Organic Macromolecules Large Carbon based molecules Crocco What
Organic Macromolecules Large Carbon based molecules Crocco

What does it mean to be organic?
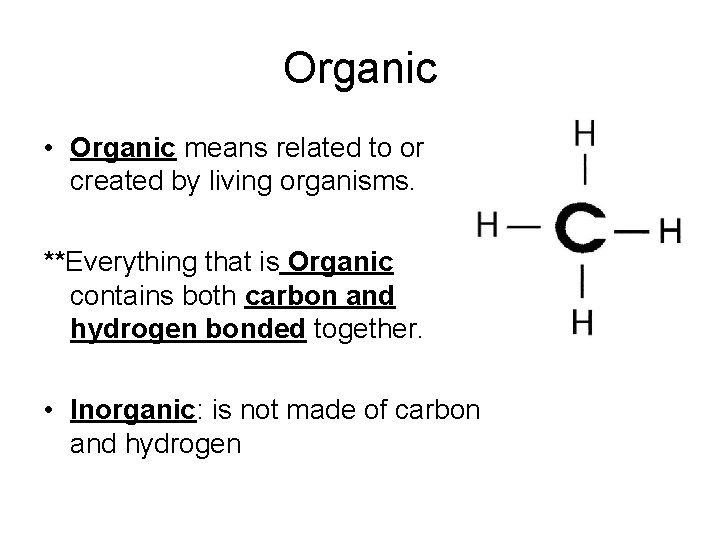
Organic • Organic means related to or created by living organisms. **Everything that is Organic contains both carbon and hydrogen bonded together. • Inorganic: is not made of carbon and hydrogen
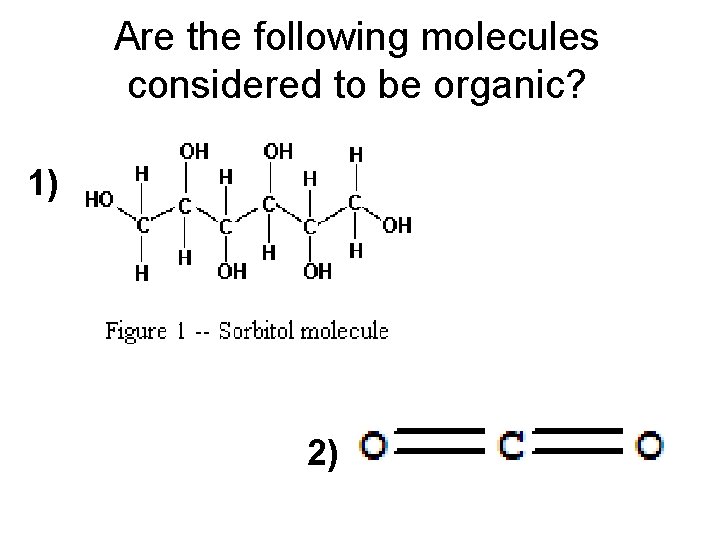
Are the following molecules considered to be organic? 1) 2)
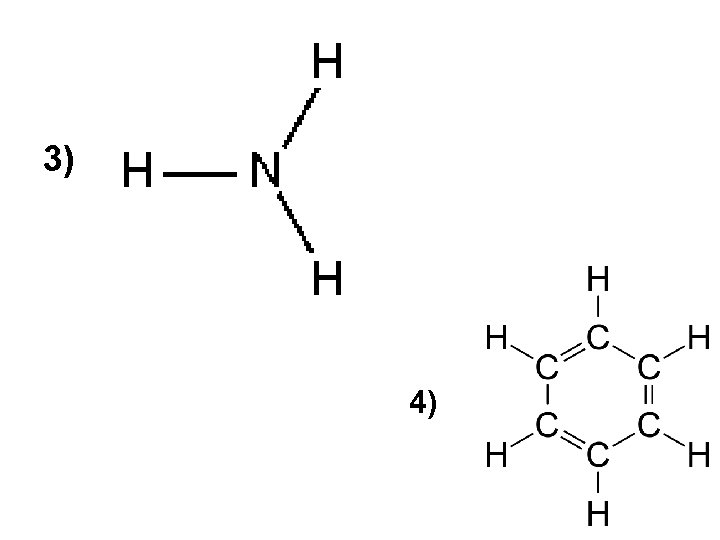
3) 4)

Is this organic or inorganic? water

organic or inorganic? • Sugar in candy

organic or inorganic? • Clouds • Mountains
organic or inorganic? • Orangutan • grass
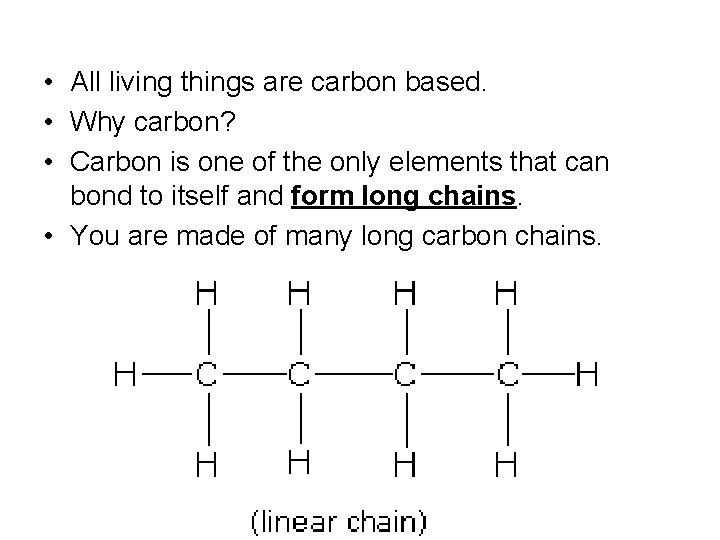
• All living things are carbon based. • Why carbon? • Carbon is one of the only elements that can bond to itself and form long chains. • You are made of many long carbon chains.
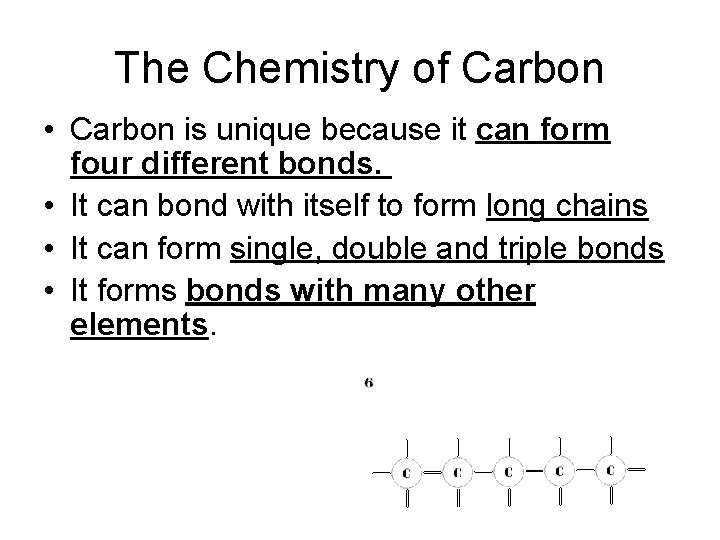
