Organic Molecules The Chemistry of Life The molecule









- Slides: 31

Organic Molecules The Chemistry of Life

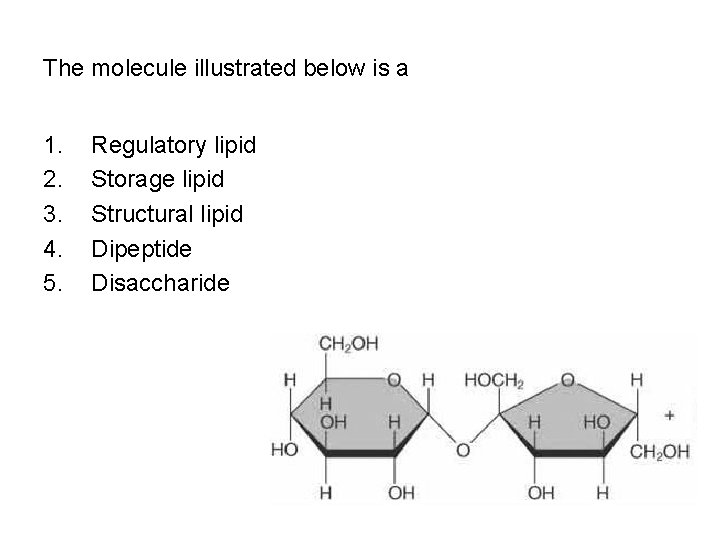
The molecule illustrated below is a 1. 2. 3. 4. 5. Regulatory lipid Storage lipid Structural lipid Dipeptide Disaccharide

I. Overview • A. Limited number of elements are found in organic molecules • B. Carbon, Hydrogen, Oxygen, Nitrogen, Sulfur, Phosphate • C. Four major classes of organic molecules with different functions – – Carbohydrates Lipids Proteins Nucleic Acids

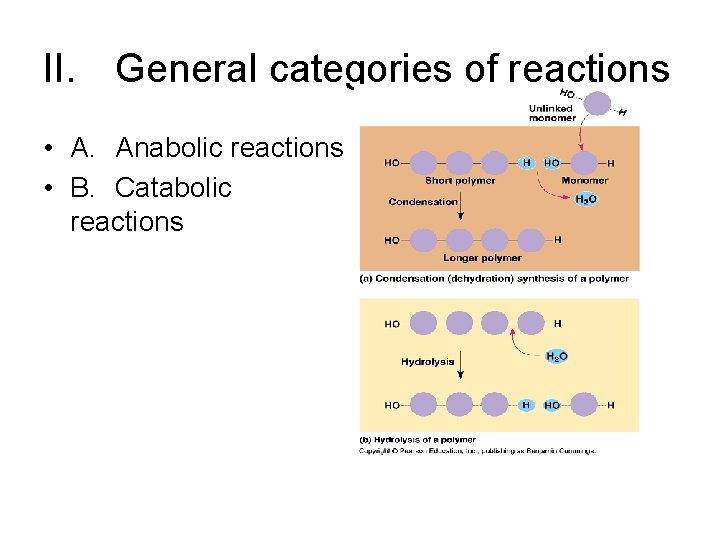
II. General categories of reactions • A. Anabolic reactions • B. Catabolic reactions

III. Carbohydrates • A. Introduction – Elements found – Generalized formula suggested by name – Names often end in –ose – Generally concerned with energy usage and storage – Some carbohydrates are structural

