Organic Molecules and Biomolecules Organic Chemistry is the
Organic Molecules and Biomolecules
• Organic Chemistry is the chemistry of living organisms • Inorganic Chemistry is the chemistry of nonliving things • Organic molecules are defined as molecules containing both carbon and hydrogen. • Biomolecules are organic compounds found in

Carbon • Carbon only has 4 electrons in its outer shell. • To fill its outer shell to 8, carbon usually combines with C, H, N, O. P, S which are the elements that make up most of the weight of living things. • Because carbon can combine with up to 4 other elements, it makes it the perfect building block for biomolecules.

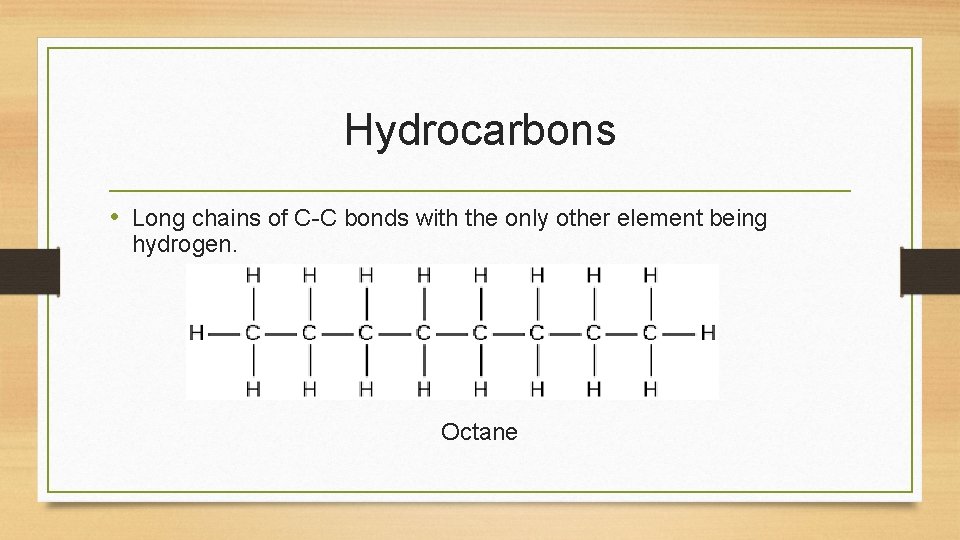
Hydrocarbons • Long chains of C-C bonds with the only other element being hydrogen. Octane
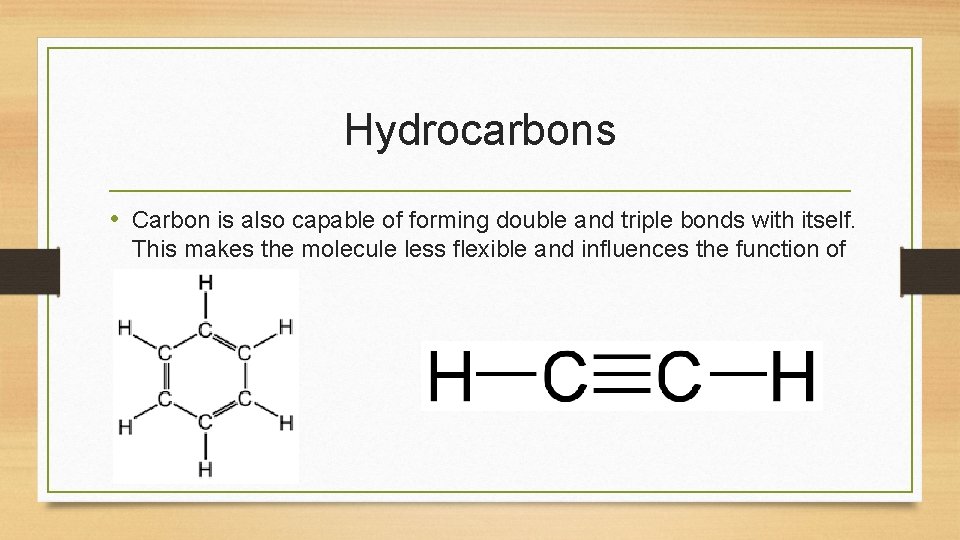
Hydrocarbons • Carbon is also capable of forming double and triple bonds with itself. This makes the molecule less flexible and influences the function of the molecule
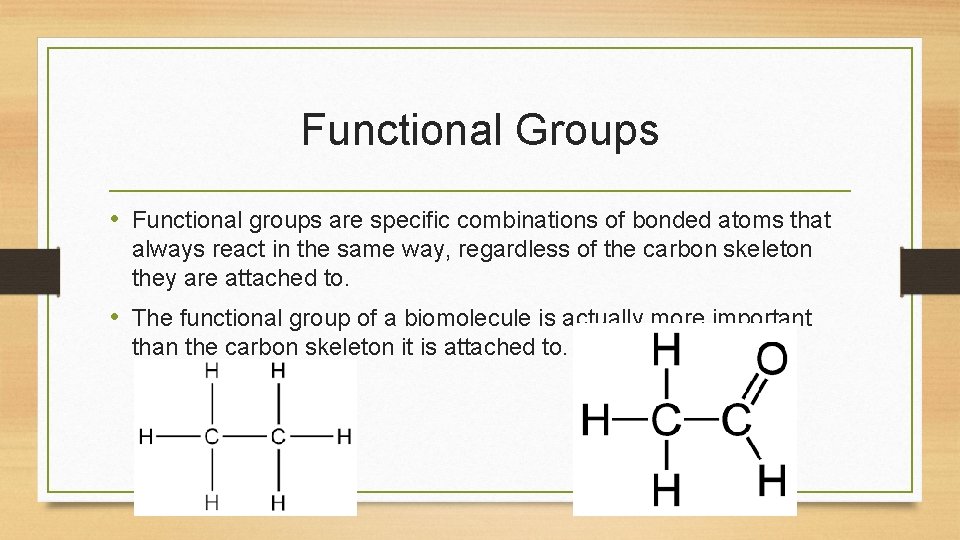
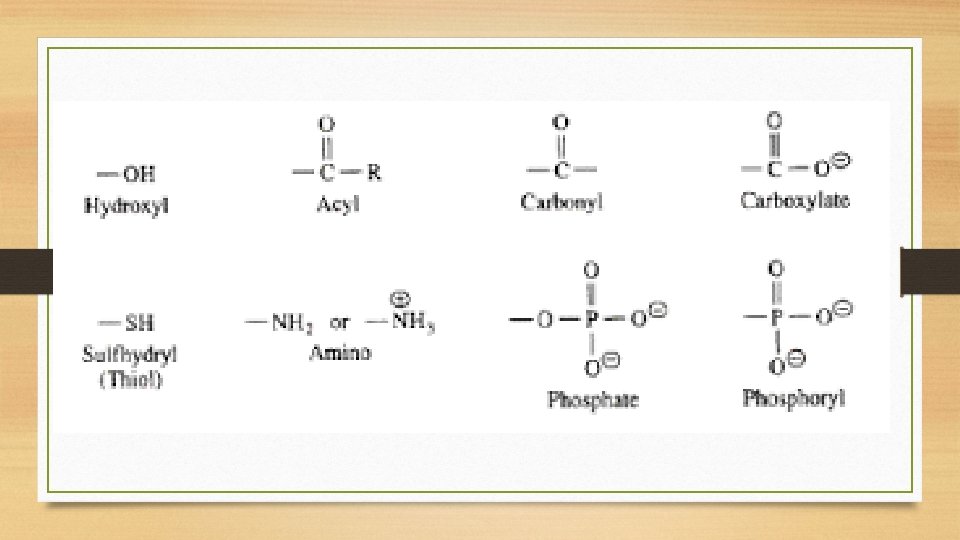
Functional Groups • Functional groups are specific combinations of bonded atoms that always react in the same way, regardless of the carbon skeleton they are attached to. • The functional group of a biomolecule is actually more important than the carbon skeleton it is attached to.
