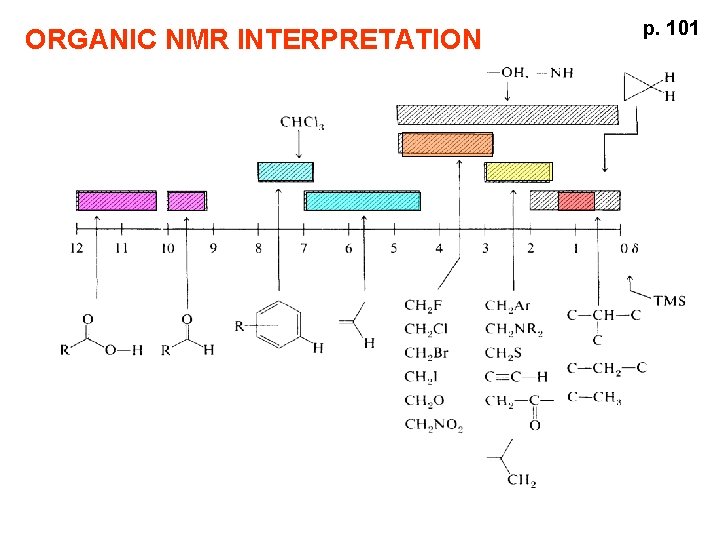
ORGANIC NMR INTERPRETATION p 101 ALKANES AND ALKYL

- Slides: 43

ORGANIC NMR INTERPRETATION p. 101

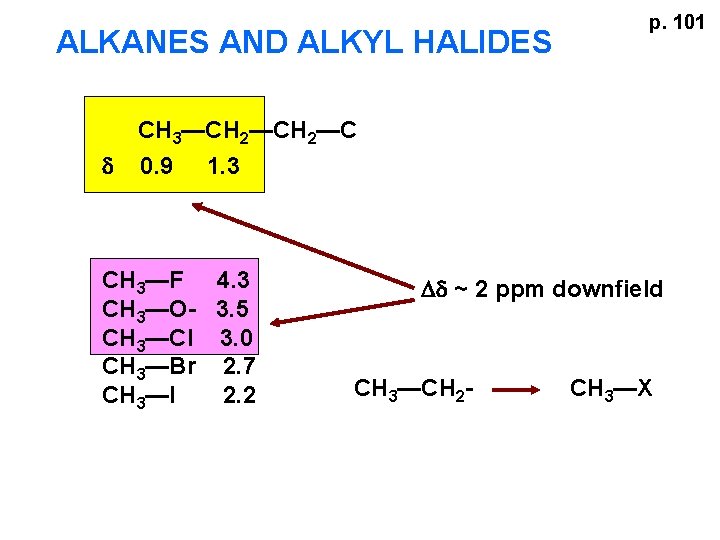
ALKANES AND ALKYL HALIDES d p. 101 CH 3—CH 2—C 0. 9 1. 3 CH 3—F 4. 3 CH 3—O- 3. 5 CH 3—Cl 3. 0 CH 3—Br 2. 7 CH 3—I 2. 2 Dd ~ 2 ppm downfield CH 3—CH 2 - CH 3—X

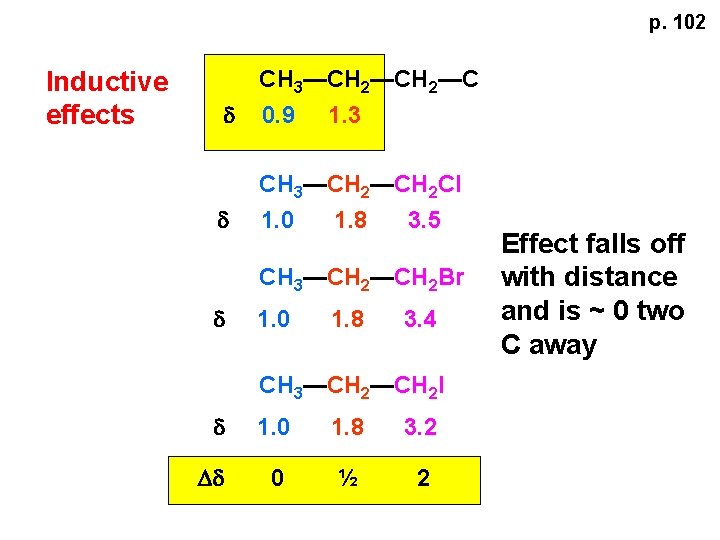
p. 102 Inductive effects d CH 3—CH 2—C 0. 9 1. 3 d CH 3—CH 2 Cl 1. 0 1. 8 3. 5 CH 3—CH 2 Br d 1. 0 1. 8 3. 4 CH 3—CH 2 I d 1. 0 1. 8 3. 2 Dd 0 ½ 2 Effect falls off with distance and is ~ 0 two C away

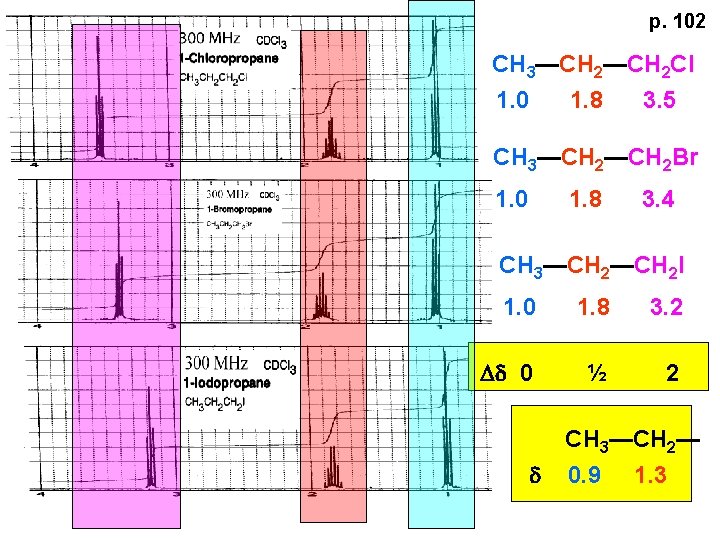
p. 102 CH 3—CH 2 Cl 1. 0 1. 8 3. 5 CH 3—CH 2 Br 1. 0 1. 8 3. 4 CH 3—CH 2 I 1. 0 1. 8 3. 2 Dd 0 ½ 2 d CH 3—CH 2— 0. 9 1. 3

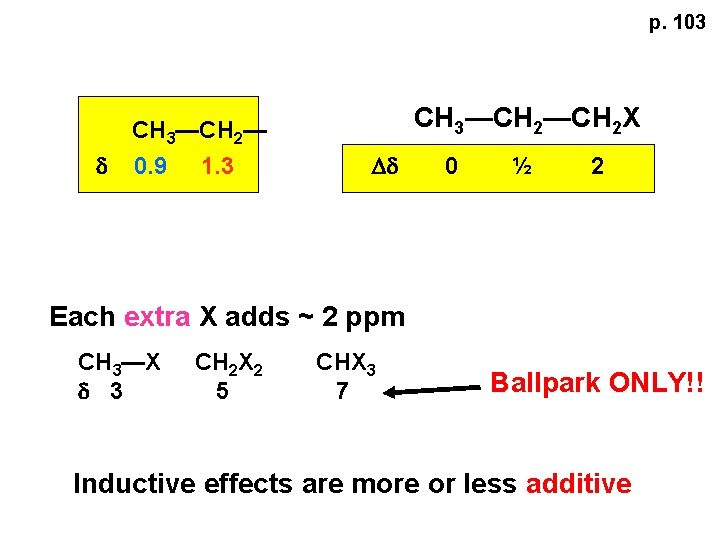
p. 103 d CH 3—CH 2— 0. 9 1. 3 CH 3—CH 2 X Dd 0 ½ 2 Each extra X adds ~ 2 ppm CH 3—X d 3 CH 2 X 2 5 CHX 3 7 Ballpark ONLY!! Inductive effects are more or less additive

