Organic Molecules The Chemistry of Life What makes























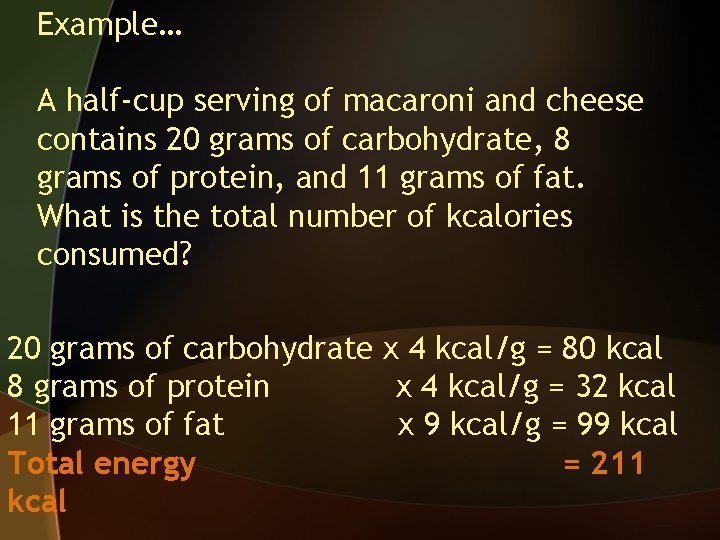





- Slides: 64

Organic Molecules The Chemistry of Life

What makes it ORGANIC? • Molecules made up of mainly CARBONs and HYDROGENs – (w/other elements at times like O, N, P) – Carbon’s special trait: Making 4 bonds

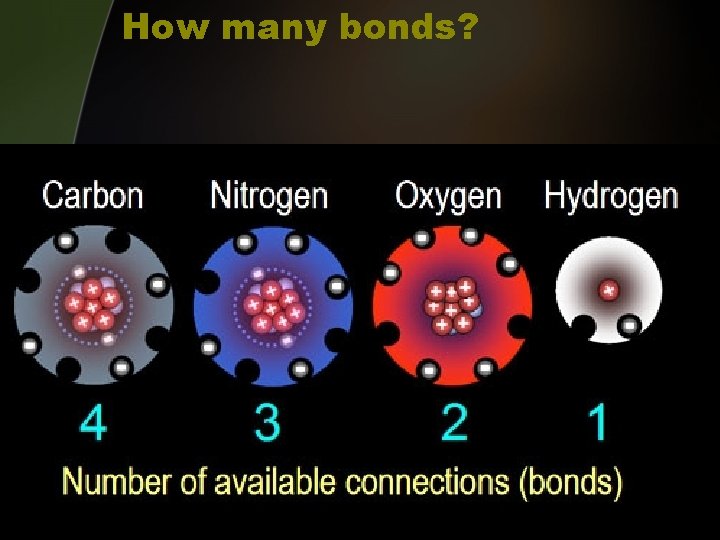
How many bonds?

4 Major Categories 1 st: Carbohydrates

4 Major Categories 2 nd: Proteins

4 Major Categories 3 rd: Lipids

4 Major Categories 4 th: Nucleic Acids RNA and DNA

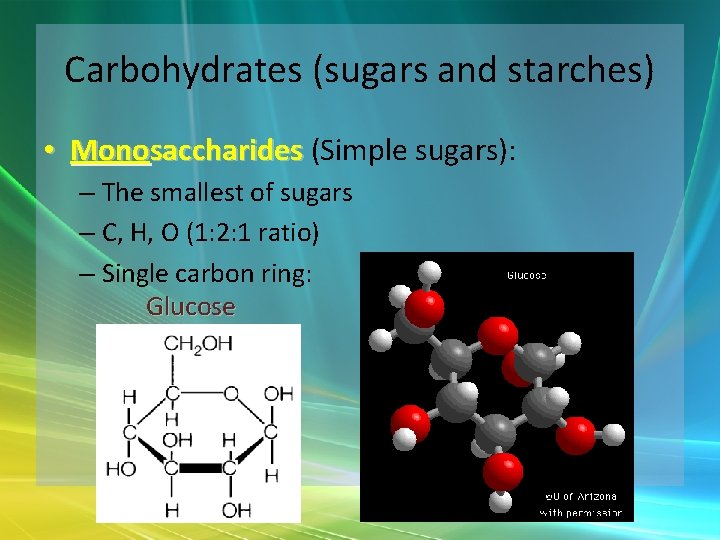
Carbohydrates (sugars and starches) • Monosaccharides (Simple sugars): – The smallest of sugars – C, H, O (1: 2: 1 ratio) – Single carbon ring: Glucose

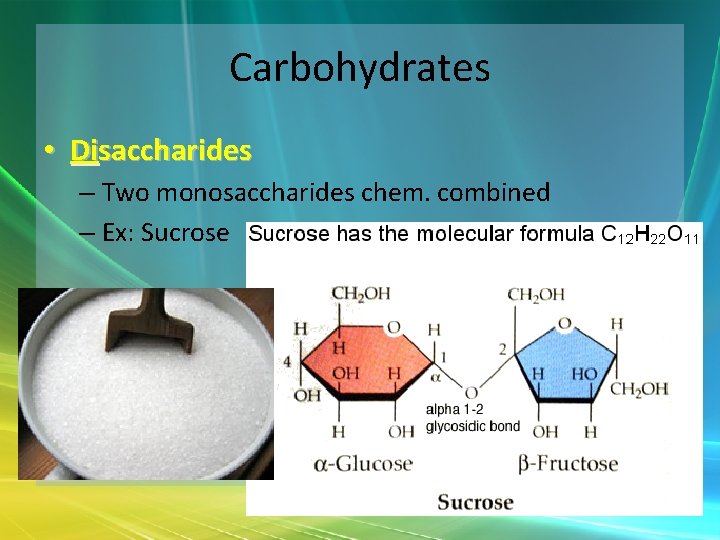
Carbohydrates • Disaccharides – Two monosaccharides chem. combined – Ex: Sucrose