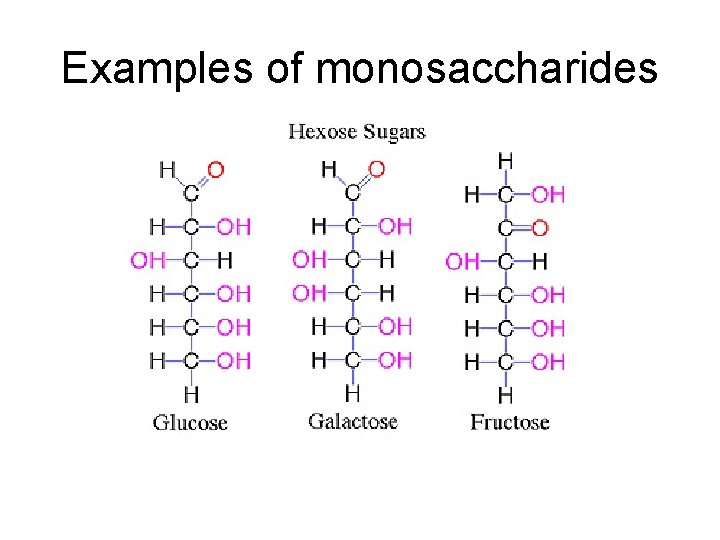
III. Carbohydrates • B. Monosaccharides – 1. – 2. – 3. – 4. trioses, pentoses, and hexoses most common examples are glucose, fructose, and galactose structural isomers of each other

Examples of monosaccharides

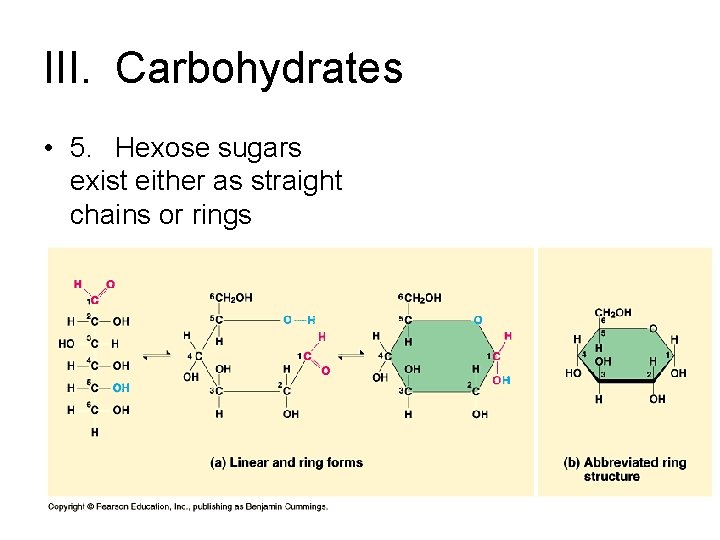
III. Carbohydrates • 5. Hexose sugars exist either as straight chains or rings

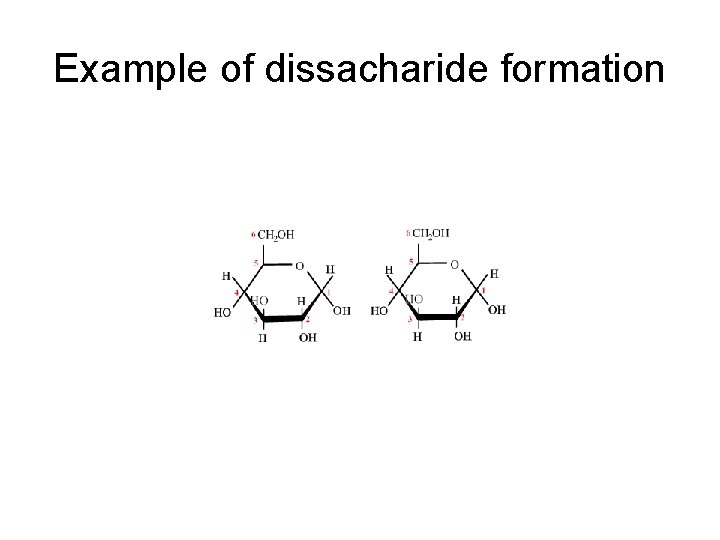
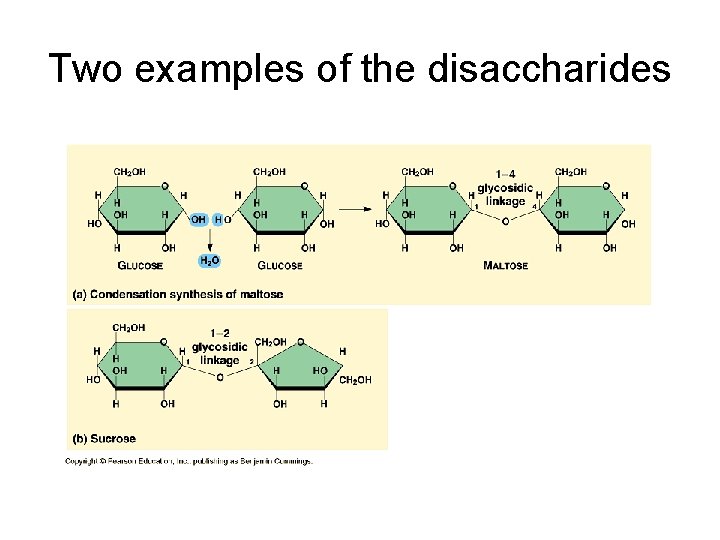
III. Carbohydrates • B. Disaccharides – 1. two monosaccharides joined by a dehydration synthesis – 2. three specific examples of dissacharides to memorize • Sucrose • Maltose • Lactose