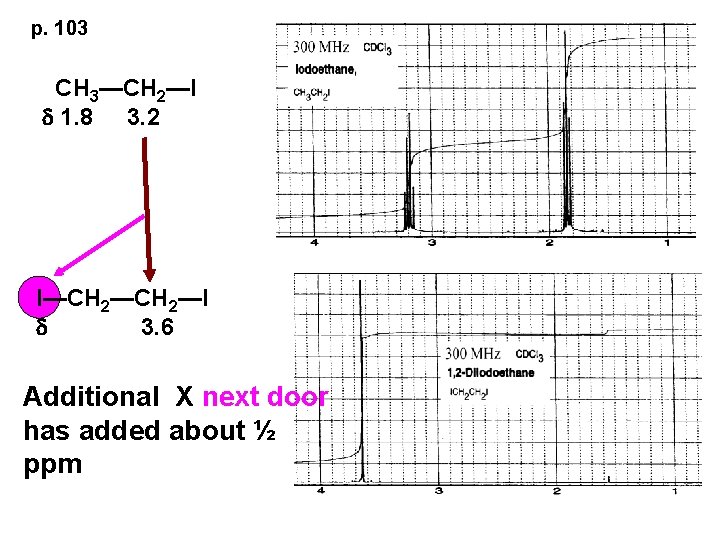
p. 103 CH 3—CH 2—I d 1. 8 3. 2 I—CH 2—I d 3. 6 Additional X next door has added about ½ ppm

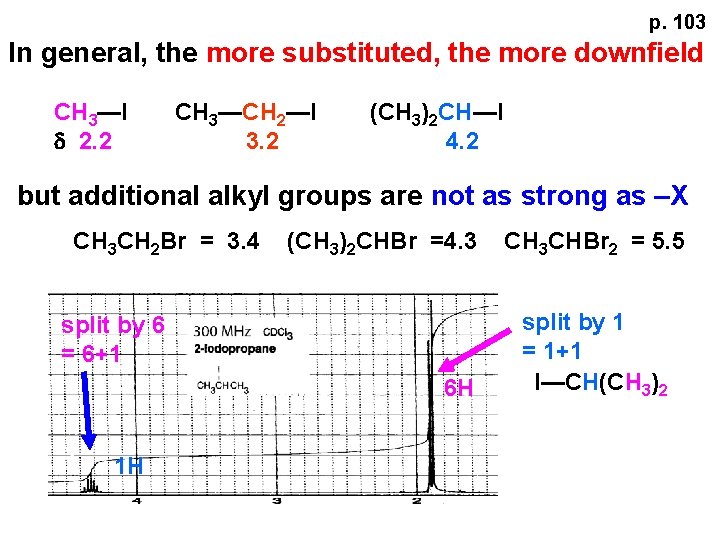
p. 103 In general, the more substituted, the more downfield CH 3—I d 2. 2 CH 3—CH 2—I 3. 2 (CH 3)2 CH—I 4. 2 but additional alkyl groups are not as strong as –X CH 3 CH 2 Br = 3. 4 (CH 3)2 CHBr =4. 3 split by 6 = 6+1 6 H 1 H CH 3 CHBr 2 = 5. 5 split by 1 = 1+1 I—CH(CH 3)2

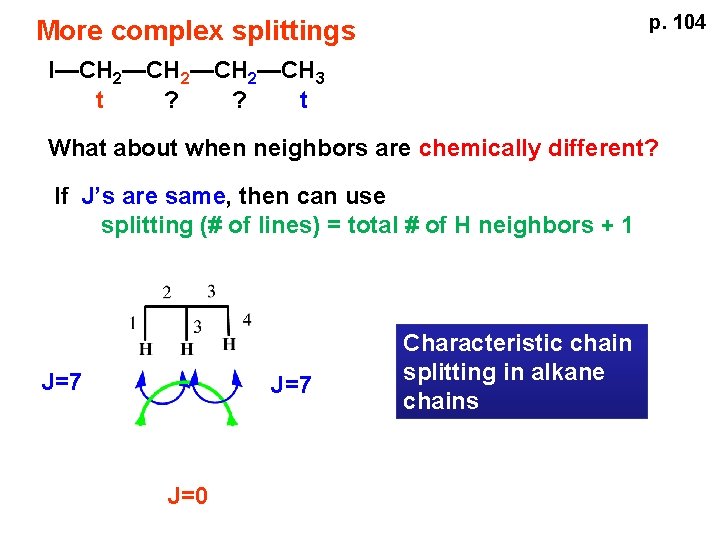
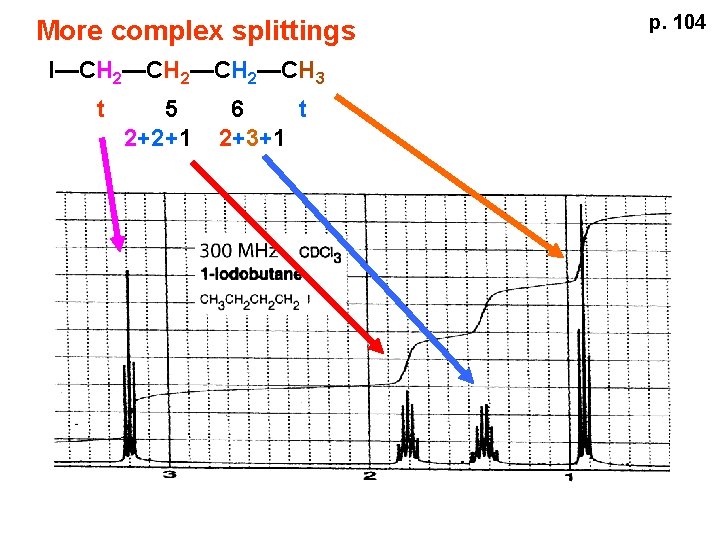
p. 104 More complex splittings I—CH 2—CH 3 t ? ? t What about when neighbors are chemically different? If J’s are same, then can use splitting (# of lines) = total # of H neighbors + 1 J=7 J=0 Characteristic chain splitting in alkane chains

More complex splittings I—CH 2—CH 3 t 5 2+2+1 6 t 2+3+1 p. 104

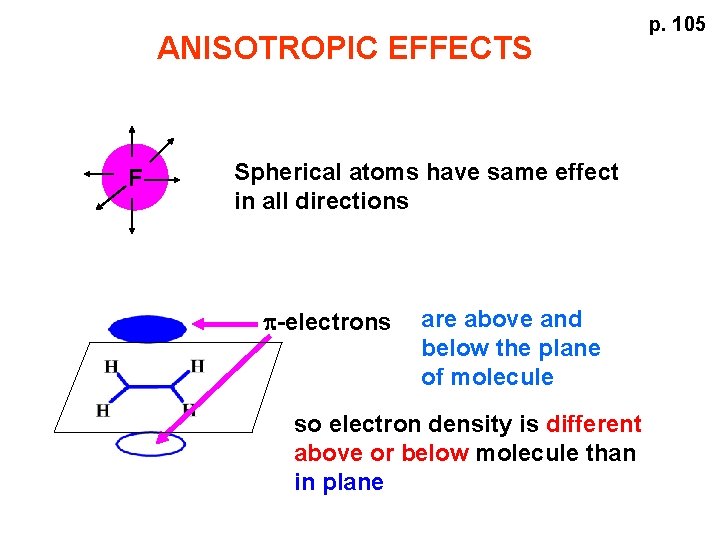
ANISOTROPIC EFFECTS F Spherical atoms have same effect in all directions p-electrons are above and below the plane of molecule so electron density is different above or below molecule than in plane p. 105