The Chemistry of Carbon • Carbon is unique because it can form four different bonds. • It can bond with itself to form long chains • It can form single, double and triple bonds • It forms bonds with many other elements.
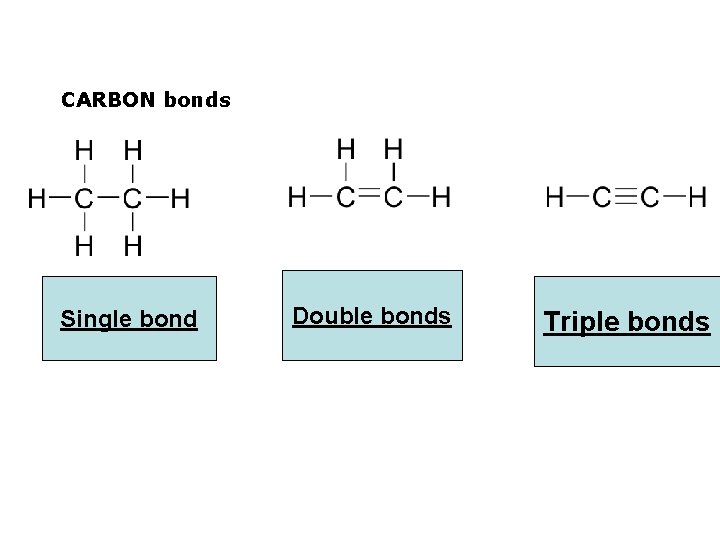
CARBON bonds Single bond Go to Section: Double bonds Triple bonds
We are going to discuss four organic macromolecules 1. carbohydrates 2. lipids 3. proteins 4. nucleic acids
• Lets begin by defining the word Macromolecule. • Macromolecules are very large molecules • Micro means small, macro mean large
How are Macromolecules formed. • Macromolecules are formed by a process called polymerization. • Polymerization: Process of joining small molecules to produce a BIG one. • The small molecules are called monomers • Mono means one
• Many monomers linked together are called a polymer. • Polymers are big so they are a type of macromolecule. – Monomer: small molecule (single unit) – Polymer: BIG molecule made of many small repeating units called monomers.