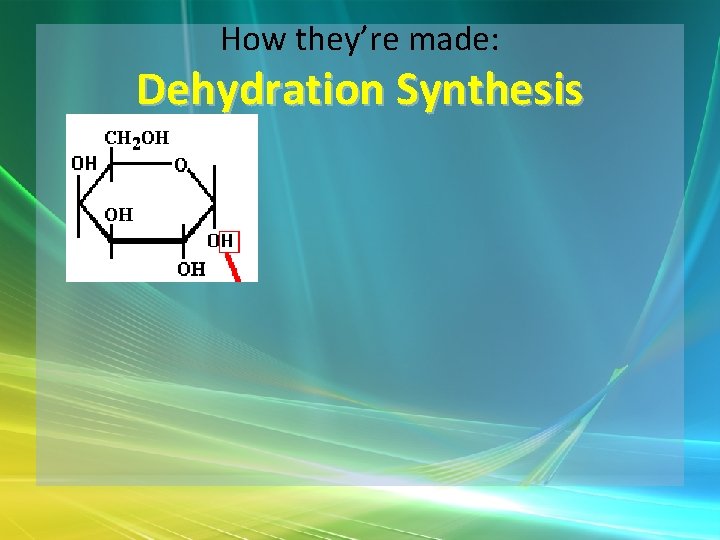
How they’re made: Dehydration Synthesis

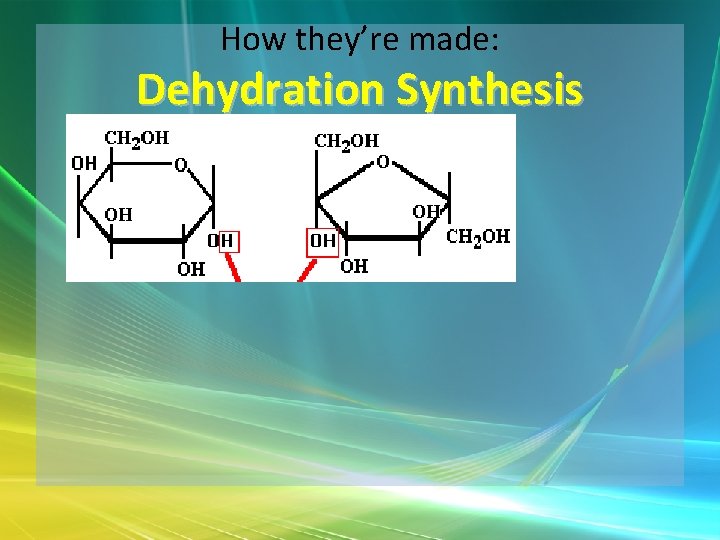
How they’re made: Dehydration Synthesis

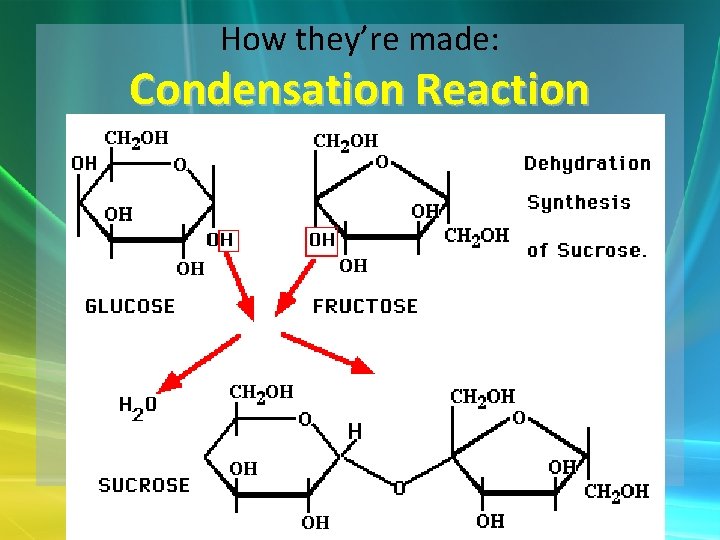
How they’re made: Condensation Reaction

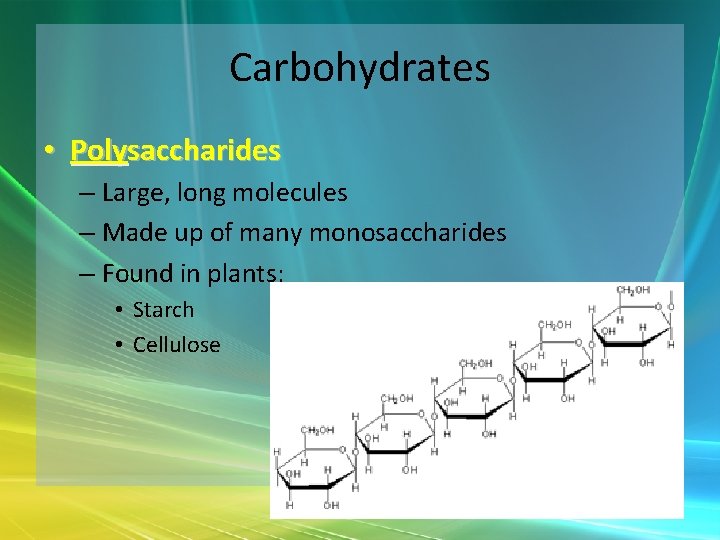
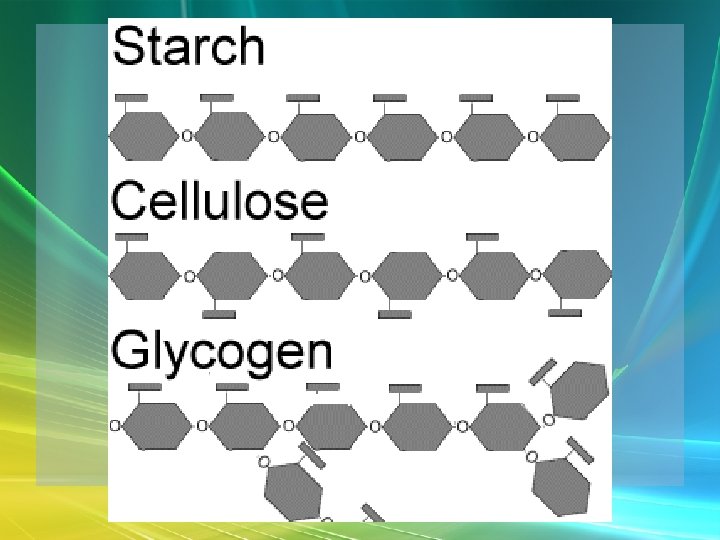
Carbohydrates • Polysaccharides – Large, long molecules – Made up of many monosaccharides – Found in plants: • Starch • Cellulose