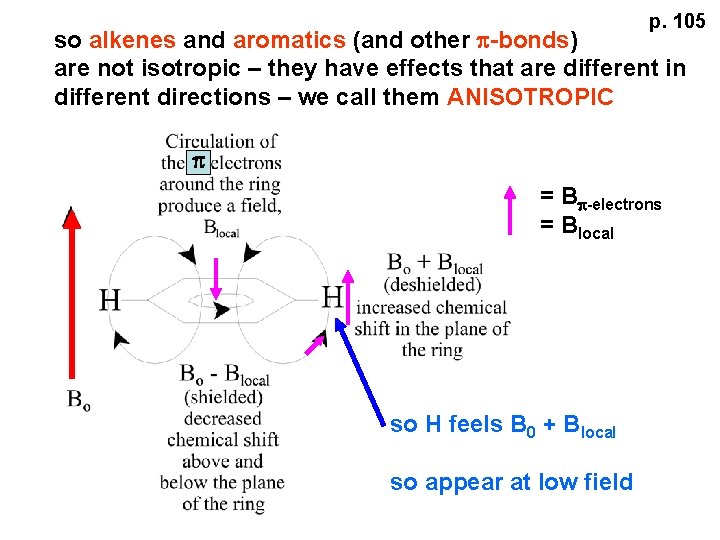
p. 105 so alkenes and aromatics (and other p-bonds) are not isotropic – they have effects that are different in different directions – we call them ANISOTROPIC p = Bp-electrons = Blocal so H feels B 0 + Blocal so appear at low field

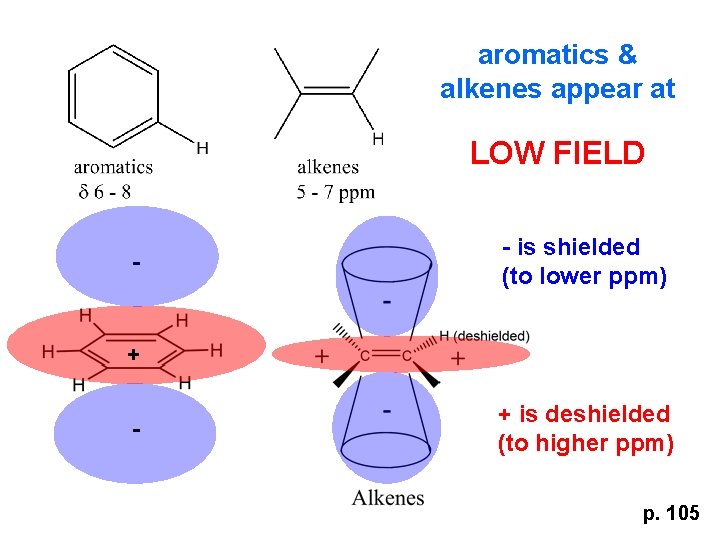
aromatics & alkenes appear at LOW FIELD - - is shielded (to lower ppm) + - + is deshielded (to higher ppm) p. 105

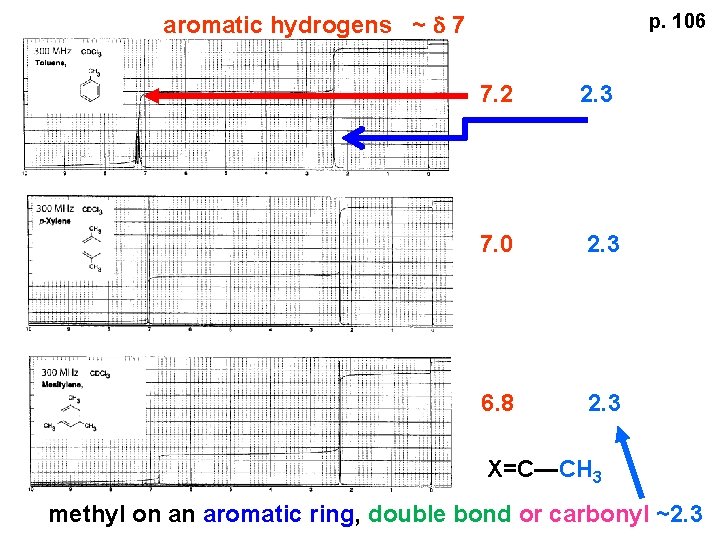
aromatic hydrogens ~ d 7 p. 106 7. 2 2. 3 7. 0 2. 3 6. 8 2. 3 X=C—CH 3 methyl on an aromatic ring, double bond or carbonyl ~2. 3

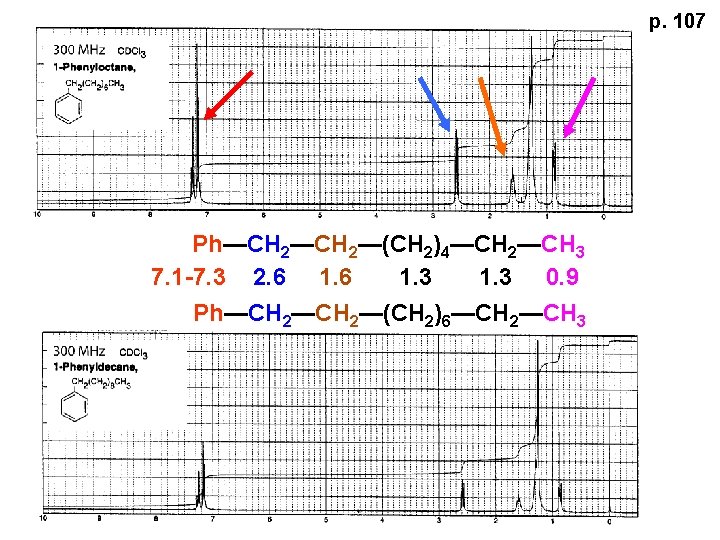
p. 107 Ph—CH 2—(CH 2)4—CH 2—CH 3 7. 1 -7. 3 2. 6 1. 3 0. 9 Ph—CH 2—(CH 2)6—CH 2—CH 3

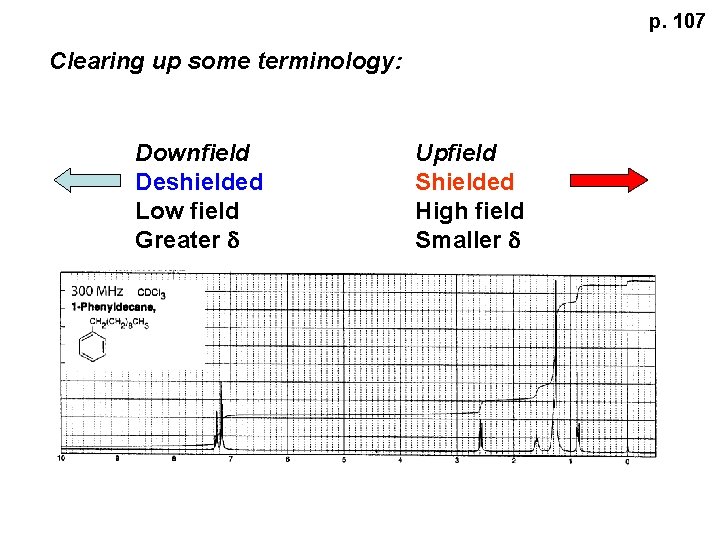
p. 107 Clearing up some terminology: Downfield Deshielded Low field Greater d Upfield Shielded High field Smaller d