Example of dissacharide formation

Two examples of the disaccharides

The empirical formula of a disaccharide is C 12 H 24 O 12. 1. True 2. False

What is the correct answer?

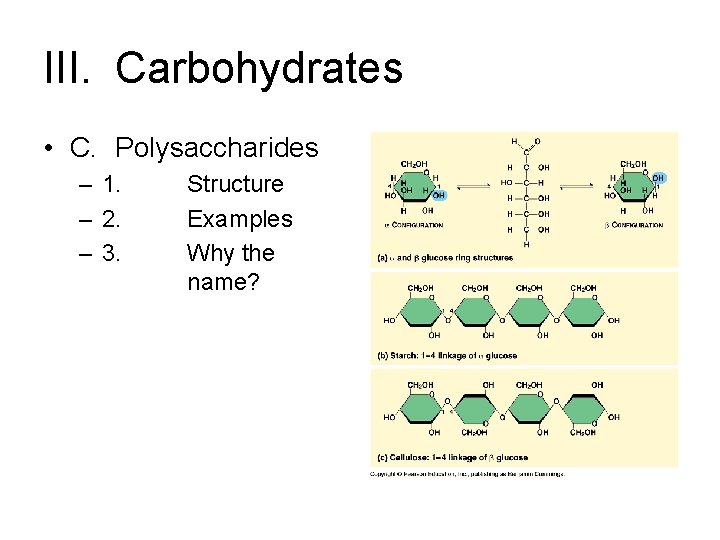
III. Carbohydrates • C. Polysaccharides – 1. – 2. – 3. Structure Examples Why the name?

