Organic Molecules Depiction of Structure The Basics Organic
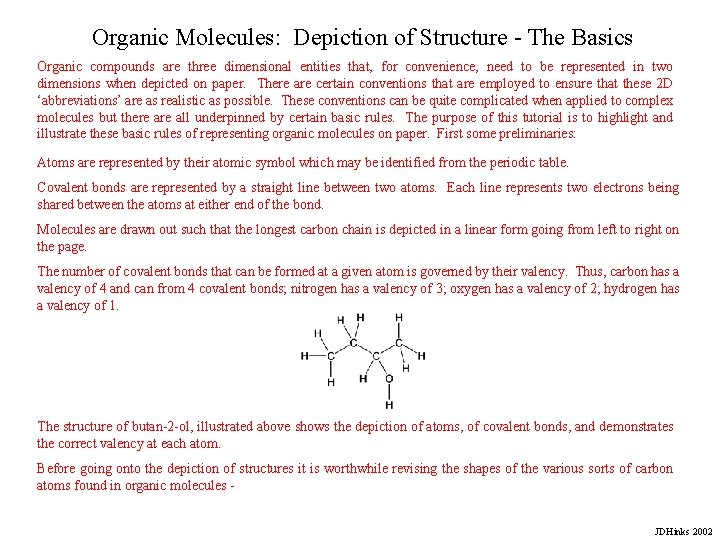
Organic Molecules: Depiction of Structure - The Basics Organic compounds are three dimensional entities that, for convenience, need to be represented in two dimensions when depicted on paper. There are certain conventions that are employed to ensure that these 2 D ‘abbreviations’ are as realistic as possible. These conventions can be quite complicated when applied to complex molecules but there all underpinned by certain basic rules. The purpose of this tutorial is to highlight and illustrate these basic rules of representing organic molecules on paper. First some preliminaries: Atoms are represented by their atomic symbol which may be identified from the periodic table. Covalent bonds are represented by a straight line between two atoms. Each line represents two electrons being shared between the atoms at either end of the bond. Molecules are drawn out such that the longest carbon chain is depicted in a linear form going from left to right on the page. The number of covalent bonds that can be formed at a given atom is governed by their valency. Thus, carbon has a valency of 4 and can from 4 covalent bonds; nitrogen has a valency of 3; oxygen has a valency of 2; hydrogen has a valency of 1. The structure of butan-2 -ol, illustrated above shows the depiction of atoms, of covalent bonds, and demonstrates the correct valency at each atom. Before going onto the depiction of structures it is worthwhile revising the shapes of the various sorts of carbon atoms found in organic molecules JDHinks 2002

Some comments on the 3 D shape of organic compounds Alkanes are compounds containing carbon atoms that have four single bonds attaching them to four other atoms. Carbon atoms in this state are called sp 3 hybridised (hybridisation is a term that will be discussed in some depth in the early part of your degree). The shape of a sp 3 hybridised carbon atom and the atoms surrounding it is tetrahedral. That is, the central carbon atom is at the centre of an imaginary tetrahedron and each of the attached atoms are at its corners. The angle between any two of the bonds is very close to 109°. The simplest alkane, methane, is shown on the right. Alkenes are compounds containing at least two carbon atoms which are joined together by a double bond. That is, each carbon atom involved in the double bond is attached to one double bond (to another carbon atom) and two single bonds The carbon atoms involved in the double bond are sp 2 hydridised. The two carbon atoms of the double bond and the four atoms attached to the double bond are all in same plane and are separated by a bond angle of 120°. The simplest alkene, ethene, is shown on the right. Alkynes are compounds containing at least two carbon atoms which are joined together by a triple bond. That is, each carbon atom involved in the double bond is attached to one triple bond (to another carbon atom) and one single bond. The carbon atoms involved in the triple bond are sp hydridised. The two carbon atoms of the triple bond and the atoms attached to each end of the triple bond are all in same plane and the atoms are all aligned along the axis of the triple bond. The simplest alkyne, acetylene, is shown on the right. JDHinks 2002