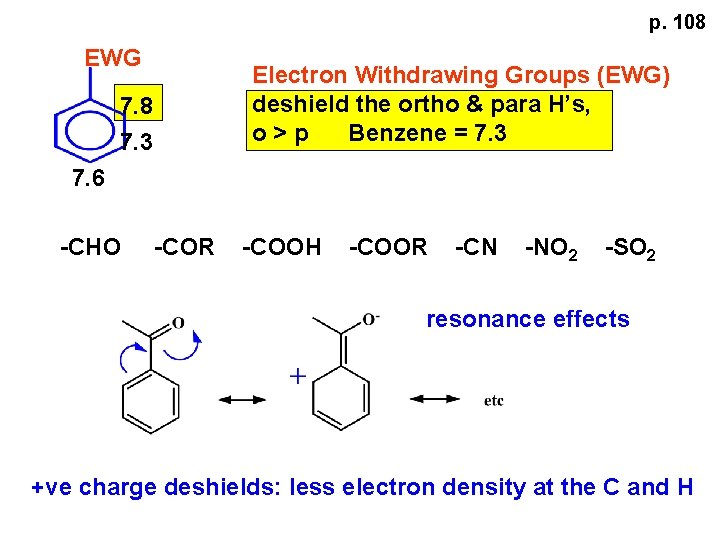
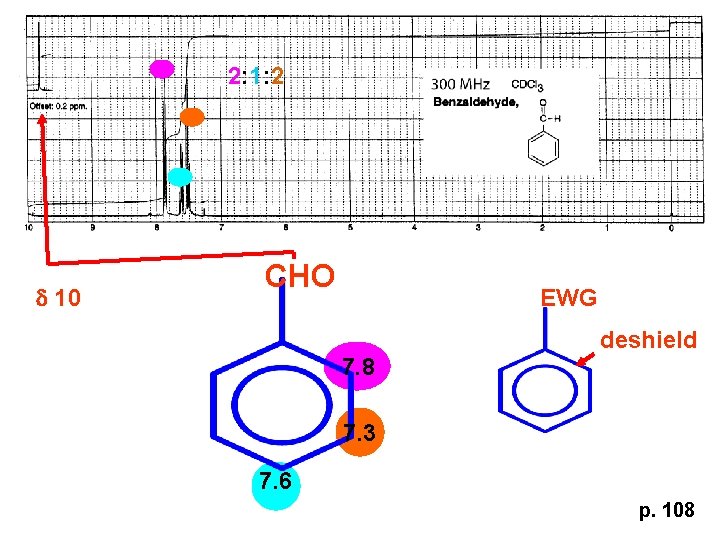
p. 108 EWG Electron Withdrawing Groups (EWG) deshield the ortho & para H’s, o>p Benzene = 7. 3 7. 8 7. 3 7. 6 -CHO -COR -COOH -COOR -CN -NO 2 -SO 2 resonance effects +ve charge deshields: less electron density at the C and H

2: 1: 2 d 10 CHO EWG deshield 7. 8 7. 3 7. 6 p. 108

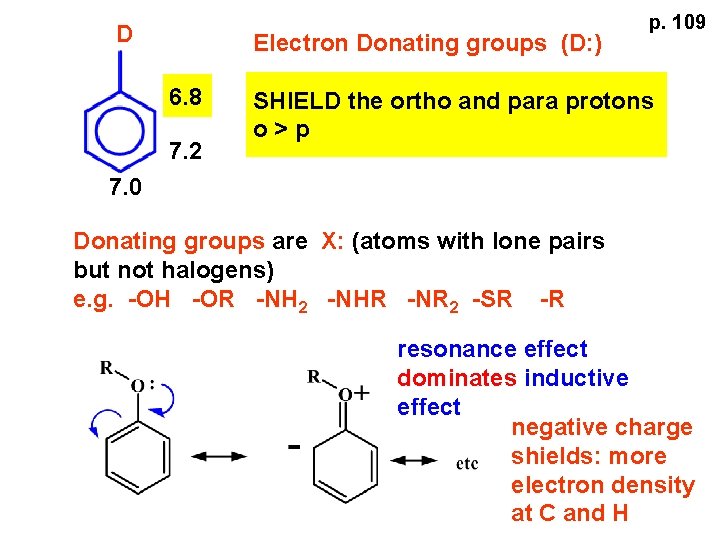
D Electron Donating groups (D: ) 6. 8 7. 2 p. 109 SHIELD the ortho and para protons o>p 7. 0 Donating groups are X: (atoms with lone pairs but not halogens) e. g. -OH -OR -NH 2 -NHR -NR 2 -SR -R resonance effect dominates inductive effect negative charge shields: more electron density at C and H

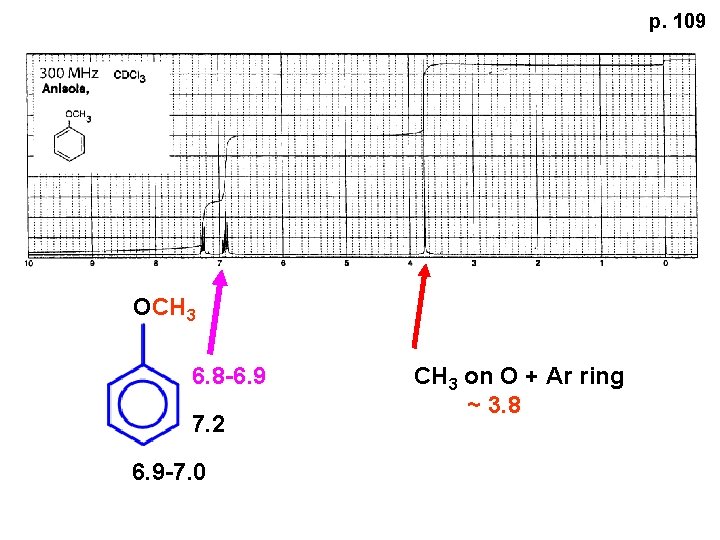
p. 109 OCH 3 6. 8 -6. 9 7. 2 6. 9 -7. 0 CH 3 on O + Ar ring ~ 3. 8

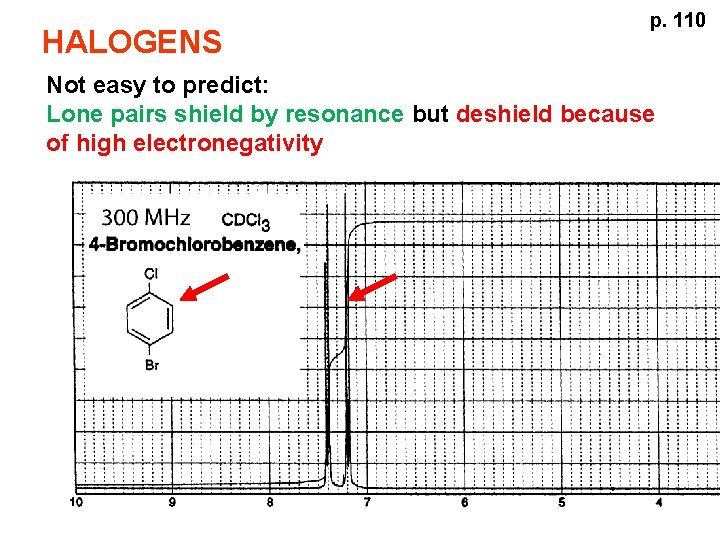
HALOGENS p. 110 Not easy to predict: Lone pairs shield by resonance but deshield because of high electronegativity