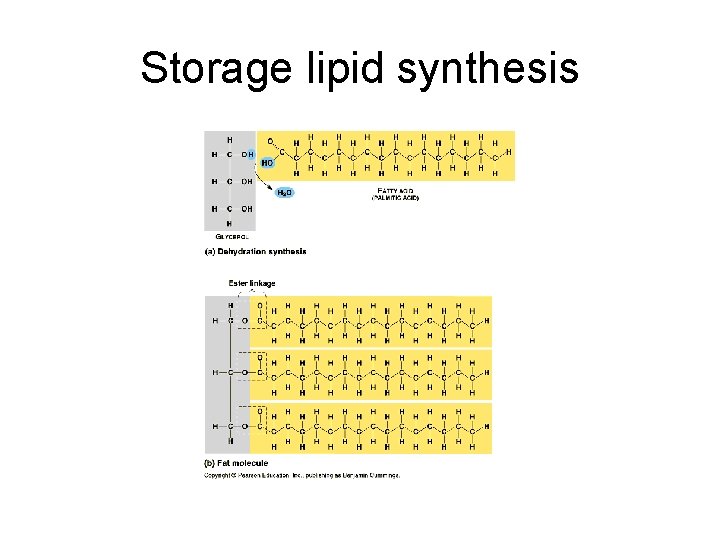
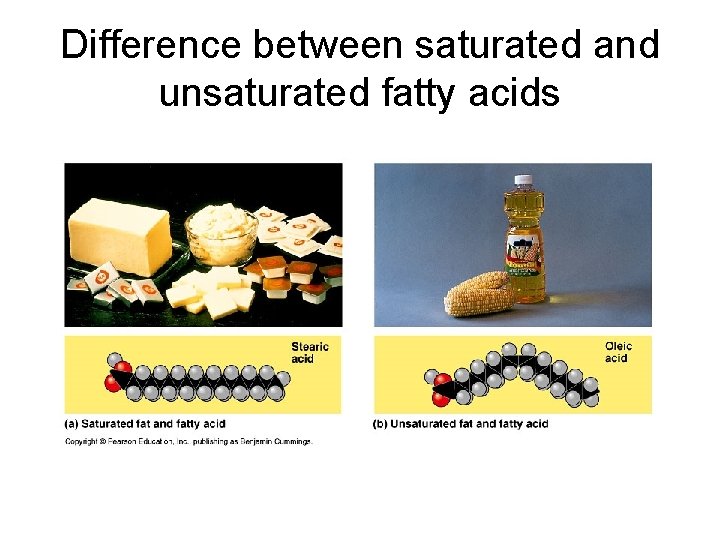
IV. Lipids-three types • A. Storage lipids (a. k. a. fat) – 1. – 2. – 3. • a. • b. component parts glycerol three fatty acids saturated unsaturated

Storage lipid synthesis

Difference between saturated and unsaturated fatty acids

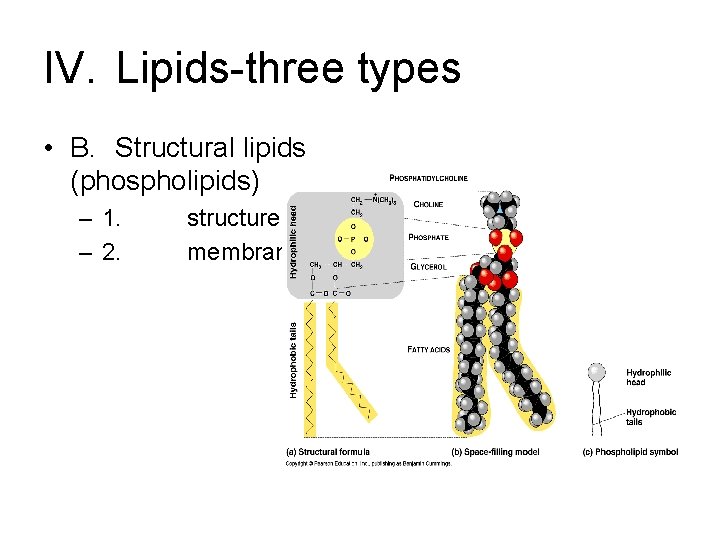
IV. Lipids-three types • B. Structural lipids (phospholipids) – 1. – 2. structure membrane

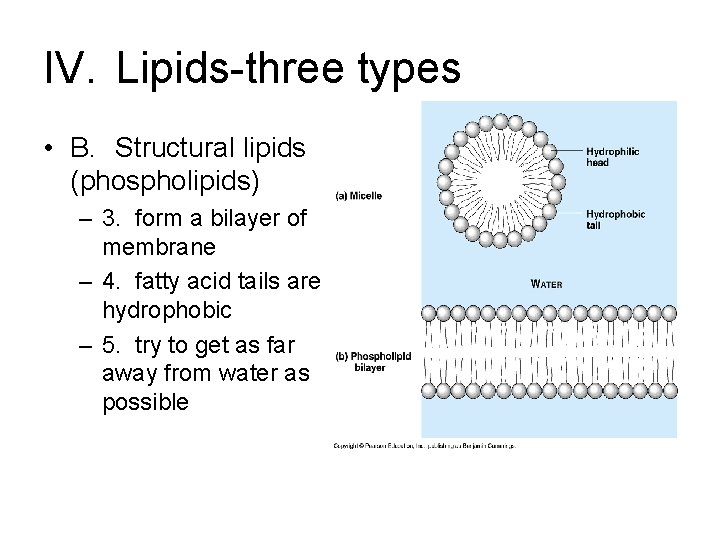
IV. Lipids-three types • B. Structural lipids (phospholipids) – 3. form a bilayer of membrane – 4. fatty acid tails are hydrophobic – 5. try to get as far away from water as possible

