Organic reaction maps Task 1 Highlight all oxidations

- Slides: 15

Organic reaction maps

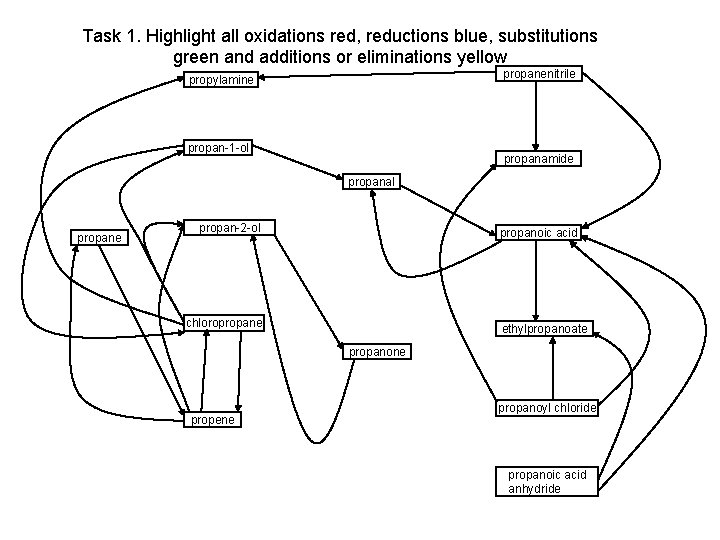
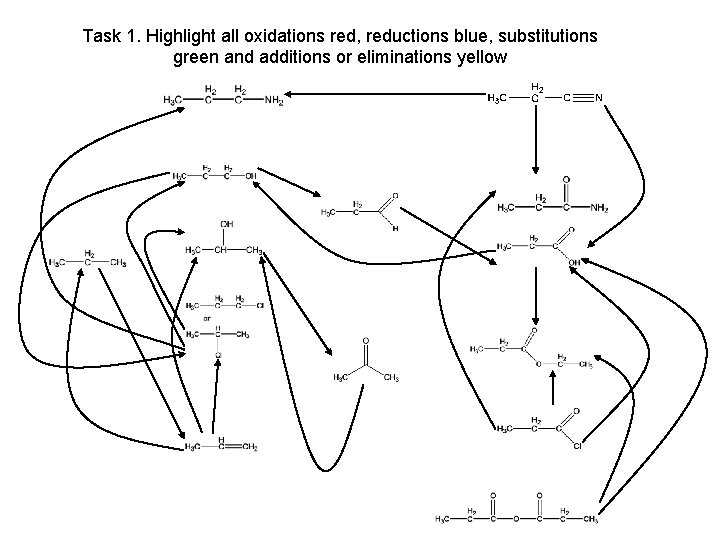
Task 1. Highlight all oxidations red, reductions blue, substitutions green and additions or eliminations yellow propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid chloropropane ethylpropanoate propanone propene propanoyl chloride propanoic acid anhydride

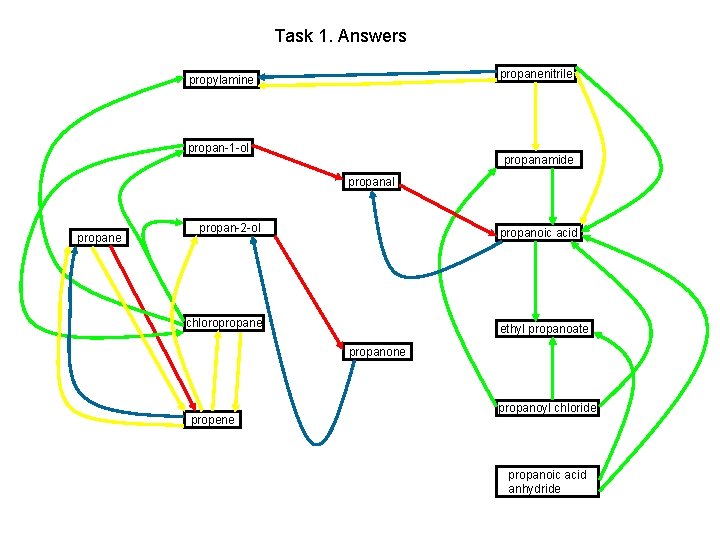
Task 1. Answers propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid chloropropane ethyl propanoate propanone propene propanoyl chloride propanoic acid anhydride