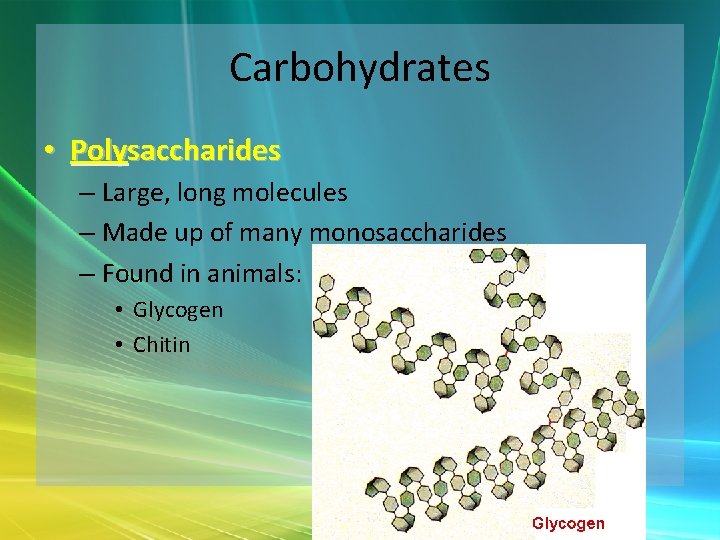
Carbohydrates • Polysaccharides – Large, long molecules – Made up of many monosaccharides – Found in animals: • Glycogen • Chitin


A note about some big molecules: Some very large molecules are actually built out of small, repeating units. Ex: This (*************) is made up of a bunch of these: (*) The repeating units are referred to as MONOMERS. The large molecule is a POLYMER So, starch is a polymer made of monomers called ________.

Carbohydrate Functions: Main source of energy for the cell Primary source of fuel for cellular respiration Used to store energy for short periods of time In plants, carbohydrates like cellulose are used as structural material in the cell wall

Carbohydrates Other Info: Foods: sugar, pasta, rice, bread, grains Chemical indicator: Starches-Lugol’s Iodine Solution turns blue/black Sugars-Benedicts turns brick red

What’s a calorie? Unit used to measure energy The amount of energy it takes to raise 1 g of water 1ºC = 1 calorie Kcal is what’s on food labels.

Caloric values… Carbohydrates and Proteins have about the same caloric values per unit vol. 4 kcal/g

Example… A half-cup serving of macaroni and cheese contains 20 grams of carbohydrate, 8 grams of protein, and 11 grams of fat. What is the total number of kcalories consumed? 20 grams of carbohydrate x 4 kcal/g = 80 kcal

Lipids Fatty, greasy, oily, or waxy Made of C, H, & less oxygen than in carbs Insoluble in water Monomer=glycerol and 3 fatty acids Foods: butter, shortening, oil, wax

Lipids: 4 categories Triglycerides/fats Phospholipids Waxes Steroids Indicator: Leaves dark spot on brown paper bag Sudan IV-changes to red

Importance of LIPIDS to humans: Energy source (primary energy storage) Cushion vital organs Insulation Major part of cell membranes Needed in some vitamins and hormones

Fatty Acids Carbon chains; make up most lipids 12 -28 C long

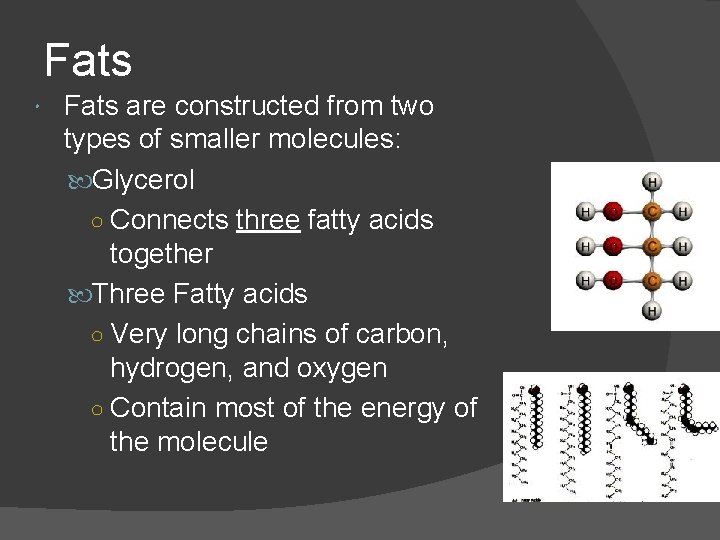
Fats are constructed from two types of smaller molecules: Glycerol ○ Connects three fatty acids together Three Fatty acids ○ Very long chains of carbon, hydrogen, and oxygen ○ Contain most of the energy of the molecule


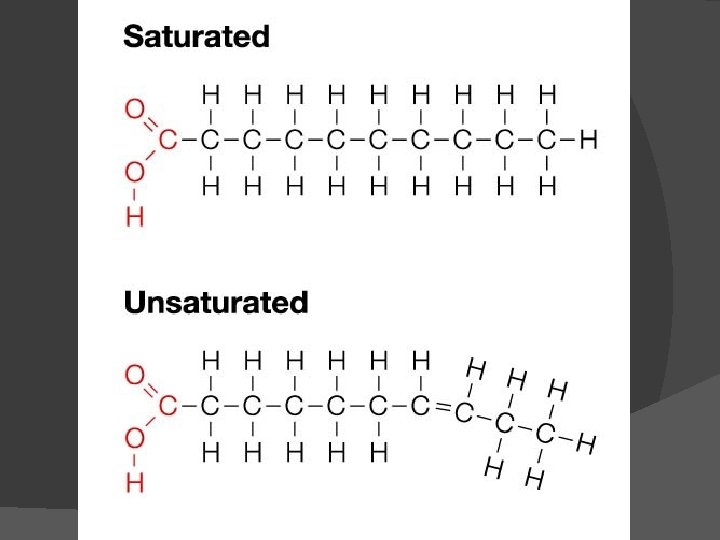
Fatty acids vary in two ways: length (number of carbons) If they have any double bonds Saturated fatty acids have the maximum number of hydrogen atoms possible and no double bonds Unsaturated fatty acids have one or more double bonds