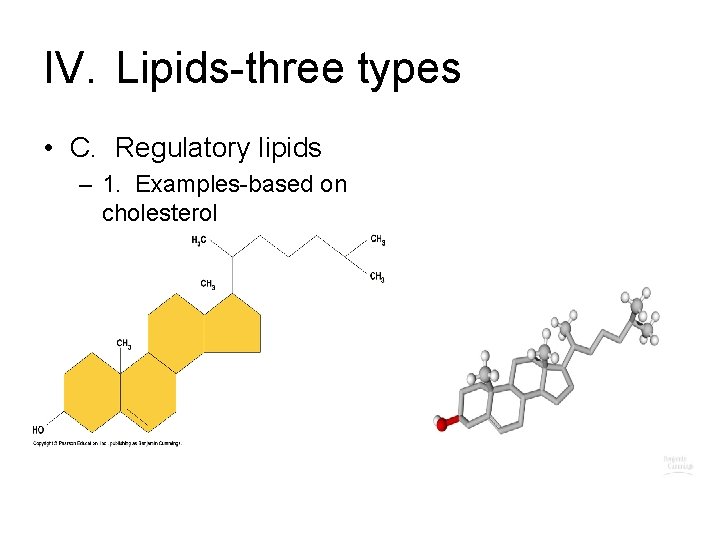
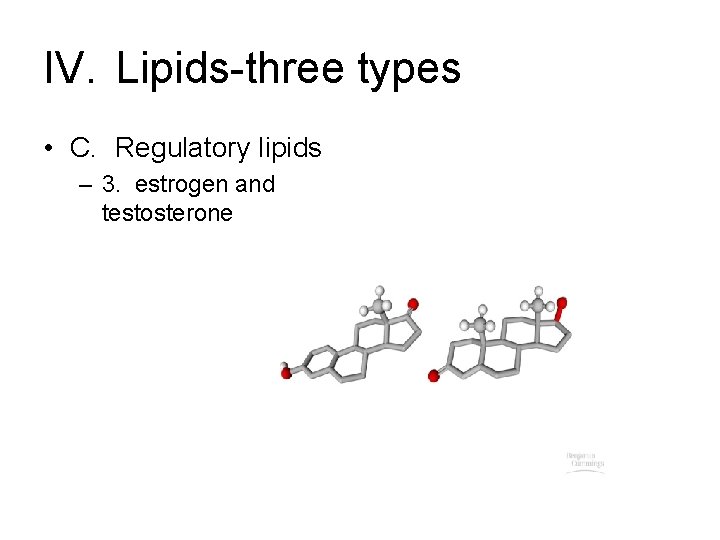
IV. Lipids-three types • C. Regulatory lipids – 1. Examples-based on cholesterol

IV. Lipids-three types • C. Regulatory lipids – 3. estrogen and testosterone

IV. Lipids-three types • C. Regulatory lipids – 4. notice that these don’t share common structure of first two – 5. share in common that they dissolve in organic solvents