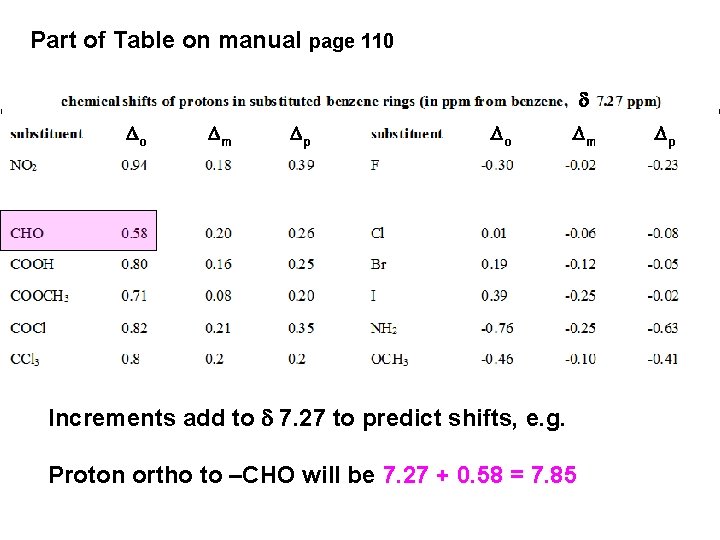
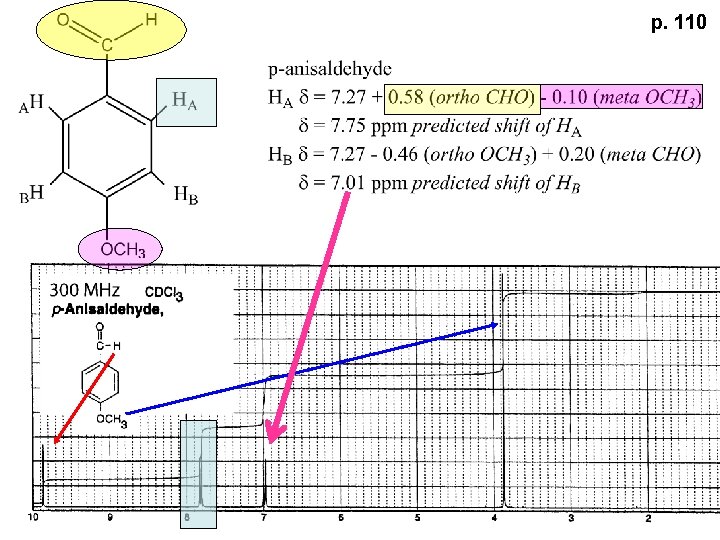
Part of Table on manual page 110 Increments add to d 7. 27 to predict shifts, e. g. Proton ortho to –CHO will be 7. 27 + 0. 58 = 7. 85

p. 110

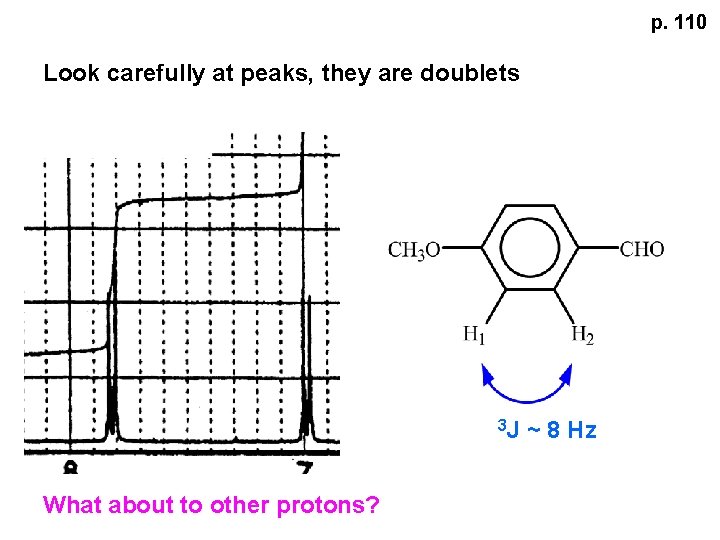
p. 110 Look carefully at peaks, they are doublets 3 J What about to other protons? ~ 8 Hz

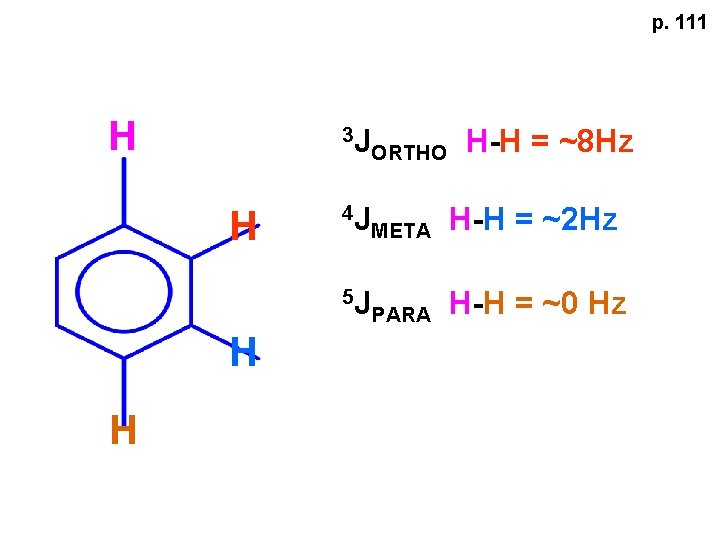
p. 111 H H 3 J ORTHO H-H = ~8 Hz 4 J META H-H = ~2 Hz 5 J PARA H-H = ~0 Hz

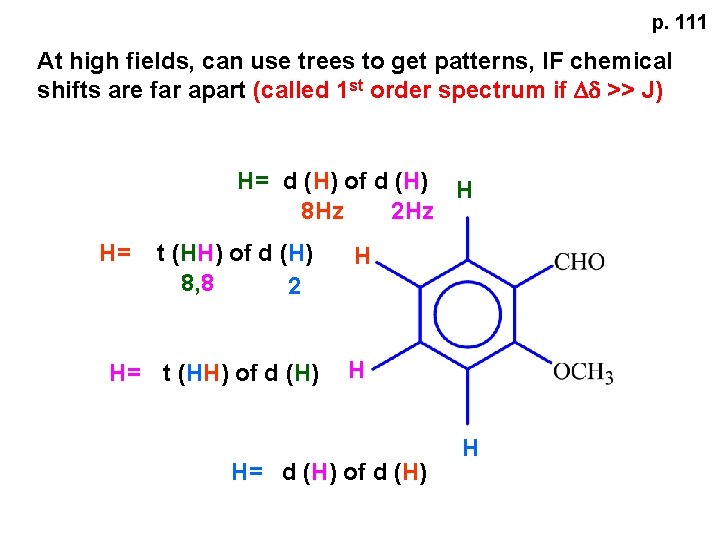
p. 111 At high fields, can use trees to get patterns, IF chemical shifts are far apart (called 1 st order spectrum if Dd >> J) H= d (H) of d (H) H 8 Hz 2 Hz H= t (HH) of d (H) 8, 8 2 H H= t (HH) of d (H) H H= d (H) of d (H) H

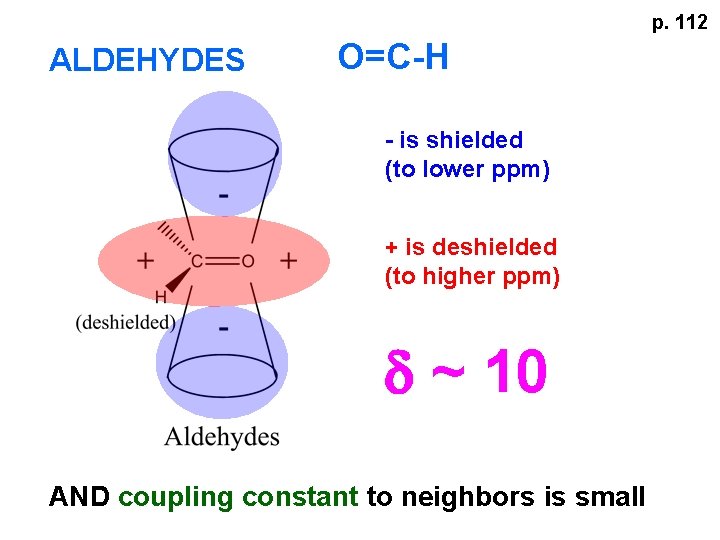
p. 112 ALDEHYDES O=C-H - is shielded (to lower ppm) + is deshielded (to higher ppm) d ~ 10 AND coupling constant to neighbors is small