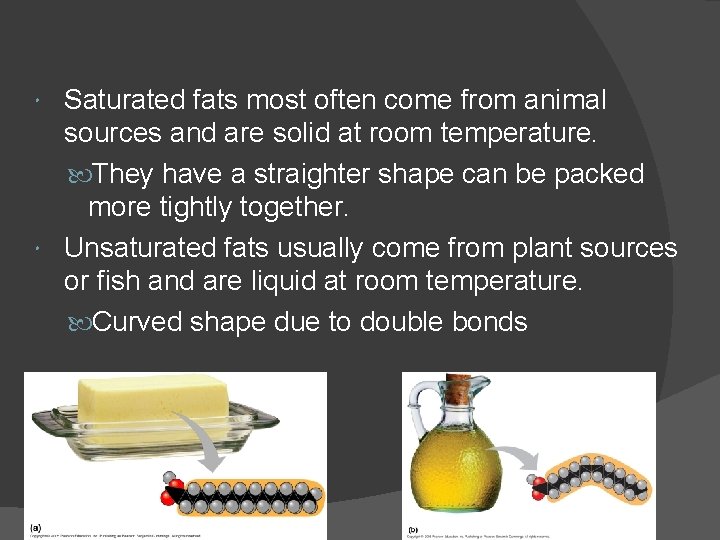
Saturated fats most often come from animal sources and are solid at room temperature. They have a straighter shape can be packed more tightly together. Unsaturated fats usually come from plant sources or fish and are liquid at room temperature. Curved shape due to double bonds


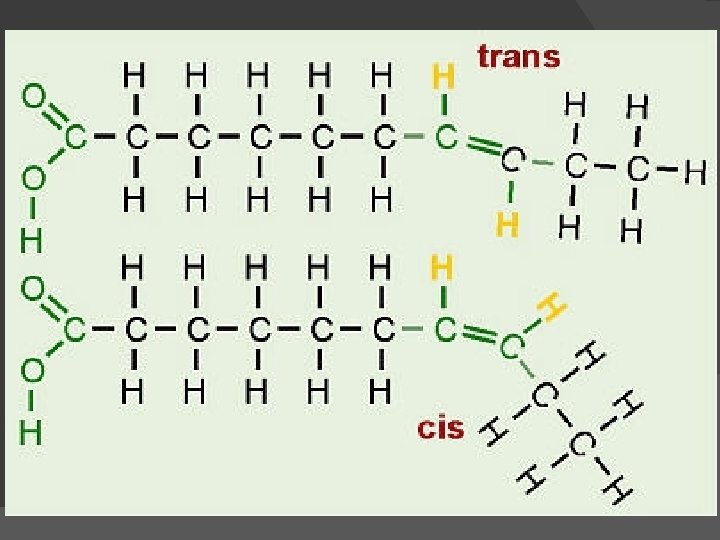
In unsaturated fatty acids, there are two ways the pieces of the hydrocarbon tail can be arranged around a C=C double bond: Cis and Trans bonds. In cis bonds, the two pieces of the carbon chain on either side of the double bond are either both “up” or both “down, ” such that both are on the same side of the molecule. In trans bonds, the two pieces of the molecule are on opposite sides of the double bond, that is, one “up” and one “down” across from each other. Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but using oil for frying causes some of the cis bonds to convert to trans bonds. If oil is used only once like when you fry an egg, only a few of the bonds do this so it’s not too bad. However, if oil is constantly reused, like in fast food French fry machines, more and more of the cis bonds are changed to trans until significant numbers of fatty acids with trans bonds build up. The reason this is of concern is that fatty acids with trans bonds are carcinogenic, or cancer-causing. The levels of trans fatty acids in highlyprocessed, lipid-containing products such as margarine are quite high. http: //redzuannorazlan. blogspot. com/2010/08/bbc 1 -k 22 -lipid. html

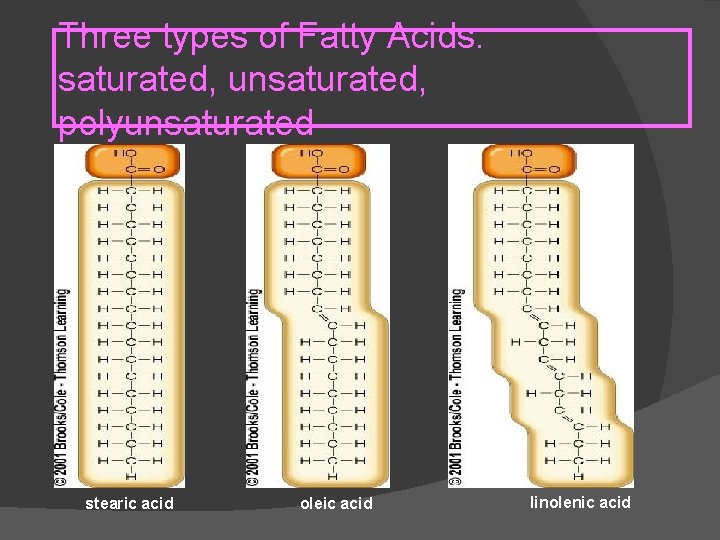
Three types of Fatty Acids: saturated, unsaturated, polyunsaturated stearic acid oleic acid linolenic acid