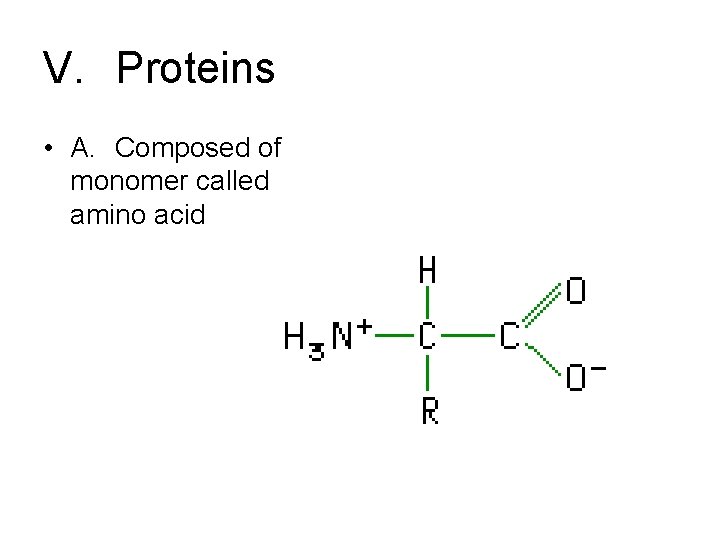
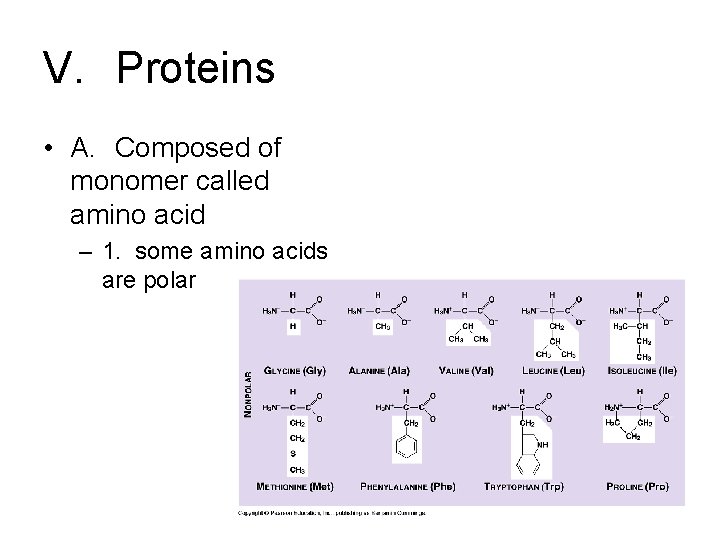
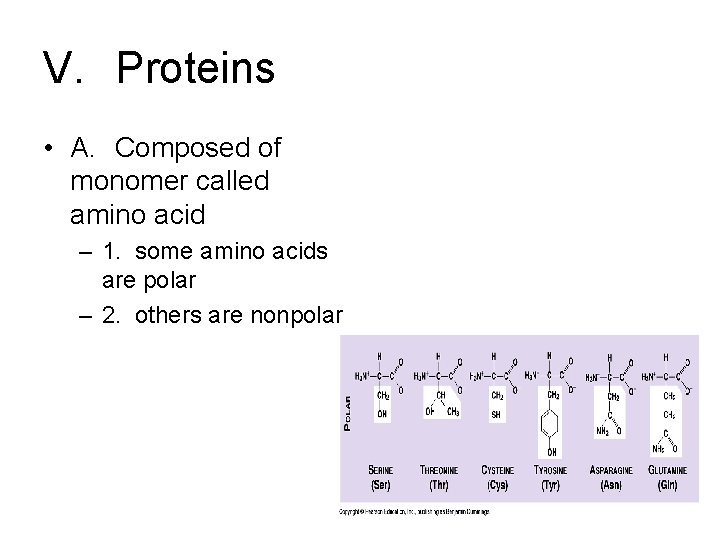
V. Proteins • A. Composed of monomer called amino acid