Polymerization • How are monomers combined to form a polymer? • the process is called dehydration synthesis. • Dehydration synthesis: the building of a polymer by joining two or more monomers and removing one water molecule • Dehydration: removing water (thirsty) • Synthesis: to build.
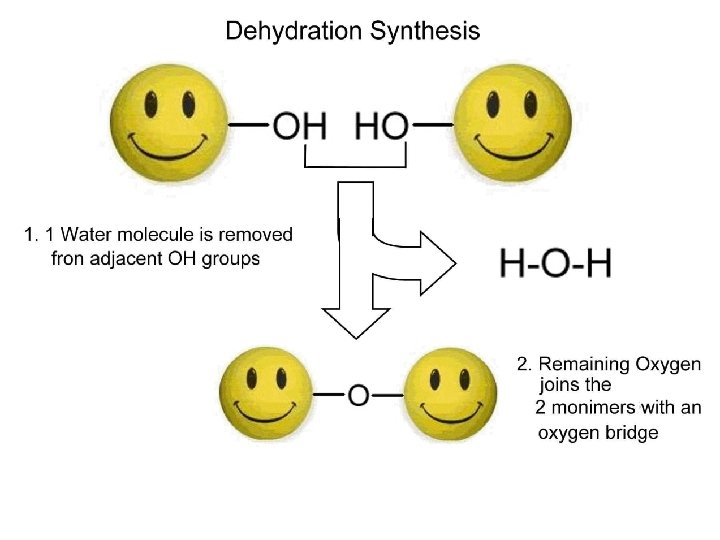
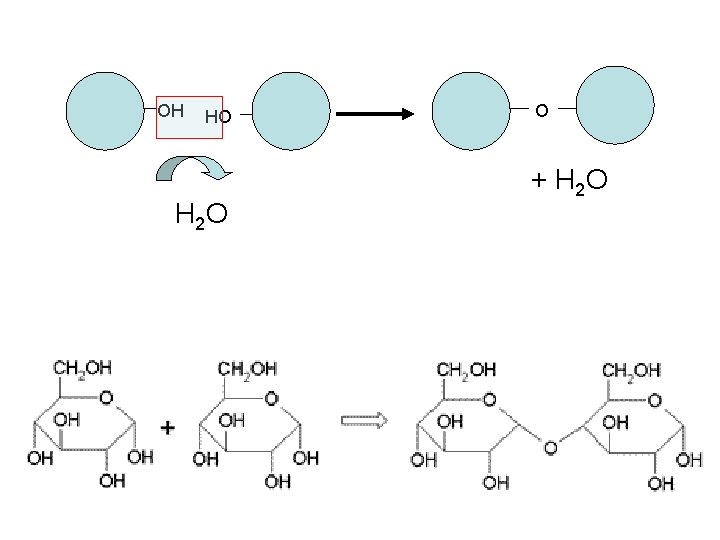
OH HO H 2 O O + H 2 O
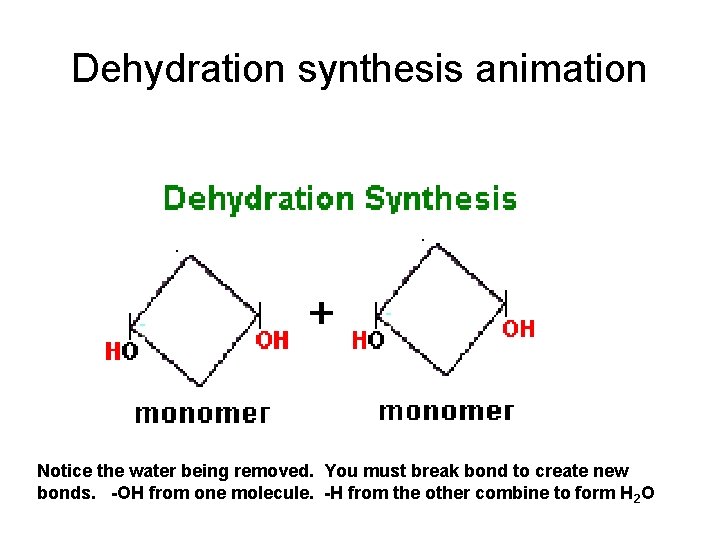
Dehydration synthesis animation Notice the water being removed. You must break bond to create new bonds. -OH from one molecule. -H from the other combine to form H 2 O
Protein synthesis is an example of dehydration synthesis. This is how your body build all of the proteins your body needs including your hair and skin pigments. Photosynthesis is another. Large sugar chains are built using the sun and carbon dioxide.