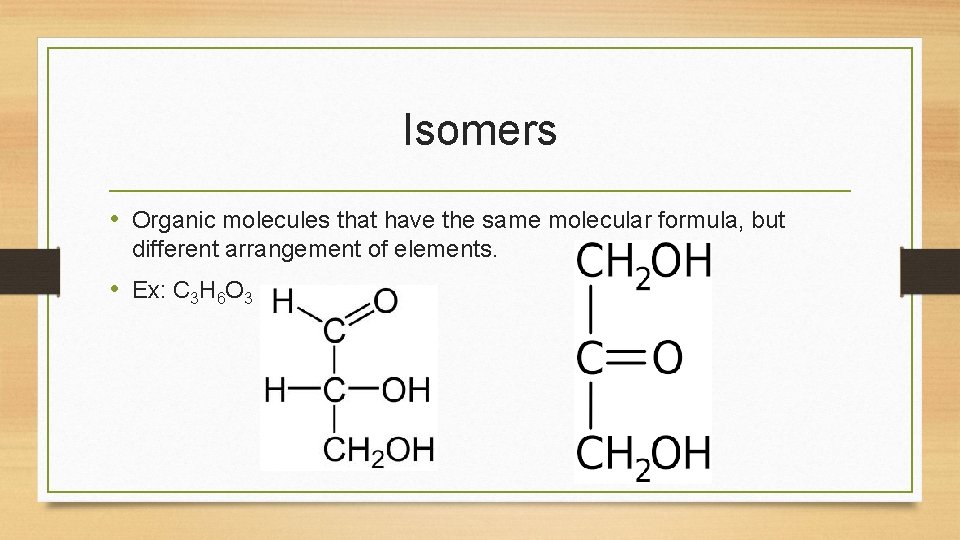
Isomers • Organic molecules that have the same molecular formula, but different arrangement of elements. • Ex: C 3 H 6 O 3

Carbohydrates • Carbohydrates are universally used as an immediate energy source and they play structural roles for a variety of organisms. • Carbohydrates have a carbon to hydrogen to oxygen ratio of 1: 2: 1 • Carbs include single sugar molecules and chains of sugars.
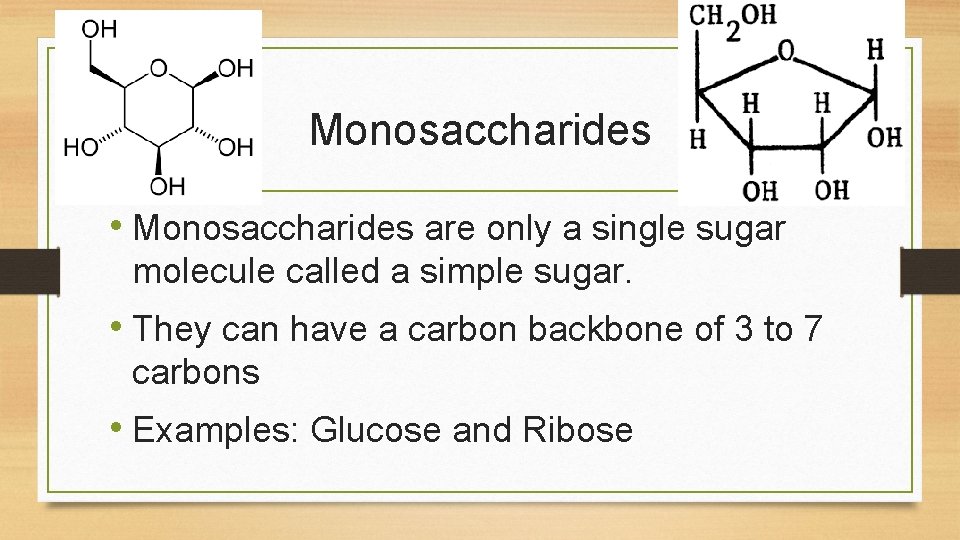
Monosaccharides • Monosaccharides are only a single sugar molecule called a simple sugar. • They can have a carbon backbone of 3 to 7 carbons • Examples: Glucose and Ribose
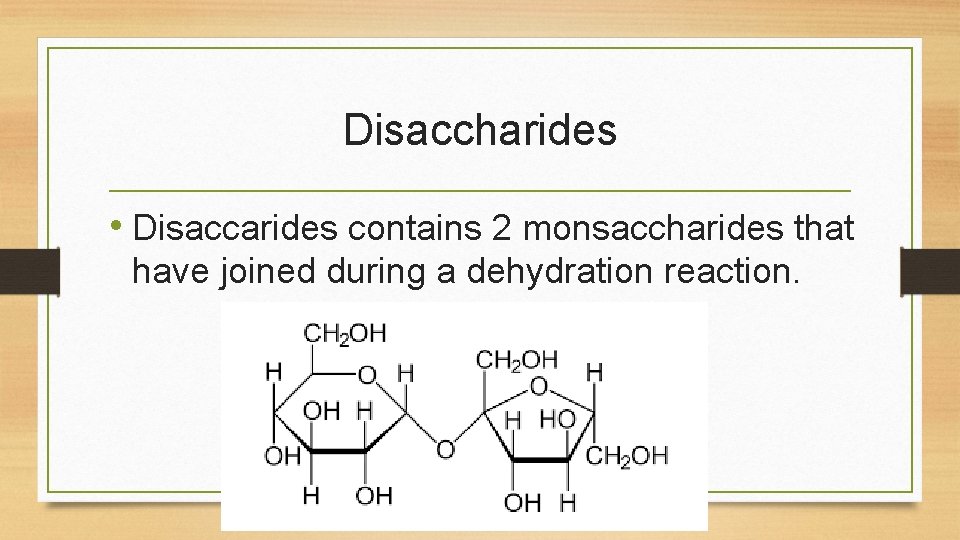
Disaccharides • Disaccarides contains 2 monsaccharides that have joined during a dehydration reaction.