V. Proteins • A. Composed of monomer called amino acid – 1. some amino acids are polar

V. Proteins • A. Composed of monomer called amino acid – 1. some amino acids are polar – 2. others are nonpolar

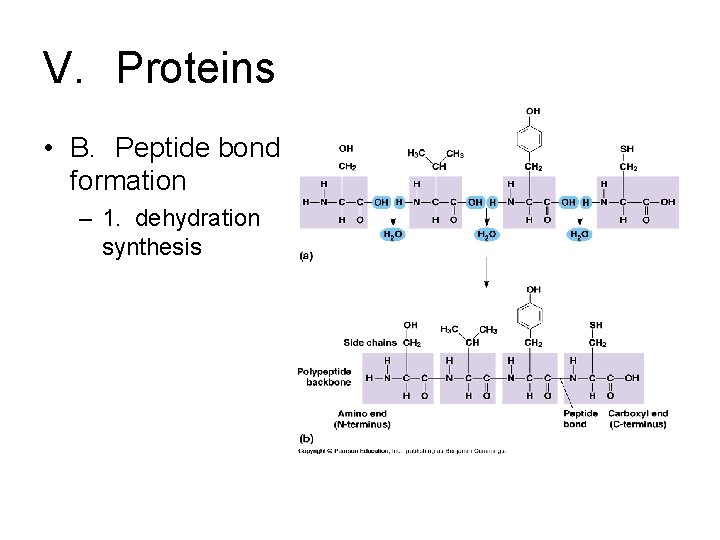
V. Proteins • B. Peptide bond formation – 1. dehydration synthesis

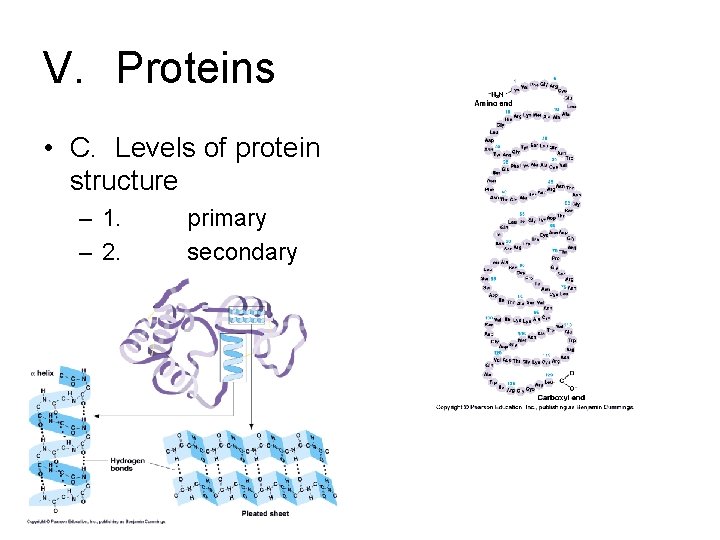
V. Proteins • C. Levels of protein structure – 1. – 2. primary secondary

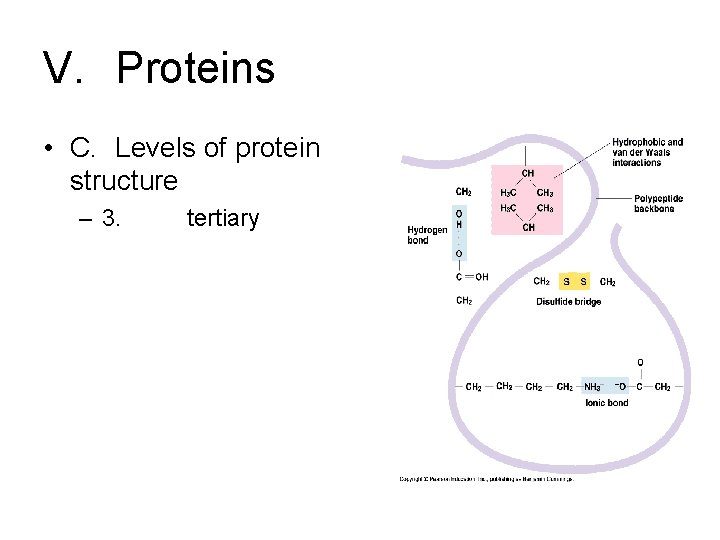
V. Proteins • C. Levels of protein structure – 3. tertiary