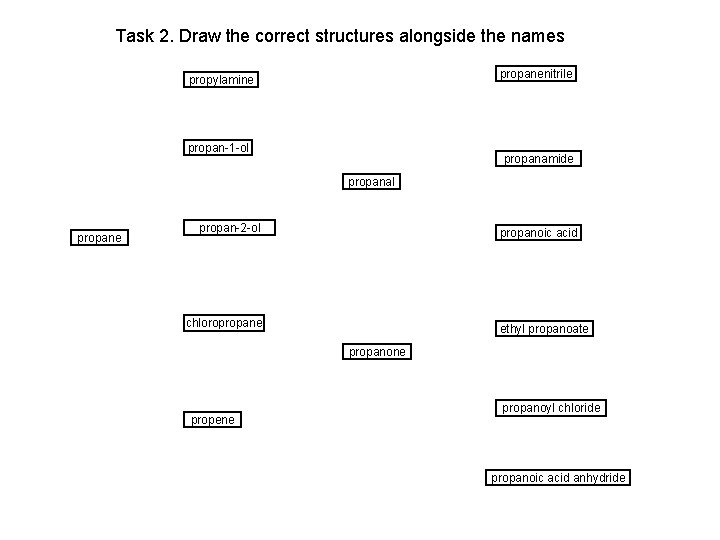
Task 2. Draw the correct structures alongside the names propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid chloropropane ethyl propanoate propanone propene propanoyl chloride propanoic acid anhydride

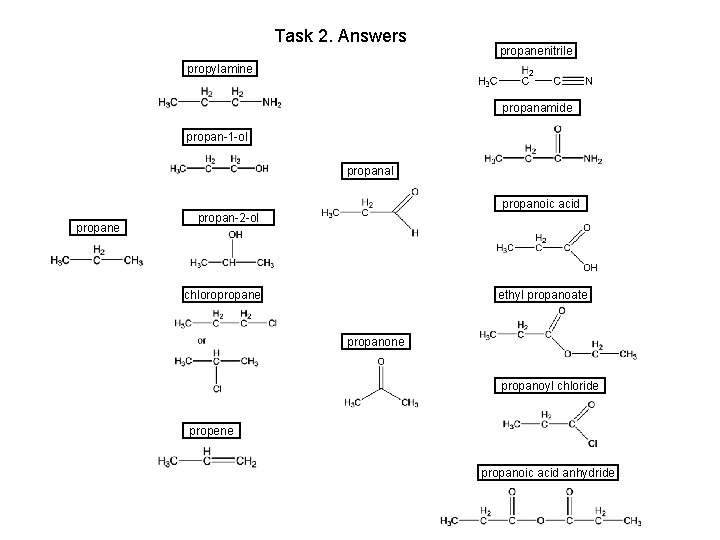
Task 2. Answers propanenitrile propylamine propanamide propan-1 -ol propanal propane propanoic acid propan-2 -ol chloropropane ethyl propanoate propanone propanoyl chloride propene propanoic acid anhydride

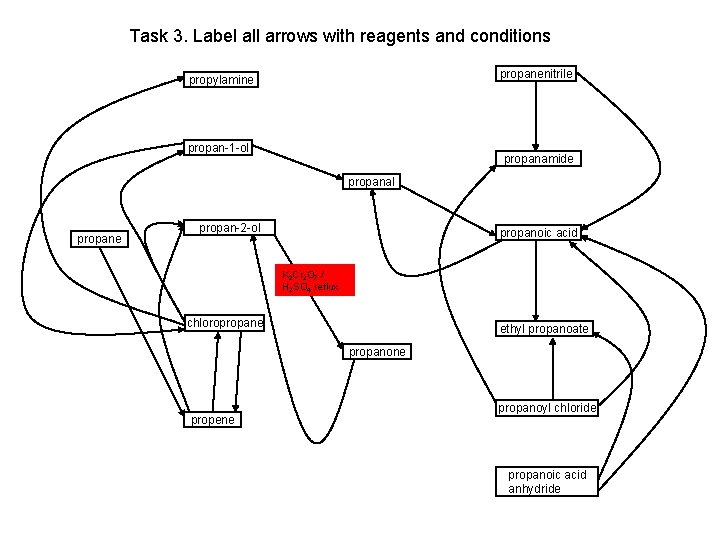
Task 3. Label all arrows with reagents and conditions propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid K 2 Cr 2 O 7 / H 2 SO 4 reflux chloropropane ethyl propanoate propanone propene propanoyl chloride propanoic acid anhydride