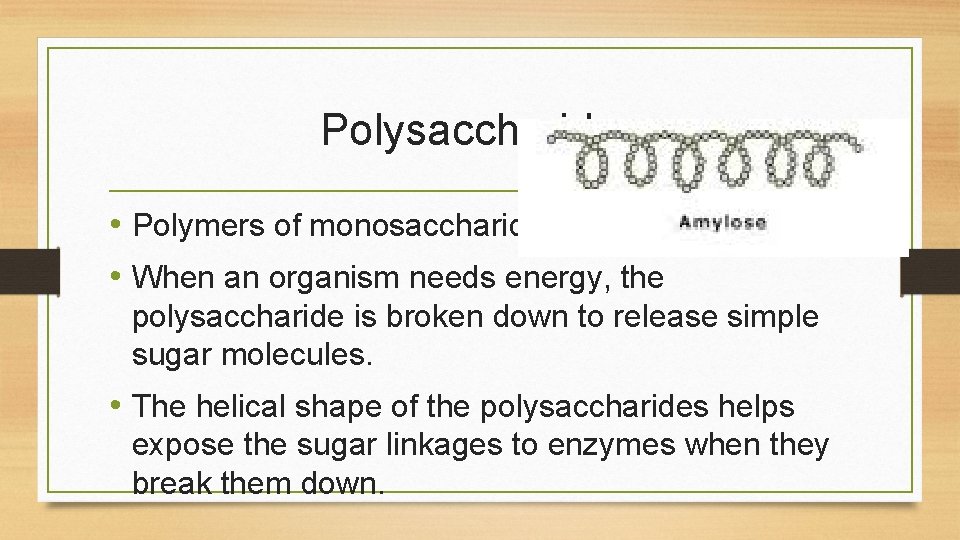
Polysaccharides • Polymers of monosaccharides. • When an organism needs energy, the polysaccharide is broken down to release simple sugar molecules. • The helical shape of the polysaccharides helps expose the sugar linkages to enzymes when they break them down.
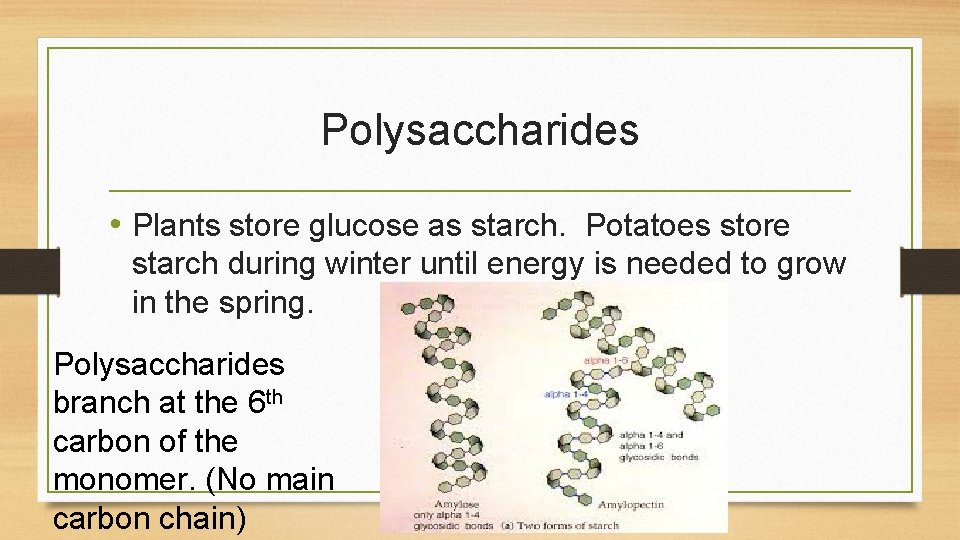
Polysaccharides • Plants store glucose as starch. Potatoes store starch during winter until energy is needed to grow in the spring. Polysaccharides branch at the 6 th carbon of the monomer. (No main carbon chain)

Polysaccharides • Animals store glucose are glycogen. Our liver cells store glycogen until energy is needed. The storage and release of glycogen is controlled by hormones.