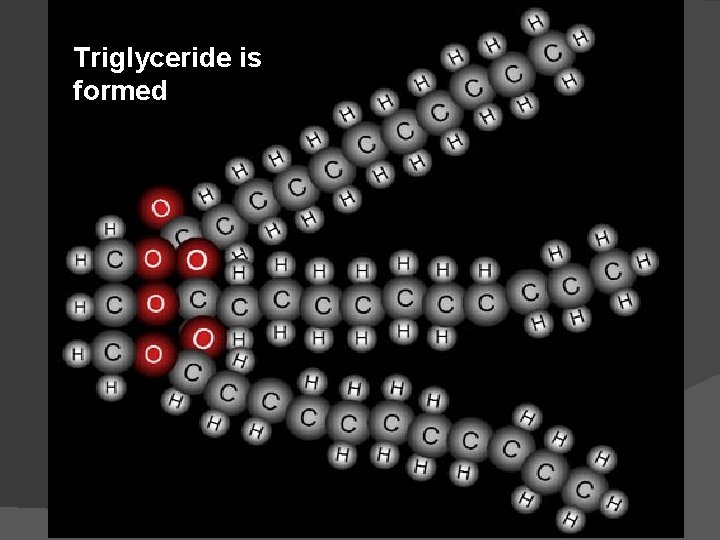
Triglyceride is formed

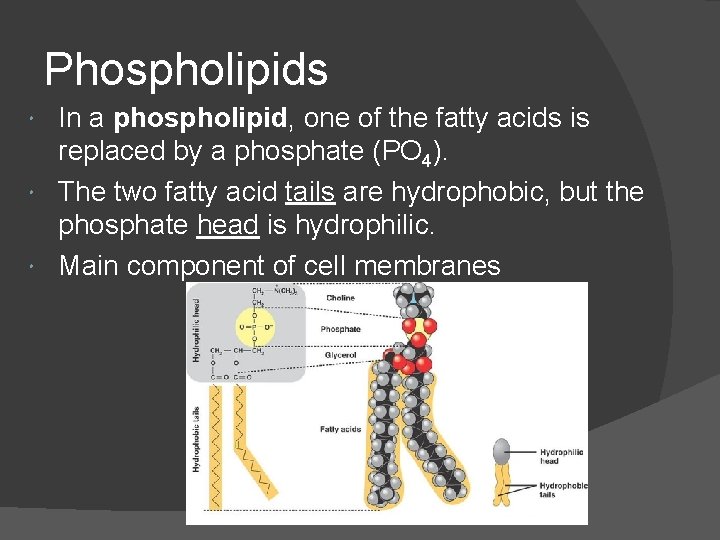
Phospholipids In a phospholipid, one of the fatty acids is replaced by a phosphate (PO 4). The two fatty acid tails are hydrophobic, but the phosphate head is hydrophilic. Main component of cell membranes

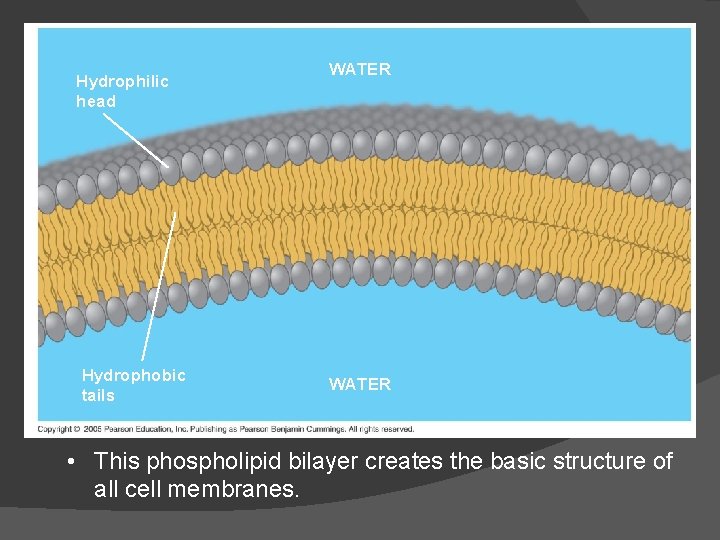
When phospholipids are added to water, they self-assemble into a bilayer The phospholipids form the outer part that is in contact with the water. The fatty acids form the inner part that is away from water.

Hydrophilic head Hydrophobic tails WATER • This phospholipid bilayer creates the basic structure of all cell membranes.

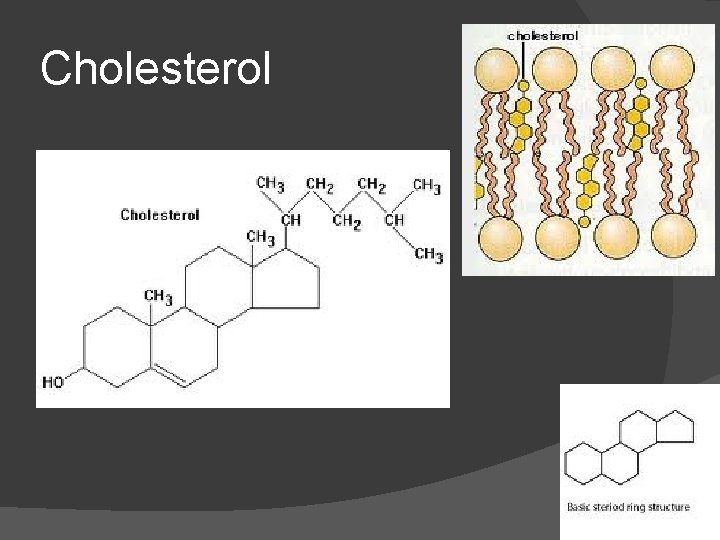
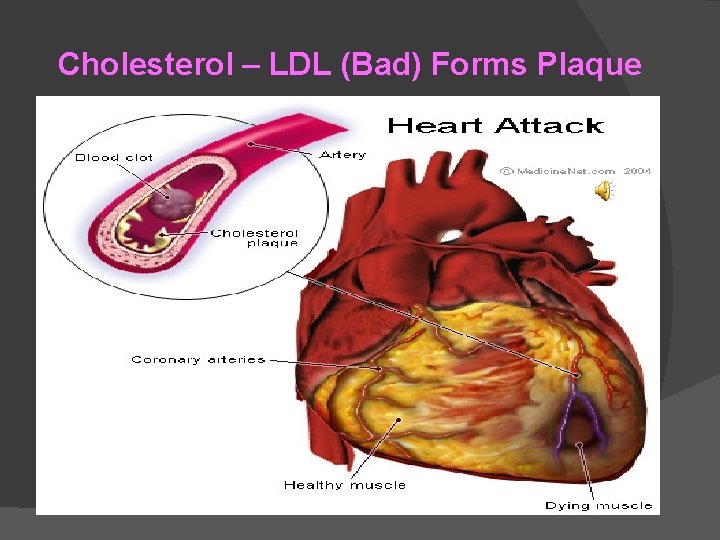
Steroids (Sterols) No fatty acid chains, but 4 fused Carbon rings Some hormones (testosterone) Cholesterol – most familiar ○ Produced in liver and eaten (meat, dairy products, etc. ) ○ Found in cell membranes (reduces fluidity of membrane) & in vertebrate brains

Cholesterol

Cholesterol – LDL (Bad) Forms Plaque

Waxes Long-chain fatty acids linked to alcohols or carbon rings Firm consistency Important in water-proofing

Caloric values… Lipids (fats), made for energy storage, have a higher caloric value. 9 kcal/g One pound of fat = 3500 Calories.

