cl H Cl phonograph records, garden hoses, pipes replace all H’s with Cl & you get Saran wrap
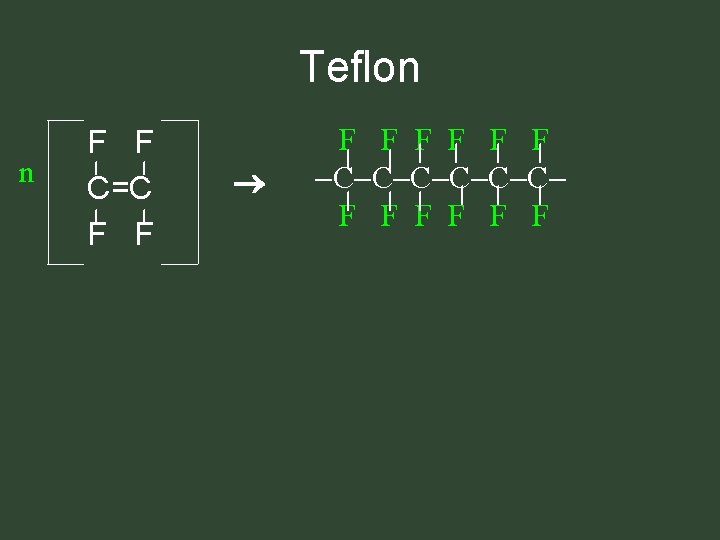
Teflon n F F C=C F F F F C C C F F F
2. Condensation Polymerization: Polymerization – combine monomers containing 2 func groups – monomer has functional group at each end – loss of a small by-product, usually H 2 O
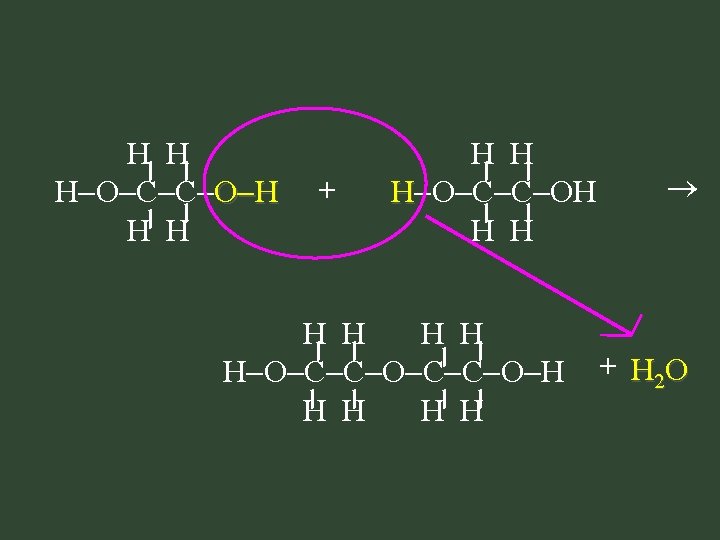
H H H O C C O H H H + H H H O C C OH H H H O C C O H H H + H 2 O
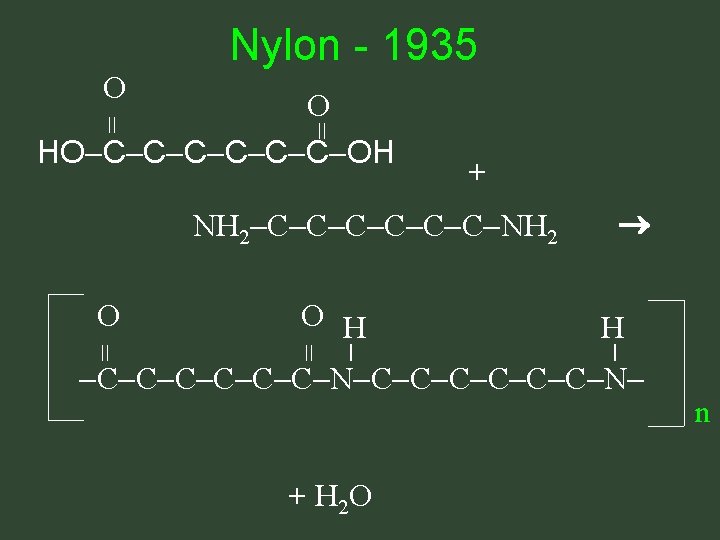
Nylon - 1935 O = + NH 2 C C C NH 2 O H = = O H = O HO C C C OH C C C N + H 2 O n

Congratulations !!!! You’re now finished with the required curriculum !
- Slides: 35