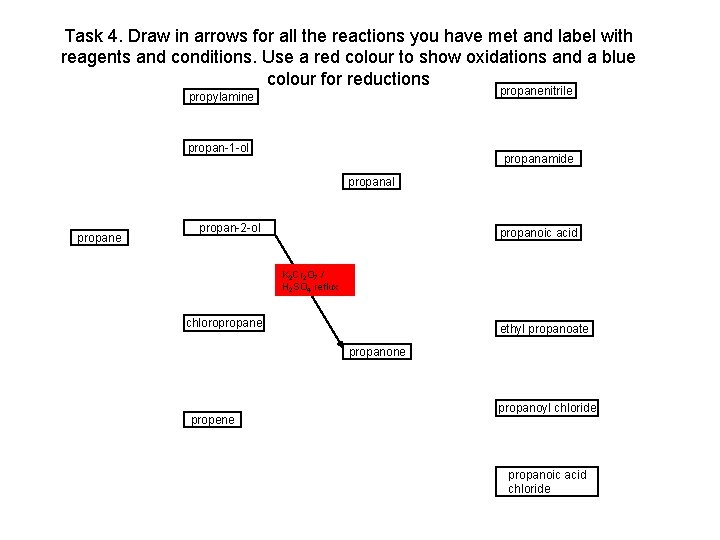
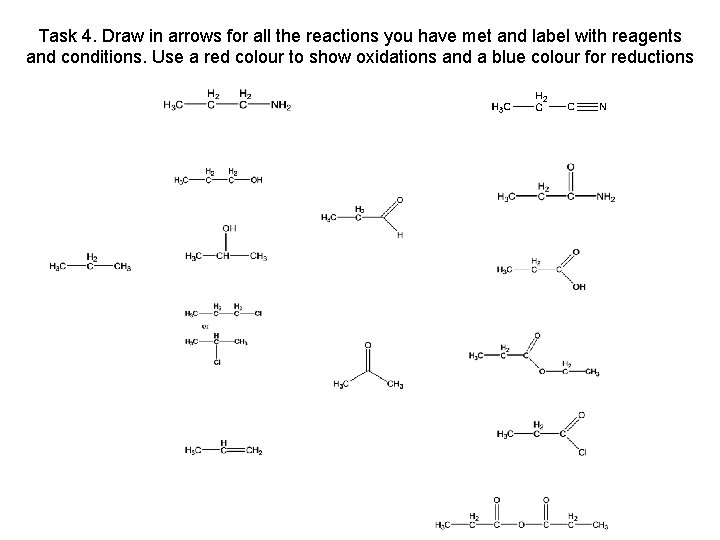
Task 4. Draw in arrows for all the reactions you have met and label with reagents and conditions. Use a red colour to show oxidations and a blue colour for reductions propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid K 2 Cr 2 O 7 / H 2 SO 4 reflux chloropropane ethyl propanoate propanone propene propanoyl chloride propanoic acid chloride