Polysaccharides • Polysaccharides are used as storage molecules because they are not soluble in water and are much larger than simple sugars.
Polysaccharides • Polysaccharides can be used as structural molecules as well. • Ex: Cellulose, Chitin, and peptidoglycan
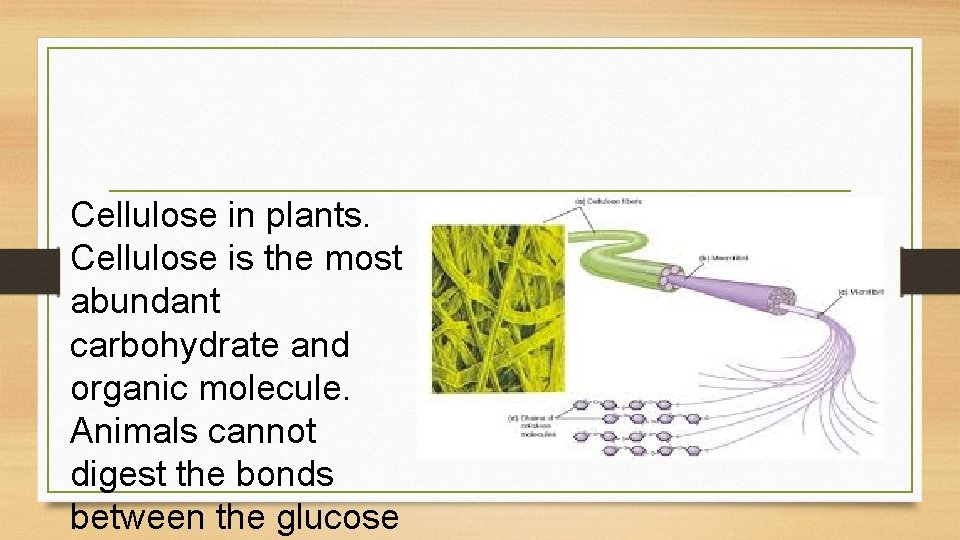
Cellulose in plants. Cellulose is the most abundant carbohydrate and organic molecule. Animals cannot digest the bonds between the glucose
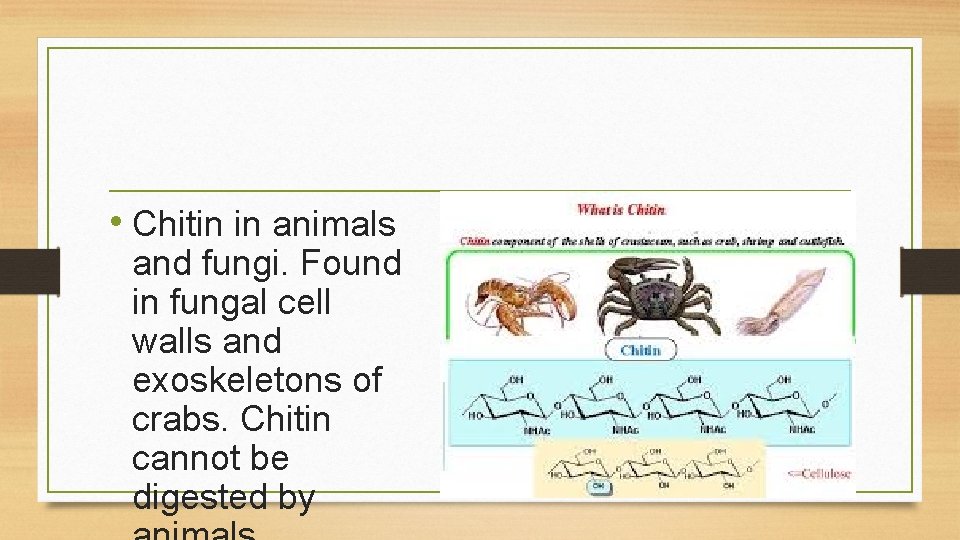
• Chitin in animals and fungi. Found in fungal cell walls and exoskeletons of crabs. Chitin cannot be digested by

Lipids • There a variety of organic compounds classified as lipids: • Fats- butter, lard • Oils – cooking oils • Phospholipids- plasma membrane • Steroids- medicines • Waxes- candles, polishes