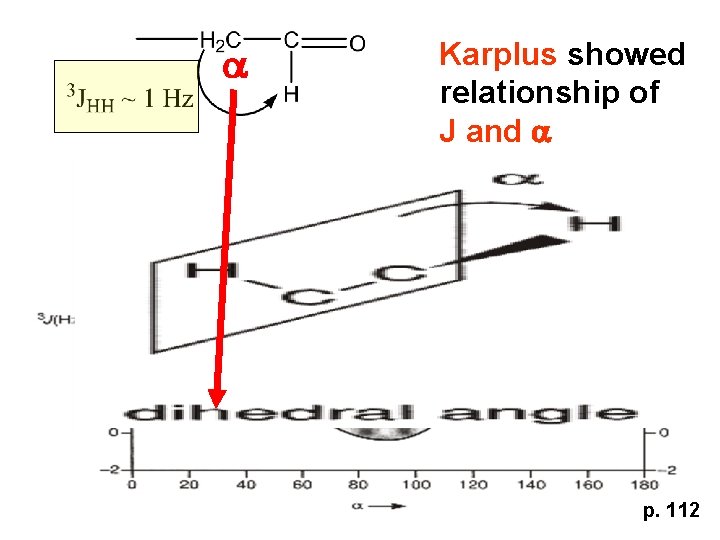
a Karplus showed relationship of J and a p. 112

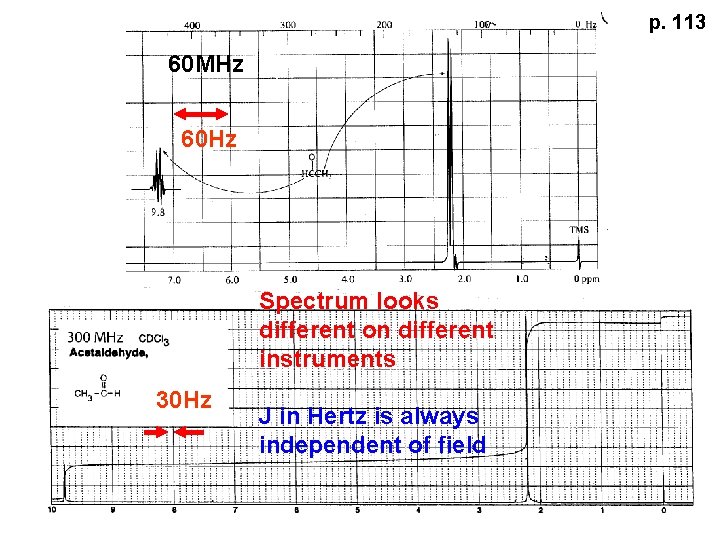
p. 113 60 MHz 60 Hz Spectrum looks different on different instruments 30 Hz J in Hertz is always independent of field

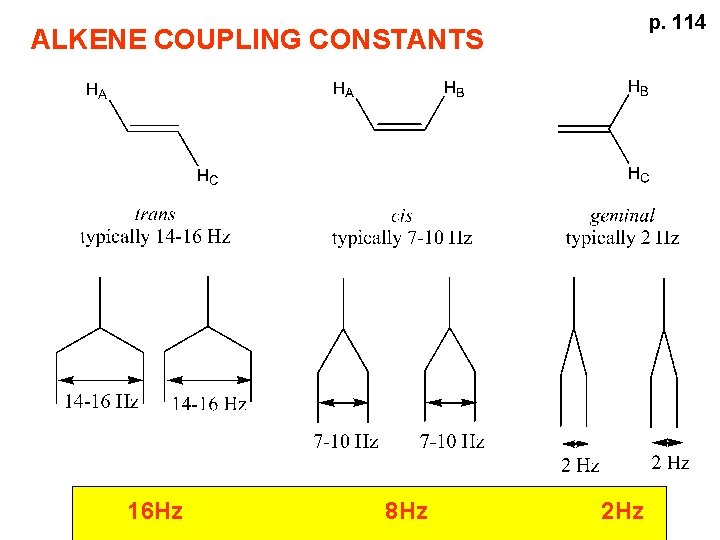
p. 114 ALKENE COUPLING CONSTANTS 16 Hz 8 Hz 2 Hz

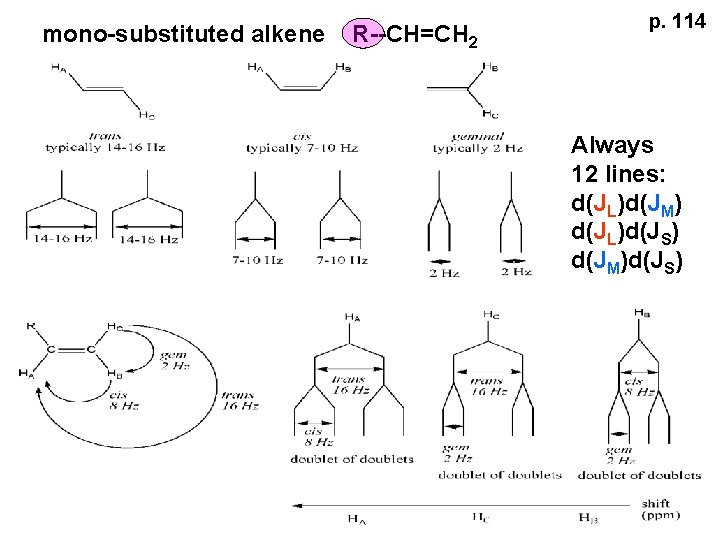
mono-substituted alkene R--CH=CH 2 p. 114 Always 12 lines: d(JL)d(JM) d(JL)d(JS) d(JM)d(JS)