Example… A half-cup serving of macaroni and cheese contains 20 grams of carbohydrate, 8 grams of protein, and 11 grams of fat. What is the total number of kcalories consumed? 11 grams of fat x 9 kcal/g = 99 kcal

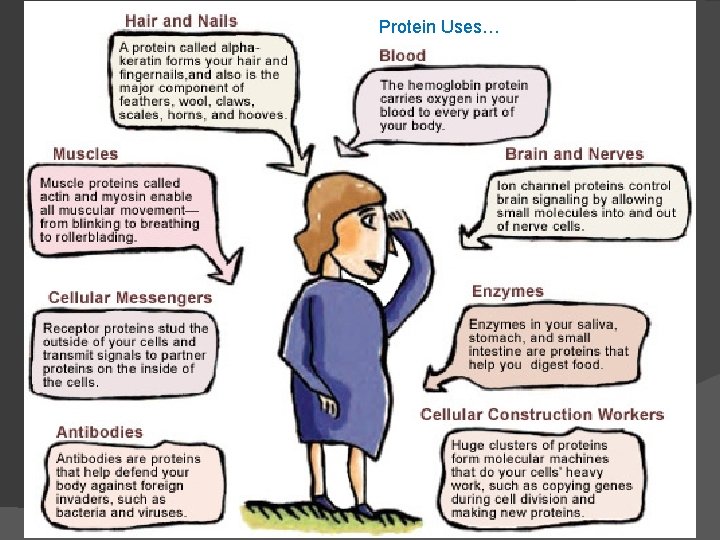
Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage, transport, cellular communications, movement, and defense against foreign substances Speed up reactions Control growth Food: meat, poultry, fish, nuts, beans, eggs, milk

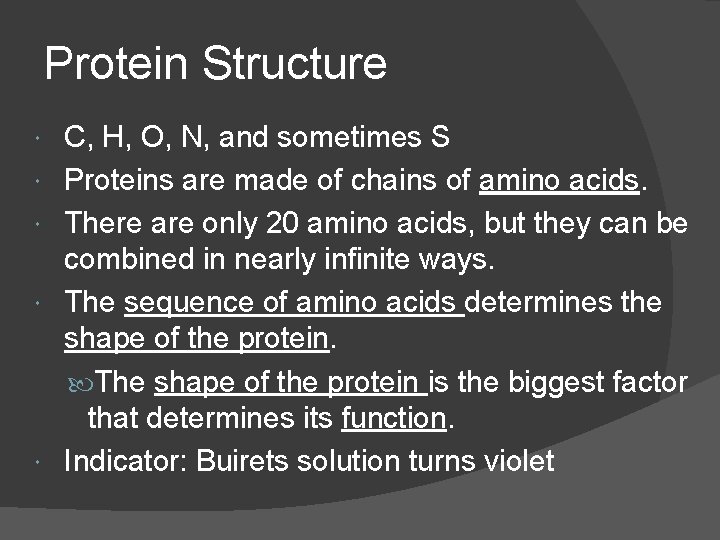
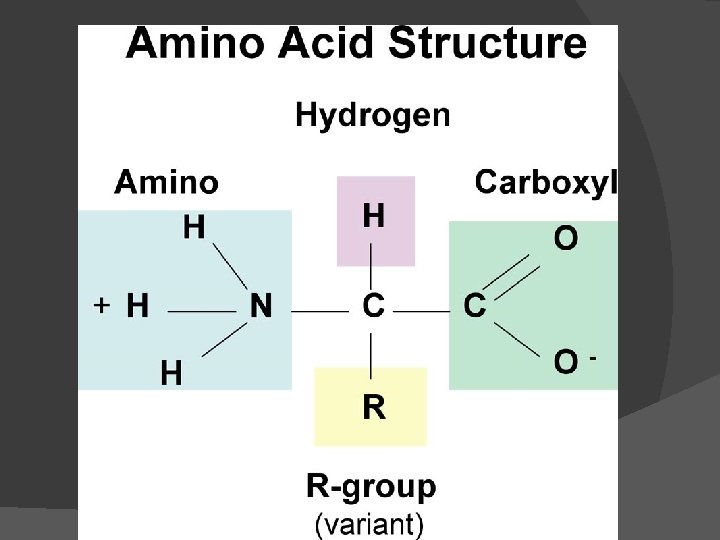
Protein Structure C, H, O, N, and sometimes S Proteins are made of chains of amino acids. There are only 20 amino acids, but they can be combined in nearly infinite ways. The sequence of amino acids determines the shape of the protein. The shape of the protein is the biggest factor that determines its function. Indicator: Buirets solution turns violet


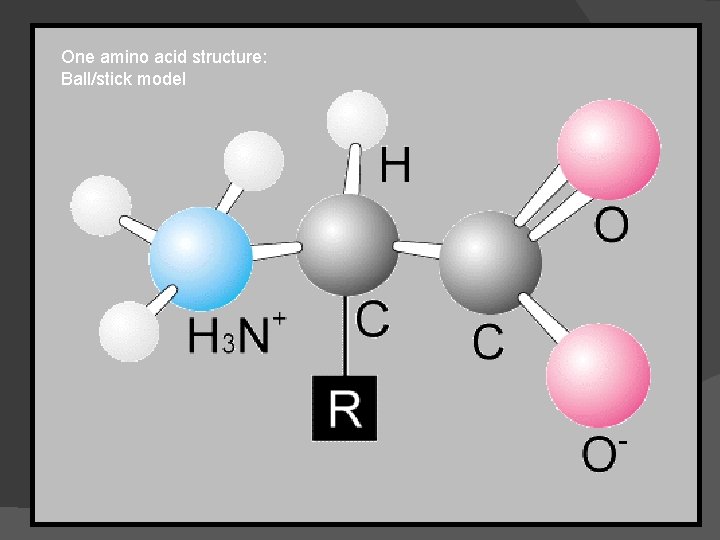
One amino acid structure: Ball/stick model

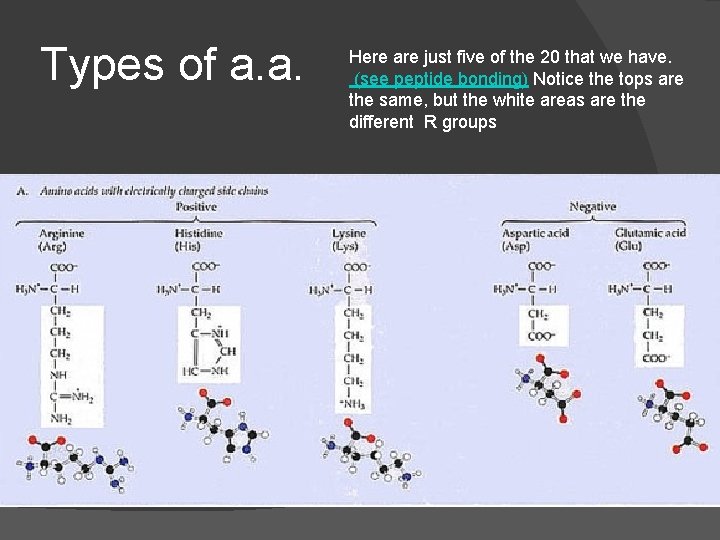
Types of a. a. Here are just five of the 20 that we have. (see peptide bonding) Notice the tops are the same, but the white areas are the different R groups