Triglycerides • Fats and Oils contain 2 subunits: fatty acids and glycerol. • Fatty acids contain an long hydrocarbon chain and a –COOH (carboxyl functional group) Most contain 16 or 18 carbons • Fatty acids can be saturated, containing no double bonds between carbons, or unsaturated, having
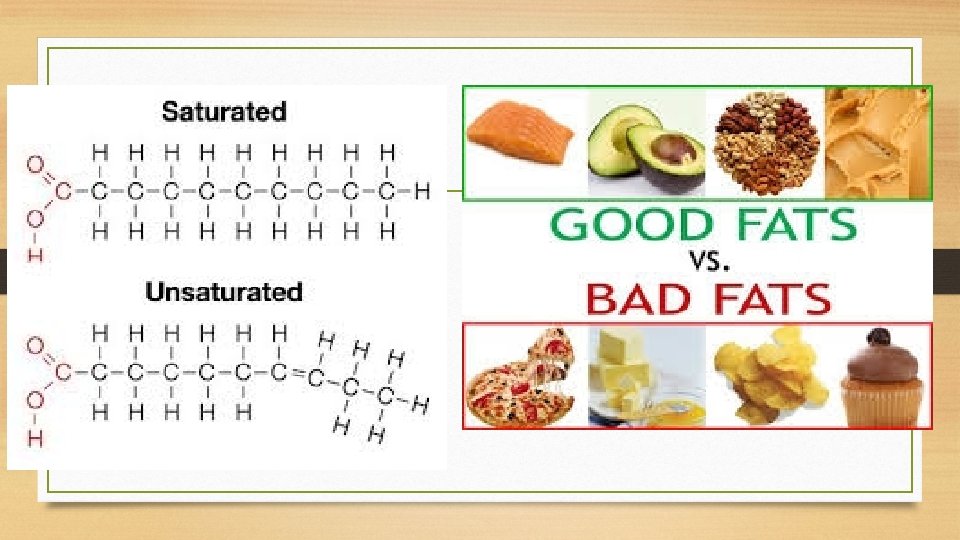

Triglycerides • Glycerol is a 3 -carbon compound with 3 –OH (hydroxyl groups) • -OH makes it polar, so therefore can mix with water.
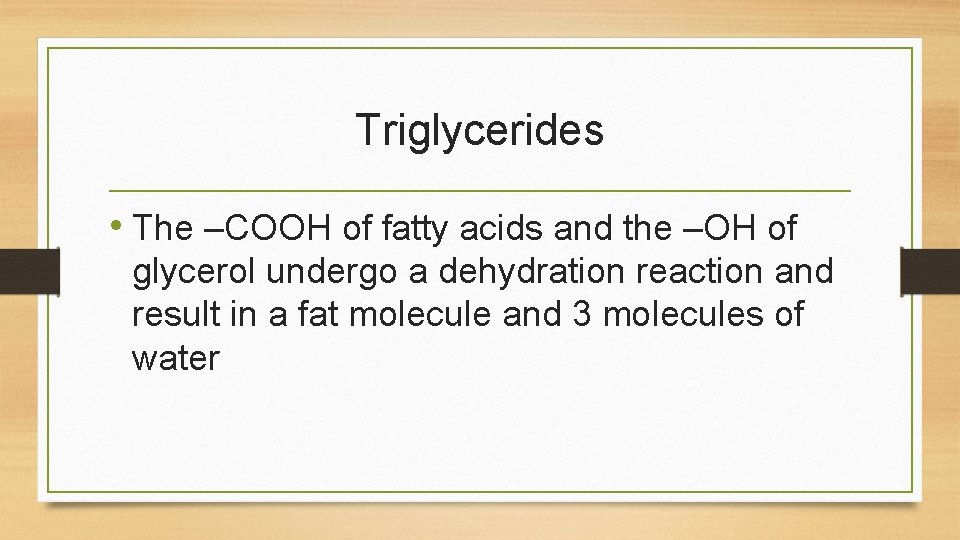
Triglycerides • The –COOH of fatty acids and the –OH of glycerol undergo a dehydration reaction and result in a fat molecule and 3 molecules of water
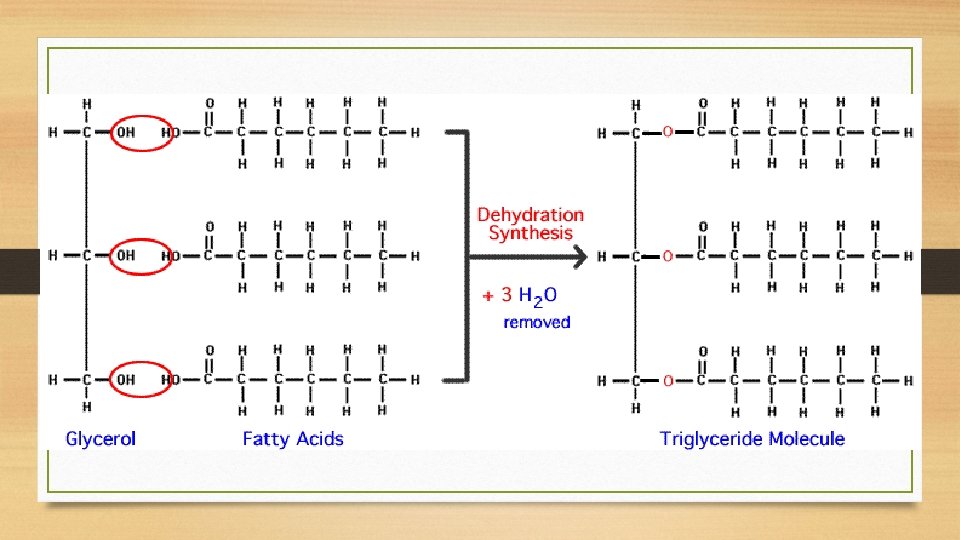