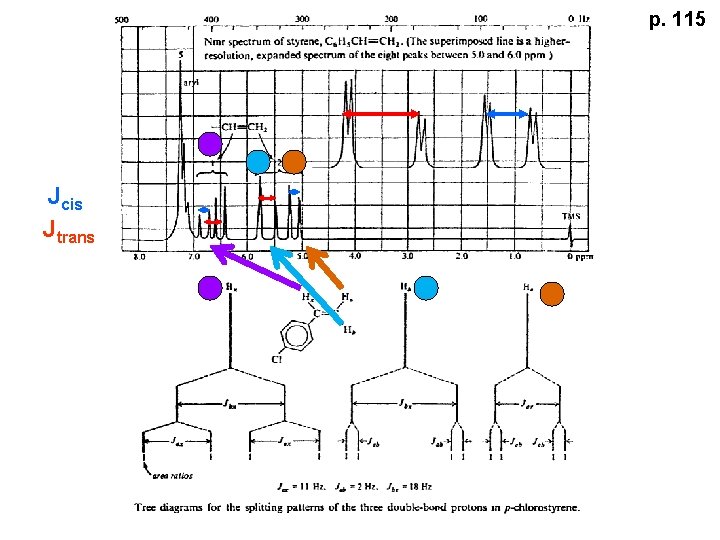
p. 115 Jcis Jtrans

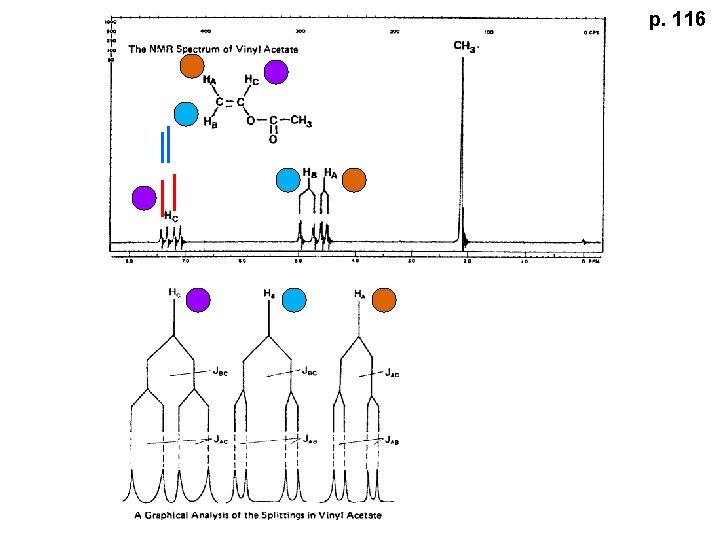
p. 116

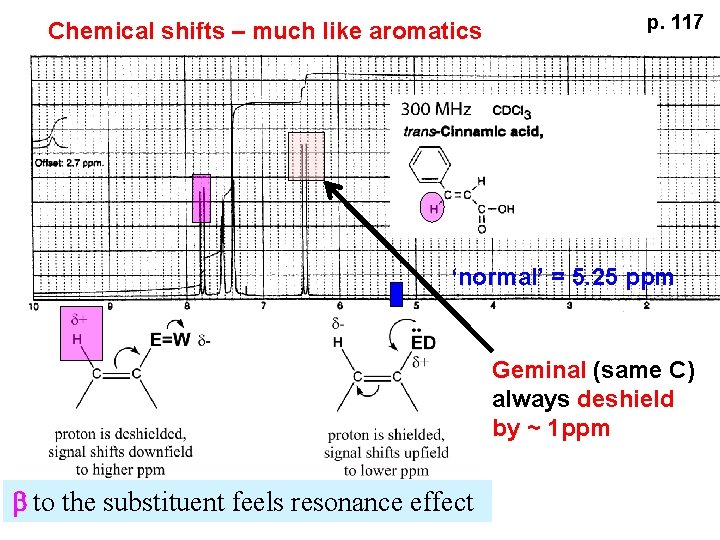
Chemical shifts – much like aromatics p. 117 ‘normal’ = 5. 25 ppm Geminal (same C) always deshield by ~ 1 ppm b to the substituent feels resonance effect

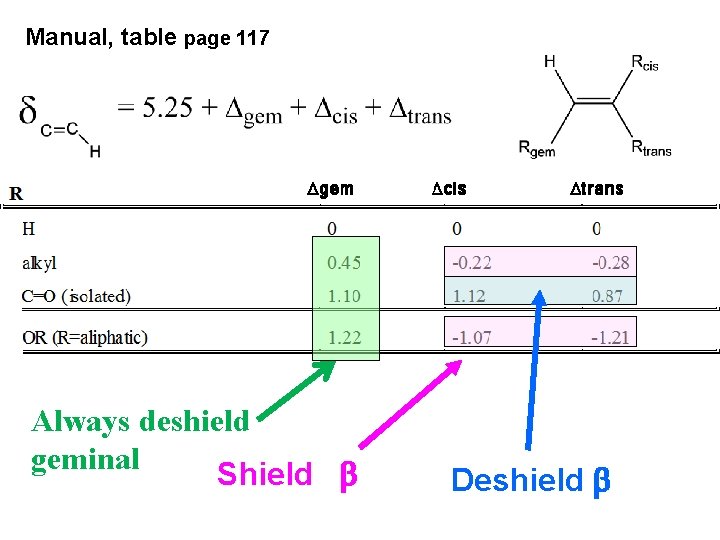
Manual, table page 117 Always deshield geminal Shield b Deshield b

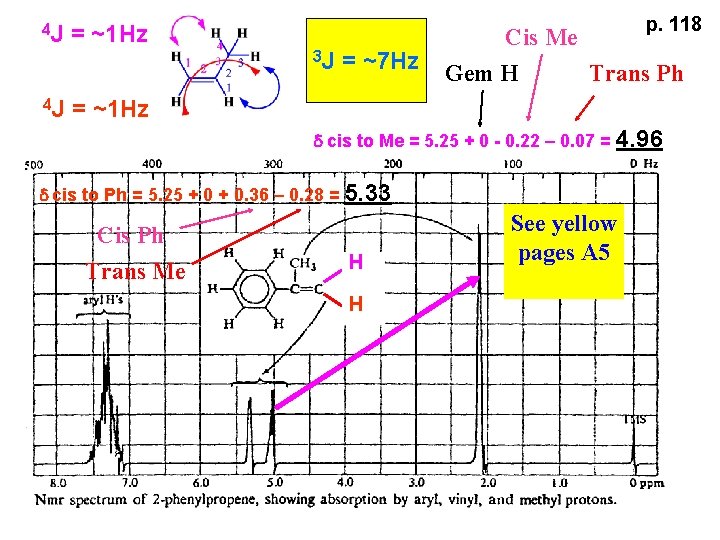
4 J p. 118 = ~1 Hz 3 J 4 J = ~7 Hz Cis Me Gem H Trans Ph = ~1 Hz d cis to Me = 5. 25 + 0 - 0. 22 – 0. 07 = 4. 96 d cis to Ph = 5. 25 + 0. 36 – 0. 28 = 5. 33 Cis Ph Trans Me H H See yellow pages A 5

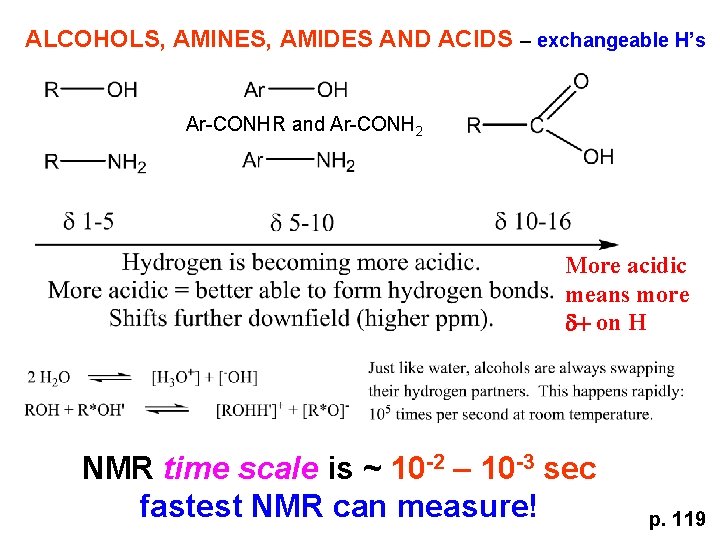
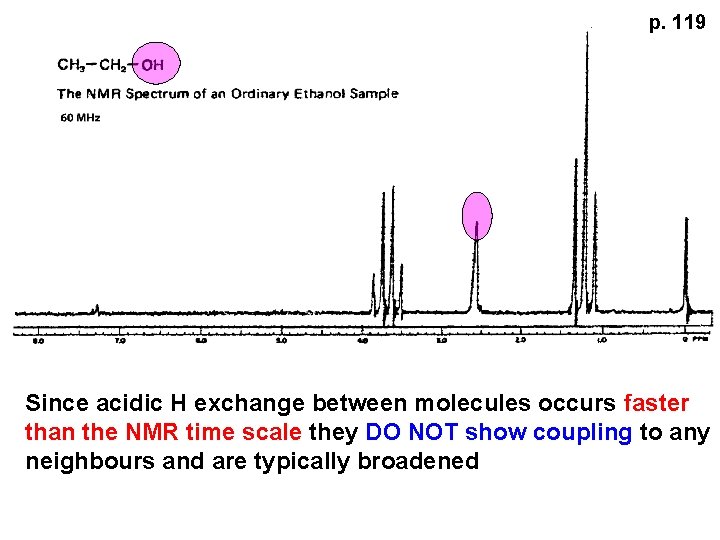
ALCOHOLS, AMINES, AMIDES AND ACIDS – exchangeable H’s Ar-CONHR and Ar-CONH 2 More acidic means more d+ on H NMR time scale is ~ 10 -2 – 10 -3 sec fastest NMR can measure! p. 119

p. 119 Since acidic H exchange between molecules occurs faster than the NMR time scale they DO NOT show coupling to any neighbours and are typically broadened