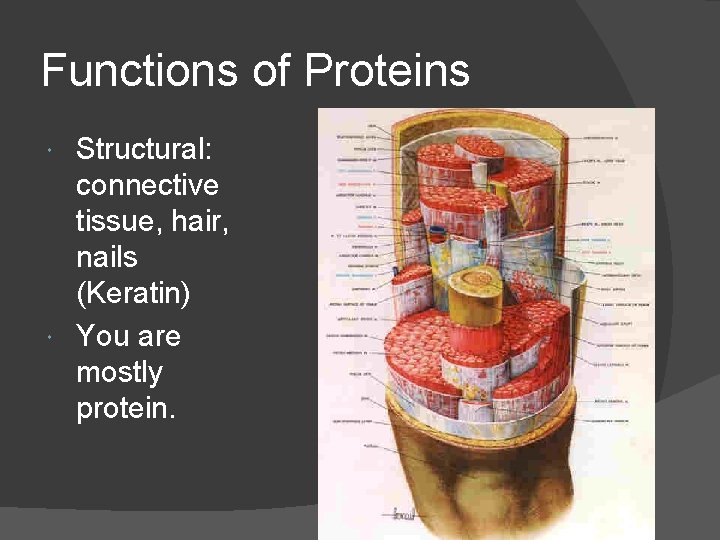
Functions of Proteins Structural: connective tissue, hair, nails (Keratin) You are mostly protein.

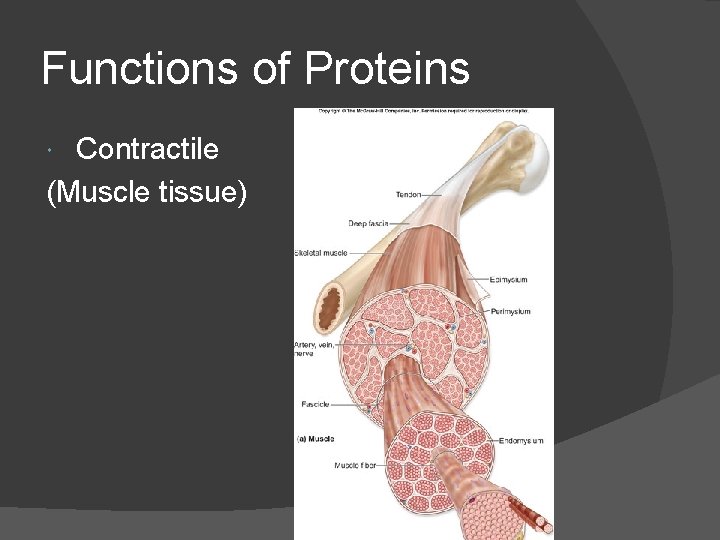
Functions of Proteins Contractile (Muscle tissue)

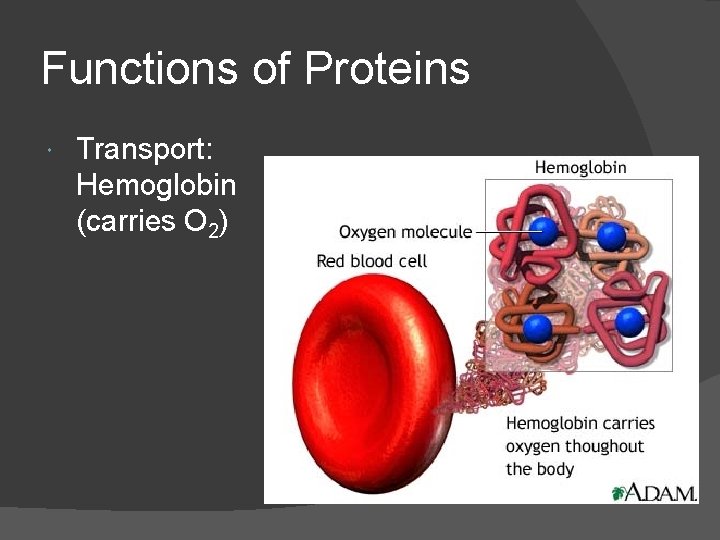
Functions of Proteins Transport: Hemoglobin (carries O 2)

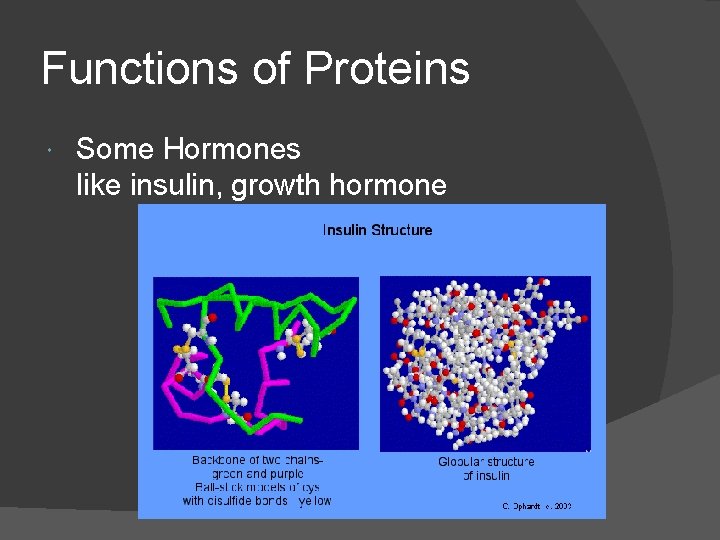
Functions of Proteins Some Hormones like insulin, growth hormone

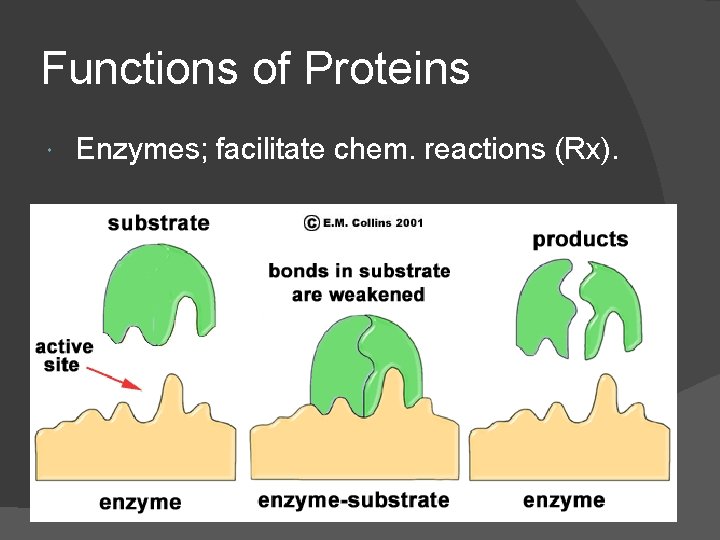
Functions of Proteins Enzymes; facilitate chem. reactions (Rx).