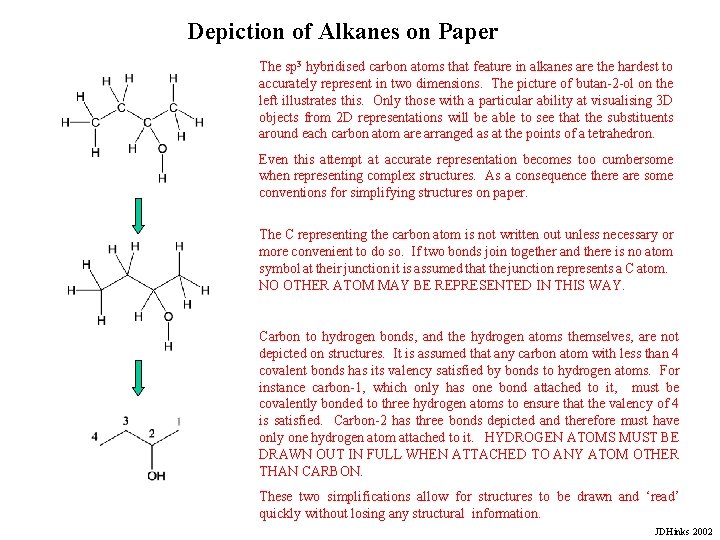
Depiction of Alkanes on Paper The sp 3 hybridised carbon atoms that feature in alkanes are the hardest to accurately represent in two dimensions. The picture of butan-2 -ol on the left illustrates this. Only those with a particular ability at visualising 3 D objects from 2 D representations will be able to see that the substituents around each carbon atom are arranged as at the points of a tetrahedron. Even this attempt at accurate representation becomes too cumbersome when representing complex structures. As a consequence there are some conventions for simplifying structures on paper. The C representing the carbon atom is not written out unless necessary or more convenient to do so. If two bonds join together and there is no atom symbol at their junction it is assumed that the junction represents a C atom. NO OTHER ATOM MAY BE REPRESENTED IN THIS WAY. Carbon to hydrogen bonds, and the hydrogen atoms themselves, are not depicted on structures. It is assumed that any carbon atom with less than 4 covalent bonds has its valency satisfied by bonds to hydrogen atoms. For instance carbon-1, which only has one bond attached to it, must be covalently bonded to three hydrogen atoms to ensure that the valency of 4 is satisfied. Carbon-2 has three bonds depicted and therefore must have only one hydrogen atom attached to it. HYDROGEN ATOMS MUST BE DRAWN OUT IN FULL WHEN ATTACHED TO ANY ATOM OTHER THAN CARBON. These two simplifications allow for structures to be drawn and ‘read’ quickly without losing any structural information. JDHinks 2002
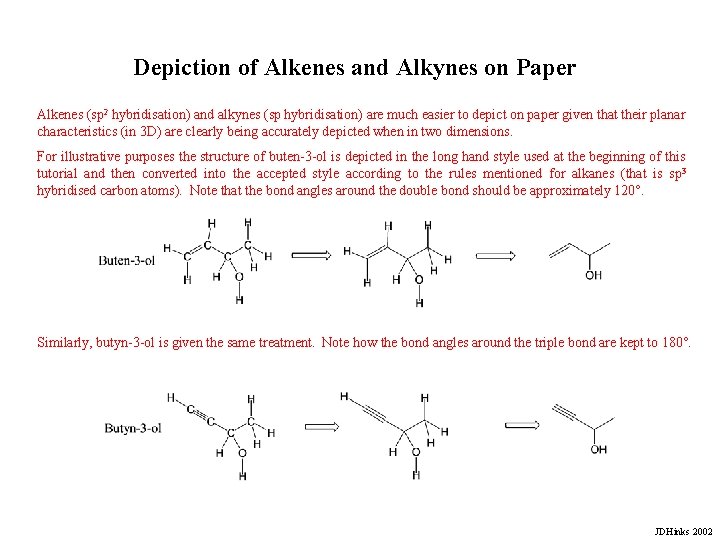
Depiction of Alkenes and Alkynes on Paper Alkenes (sp 2 hybridisation) and alkynes (sp hybridisation) are much easier to depict on paper given that their planar characteristics (in 3 D) are clearly being accurately depicted when in two dimensions. For illustrative purposes the structure of buten-3 -ol is depicted in the long hand style used at the beginning of this tutorial and then converted into the accepted style according to the rules mentioned for alkanes (that is sp 3 hybridised carbon atoms). Note that the bond angles around the double bond should be approximately 120°. Similarly, butyn-3 -ol is given the same treatment. Note how the bond angles around the triple bond are kept to 180°. JDHinks 2002
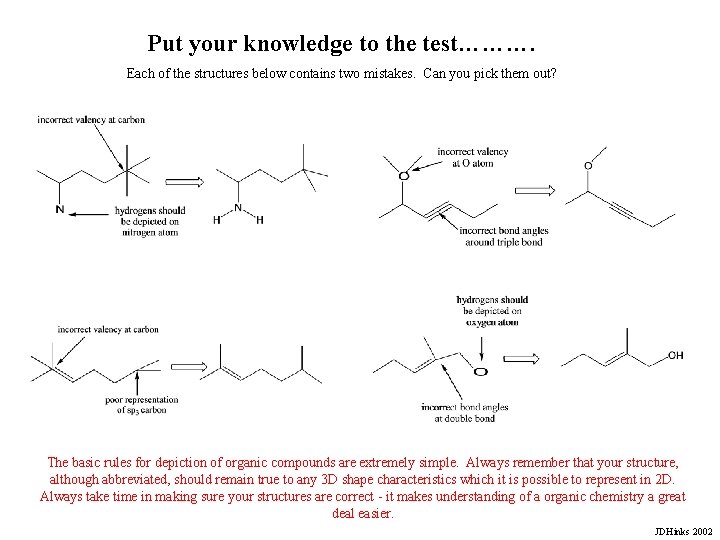
Put your knowledge to the test………. Each of the structures below contains two mistakes. Can you pick them out? The basic rules for depiction of organic compounds are extremely simple. Always remember that your structure, although abbreviated, should remain true to any 3 D shape characteristics which it is possible to represent in 2 D. Always take time in making sure your structures are correct - it makes understanding of a organic chemistry a great deal easier. JDHinks 2002
- Slides: 5