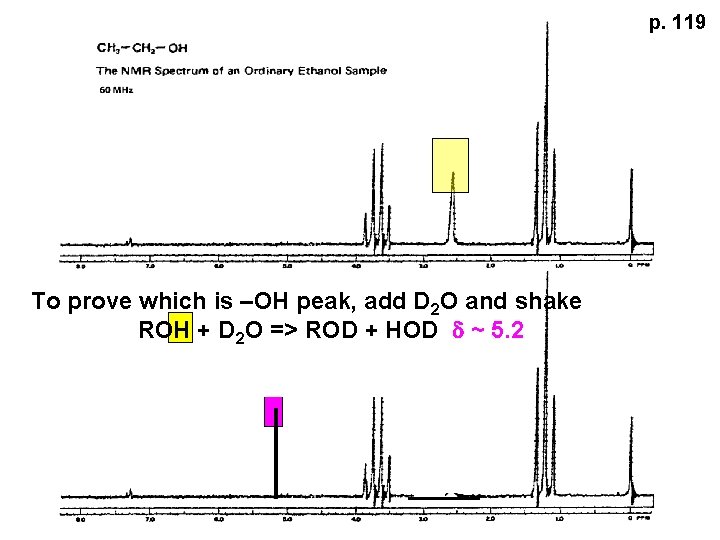
p. 119 To prove which is –OH peak, add D 2 O and shake ROH + D 2 O => ROD + HOD d ~ 5. 2

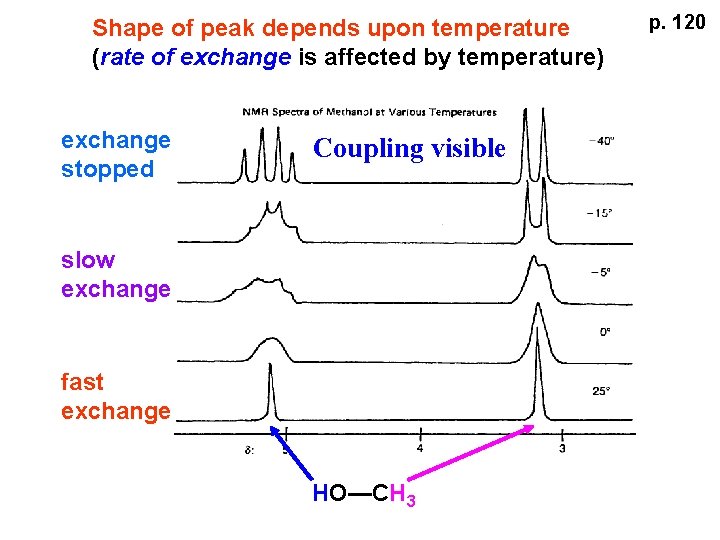
Shape of peak depends upon temperature (rate of exchange is affected by temperature) exchange stopped Coupling visible slow exchange fast exchange HO—CH 3 p. 120

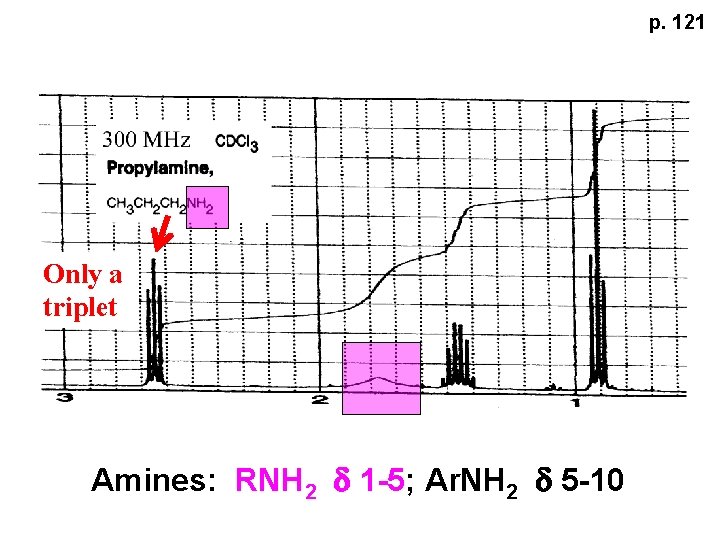
p. 121 Only a triplet Amines: RNH 2 d 1 -5; Ar. NH 2 d 5 -10

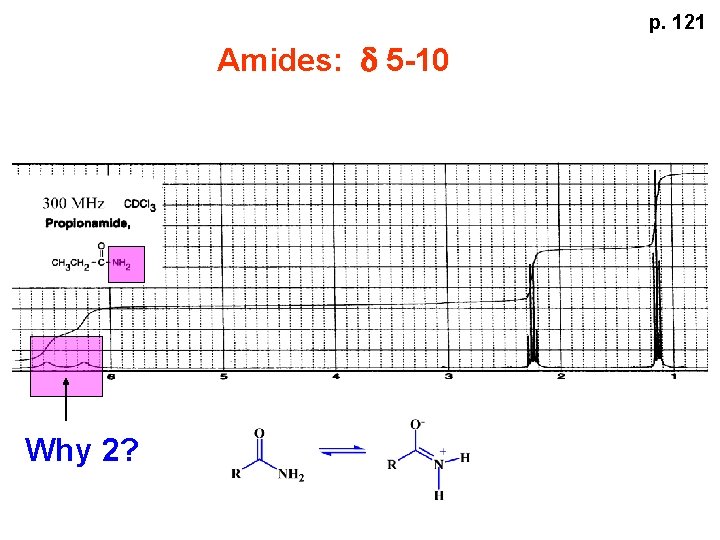
p. 121 Amides: d 5 -10 Why 2?

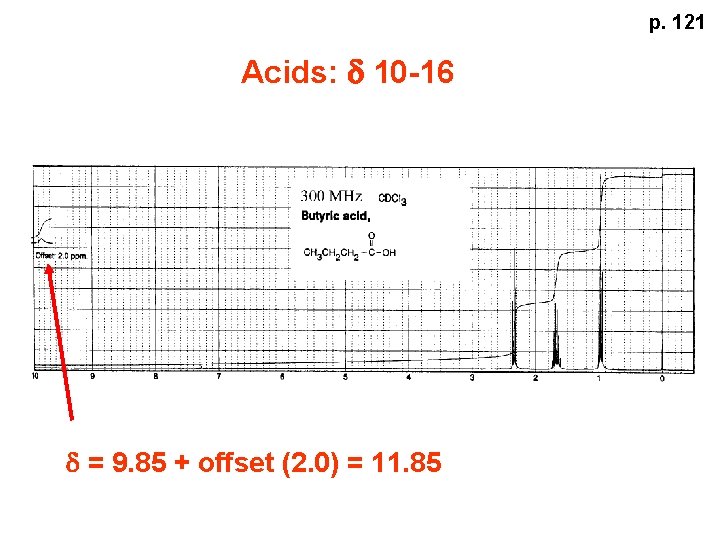
p. 121 Acids: d 10 -16 d = 9. 85 + offset (2. 0) = 11. 85

You can now start Assignment 5