Protein Uses…

Caloric values… Carbohydrates and Proteins have about the same caloric values per unit vol. 4 kcal/g

Example… A half-cup serving of macaroni and cheese contains 20 grams of carbohydrate, 8 grams of protein, and 11 grams of fat. What is the total number of kcalories consumed? 8 grams of protein x 4 kcal/g = 32 kcal

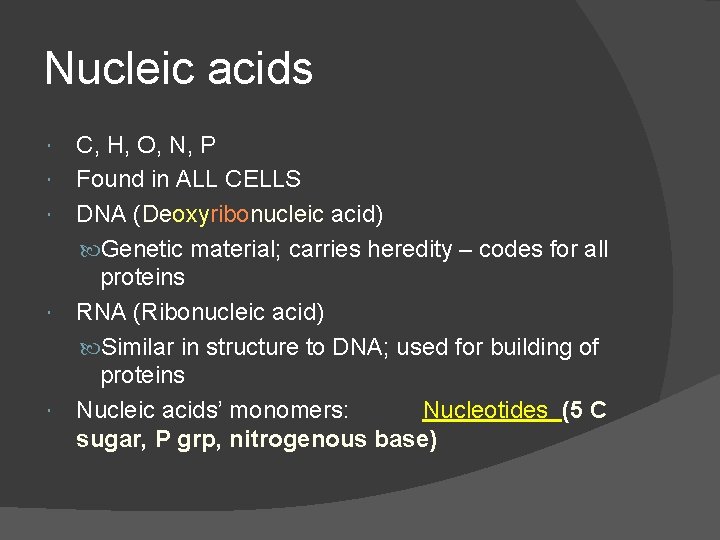
Nucleic acids C, H, O, N, P Found in ALL CELLS DNA (Deoxyribonucleic acid) Genetic material; carries heredity – codes for all proteins RNA (Ribonucleic acid) Similar in structure to DNA; used for building of proteins Nucleic acids’ monomers: Nucleotides (5 C sugar, P grp, nitrogenous base)

What’s a calorie? Unit used to measure energy The amount of energy it takes to raise 1 g of water 1ºC = 1 calorie One Kilocalorie (C) = 1000 calories (c) Kcal is what’s on food labels.

Caloric values… • Carbohydrates and Proteins have about the same caloric values per unit vol. – 4 kcal/g • Lipids (fats), made for energy storage, have a higher caloric value. – 9 kcal/g – One pound of fat = 3500 Calories.
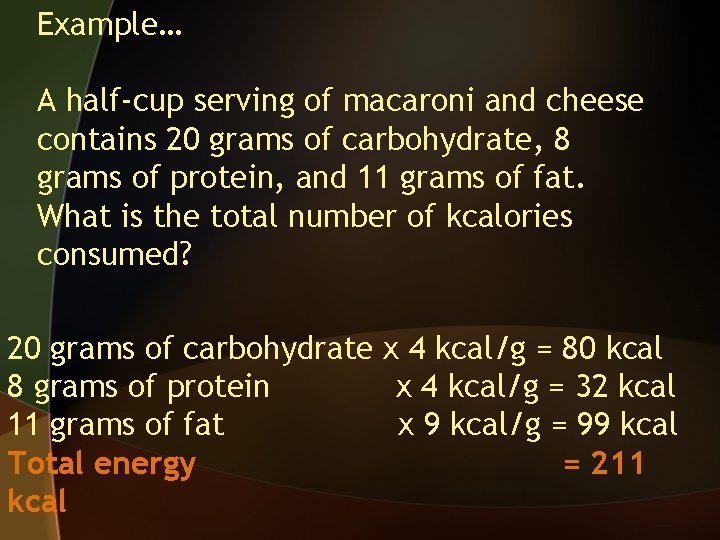
Example… A half-cup serving of macaroni and cheese contains 20 grams of carbohydrate, 8 grams of protein, and 11 grams of fat. What is the total number of kcalories consumed? 20 grams of carbohydrate x 4 kcal/g = 80 kcal 8 grams of protein x 4 kcal/g = 32 kcal 11 grams of fat x 9 kcal/g = 99 kcal Total energy = 211 kcal

Metabolism • sum of all chem Rx in a living organism Basal Metabolic Rate (BMR) • amt. of energy needed to sustain life • does not include energy needed for PA

How many Calories do you need per day? Calculating Your EER (Estimated Energy Requirement) 1. Find your weight in kg and your height in meters: Weight in kilograms = weight in pounds / 2. 2 lbs/kg (Ex. 150 pounds = 150/2. 2 = 68. 2 kg) Height in meters = height in inches x 0. 0254 in/m (Ex. 5’ 9” = 69 in x 0. 0254 in/m = 1. 75 m)

Calculating Your EER (Estimated Energy Requirement) 2. Estimate the amount of physical activity (PA) you get per day. Sedentary: Males (1. 00) Females (1. 00) Lightly active: (1. 13) (1. 16) Active: (1. 26) (1. 31) Very Active: (1. 42) (1. 56)

Calculating Your EER (Estimated Energy Requirement) 3. Choose the appropriate equation, enter your values, and write down your EER value. Females: EER = 135. 3 – (30. 8 x age in yrs) + PA [(10. 0 x Wt in kg) + (934 x Ht in m)] + 25 Males: EER = 88. 5 – (61. 9 x age in yrs) + PA [(26. 7 x Wt in kg) + (903 x Ht in m)] + 25

What are your calorie needs per day? What is your BMR (Base Metabolic Rate)? Michael Phelps, at the height of his training, requires 12, 000 Calories/day.