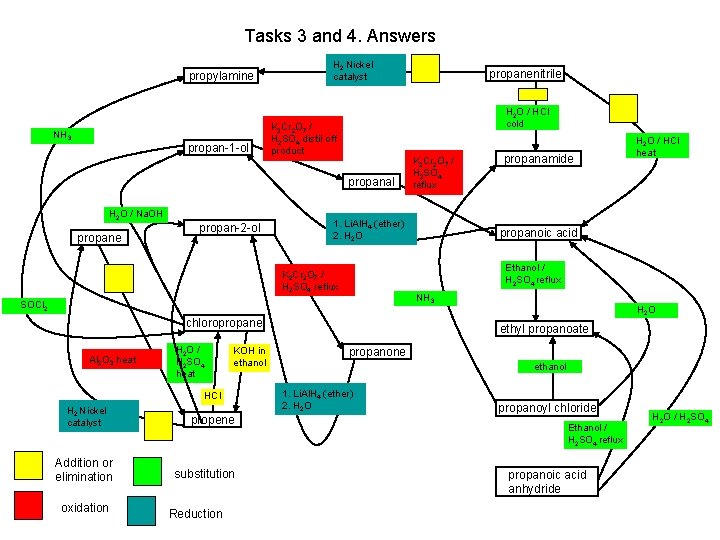
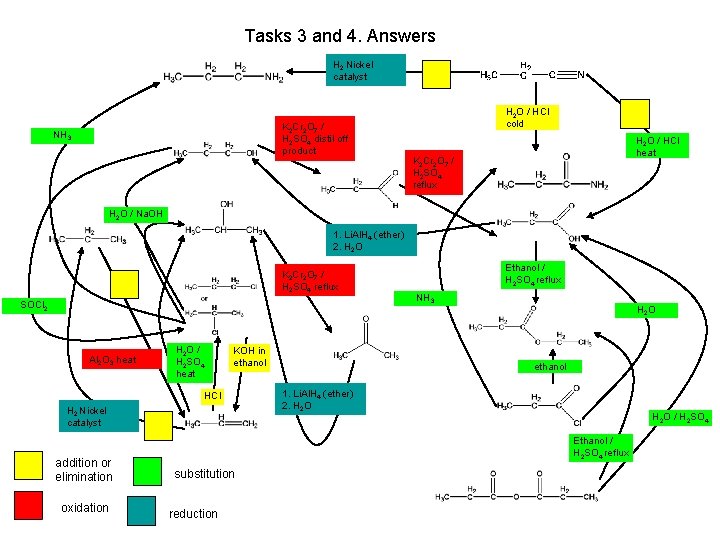
Tasks 3 and 4. Answers propylamine NH 3 propan-1 -ol H 2 Nickel catalyst H 2 O / HCl cold K 2 Cr 2 O 7 / H 2 SO 4 distil off product propanal H 2 O / Na. OH propane propan-2 -ol HCl H 2 Nickel catalyst Addition or elimination oxidation propene substitution Reduction propanoic acid NH 3 chloropropane KOH in ethanol propanamide H 2 O / HCl heat Ethanol / H 2 SO 4 reflux SOCl 2 H 2 O / H 2 SO 4 heat K 2 Cr 2 O 7 / H 2 SO 4 reflux 1. Li. Al. H 4 (ether) 2. H 2 O K 2 Cr 2 O 7 / H 2 SO 4 reflux Al 2 O 3 heat propanenitrile H 2 O ethyl propanoate propanone ethanol 1. Li. Al. H 4 (ether) 2. H 2 O propanoyl chloride Ethanol / H 2 SO 4 reflux propanoic acid anhydride H 2 O / H 2 SO 4

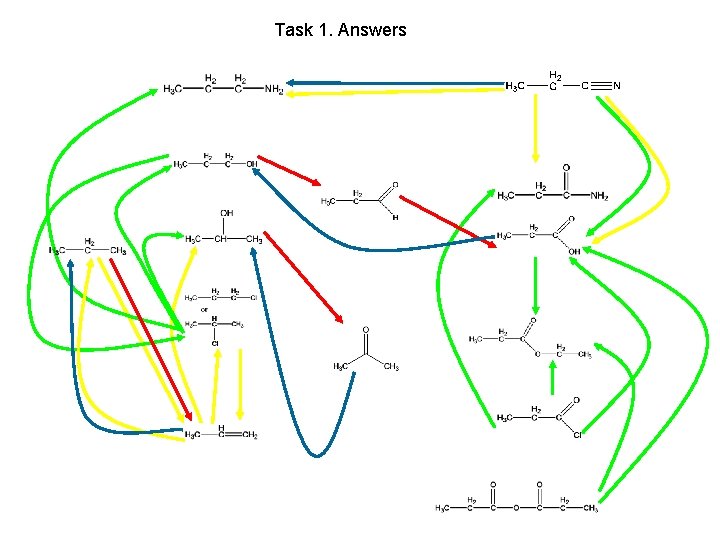
Task 1. Highlight all oxidations red, reductions blue, substitutions green and additions or eliminations yellow

Task 1. Answers

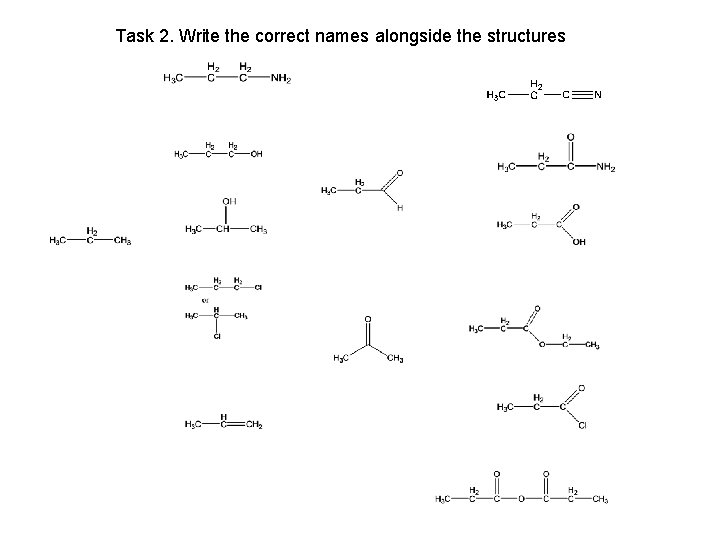
Task 2. Write the correct names alongside the structures