Phopholipids • Like triglycerides, but the third fatty acid contains a phosphate group. This causes the fatty acid to become polar and be the polar “head”

Steroids • Steroids are different from fats and have skeletons of 4 fused carbon rings. Each type of steroids differ based on the type of functional group attached to the carbons. • Cholesterol is a primary component to the cell membrane and is a precursor of several other steroids.
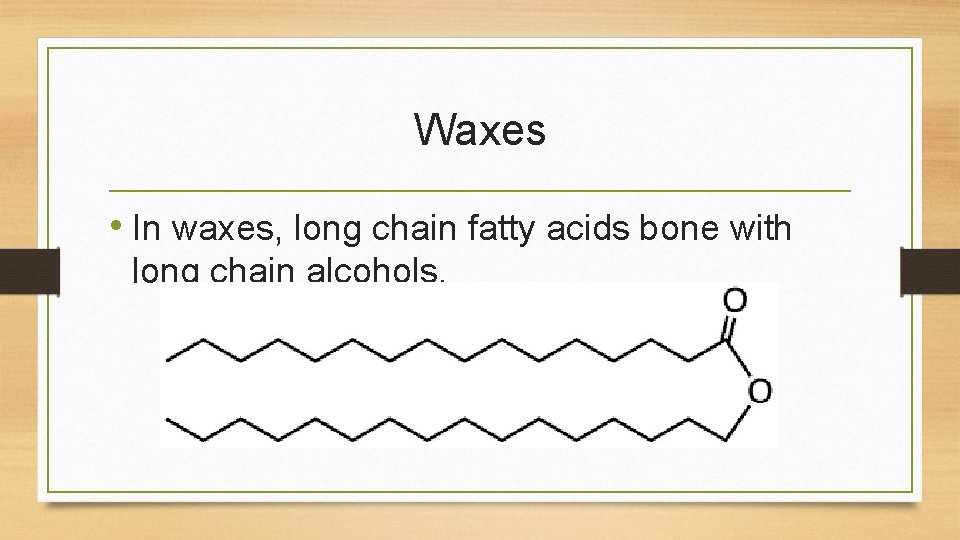
Waxes • In waxes, long chain fatty acids bone with long chain alcohols.
Proteins • Proteins are of primary importance to the structure and function of cells. • Some functions include: • Metabolism, Support, transport, defense, regulation, and motion.

Peptides
- Slides: 31