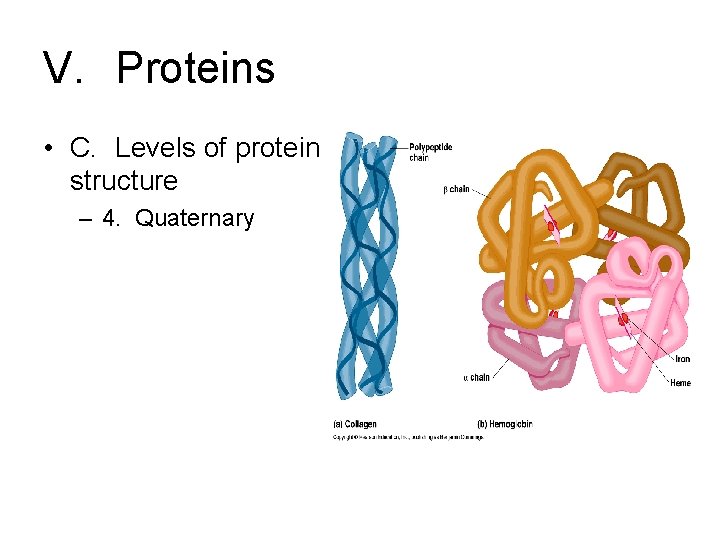
V. Proteins • C. Levels of protein structure – 4. Quaternary

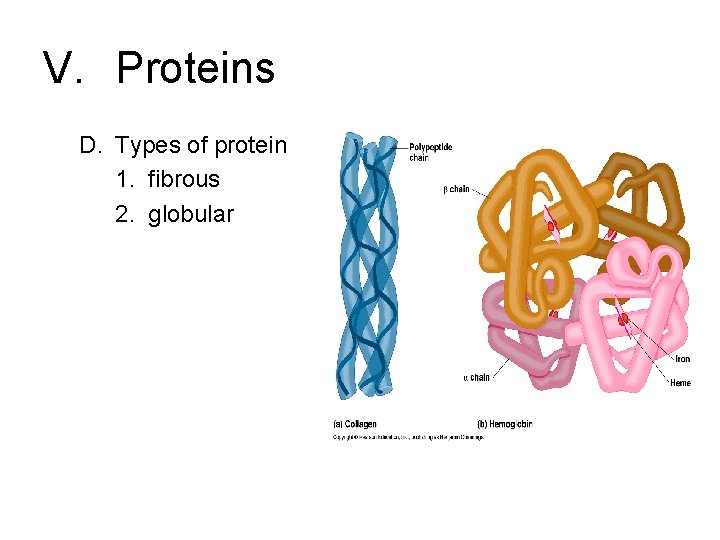
V. Proteins D. Types of protein 1. fibrous 2. globular

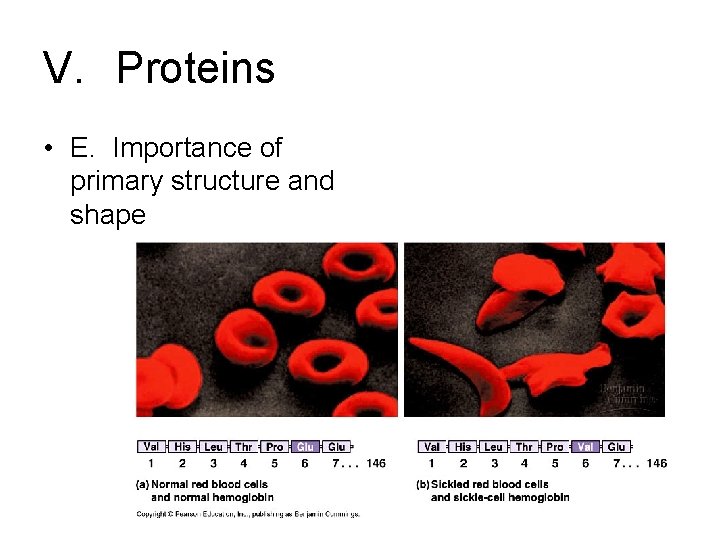
V. Proteins • E. Importance of primary structure and shape