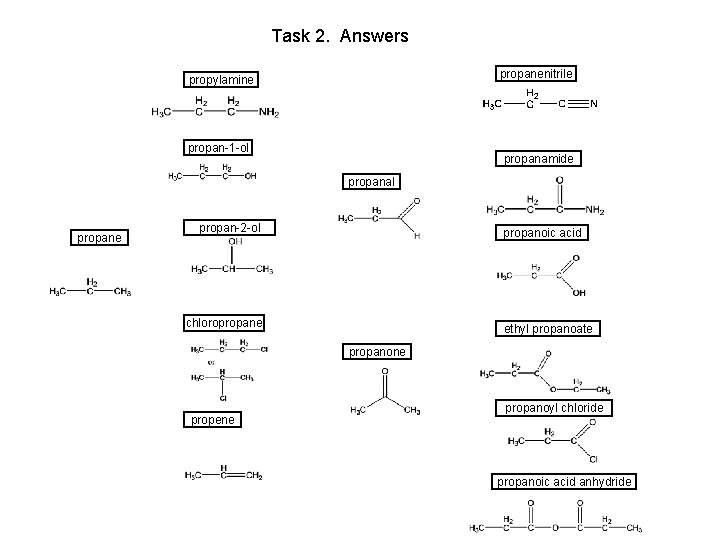
Task 2. Answers propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid chloropropane ethyl propanoate propanone propene propanoyl chloride propanoic acid anhydride

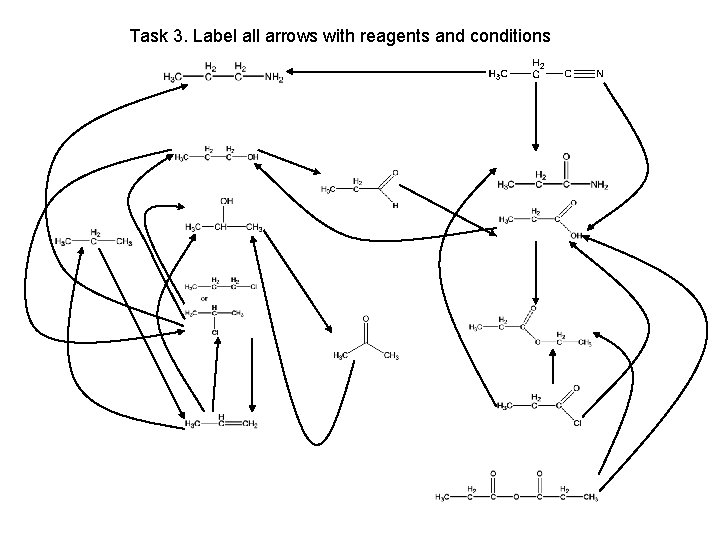
Task 3. Label all arrows with reagents and conditions

Task 4. Draw in arrows for all the reactions you have met and label with reagents and conditions. Use a red colour to show oxidations and a blue colour for reductions

Tasks 3 and 4. Answers H 2 Nickel catalyst K 2 Cr 2 O 7 / H 2 SO 4 distil off product NH 3 H 2 O / HCl cold H 2 O / HCl heat K 2 Cr 2 O 7 / H 2 SO 4 reflux H 2 O / Na. OH 1. Li. Al. H 4 (ether) 2. H 2 O K 2 Cr 2 O 7 / H 2 SO 4 reflux SOCl 2 Al 2 O 3 heat H 2 O / H 2 SO 4 heat KOH in ethanol HCl H 2 Nickel catalyst addition or elimination oxidation Ethanol / H 2 SO 4 reflux NH 3 H 2 O ethanol 1. Li. Al. H 4 (ether) 2. H 2 O / H 2 SO 4 Ethanol / H 2 SO 4 reflux substitution reduction