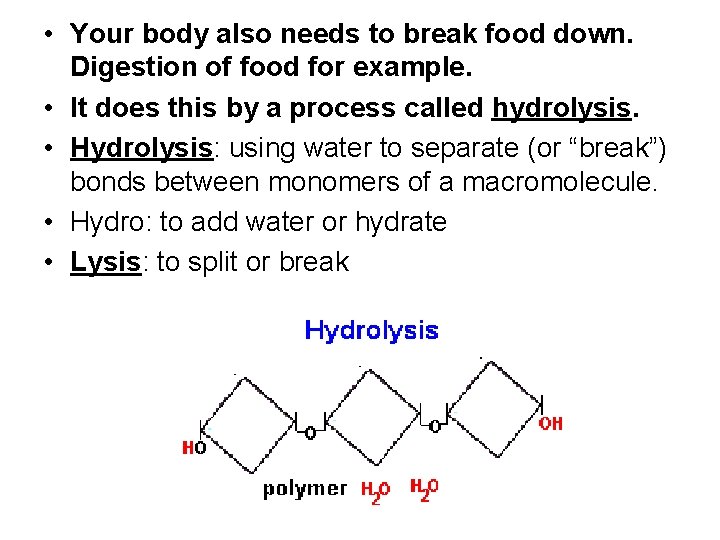
• Your body also needs to break food down. Digestion of food for example. • It does this by a process called hydrolysis. • Hydrolysis: using water to separate (or “break”) bonds between monomers of a macromolecule. • Hydro: to add water or hydrate • Lysis: to split or break

The four organic macromolecules of the living things. • Organic: C-H bond • Macromolecules: large molecules 1. carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids
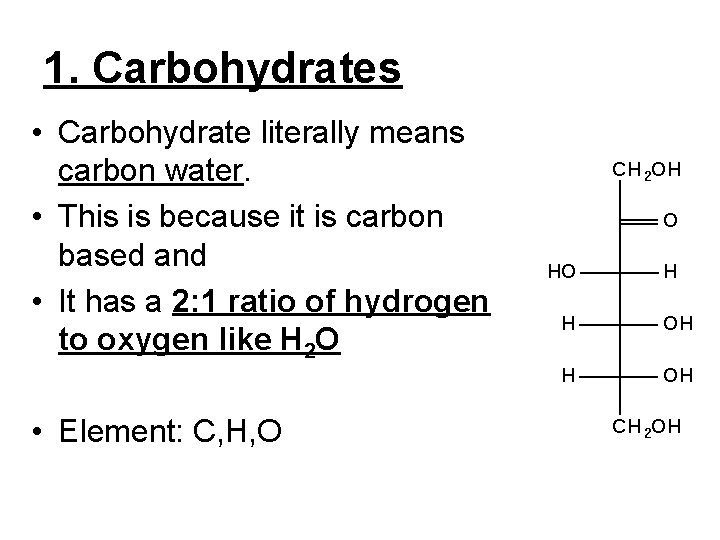
1. Carbohydrates • Carbohydrate literally means carbon water. • This is because it is carbon based and • It has a 2: 1 ratio of hydrogen to oxygen like H 2 O • Element: C, H, O
Functions of carbohydrates • Main energy source • Structural component: cell walls • receptors in the cell membrane: communication
• Each carbohydrate is a macromolecule and a polymer • They are made of monomers formed by polymerization (dehydration synthesis). • Monomers of carbohydrates are called monosaccharides or simple sugars. – Monosaccharides means one sugar: – Examples of monosaccharides are Glucose, fructose, galactose; chemical formula – Monosaccharide chemical formula= C 6 H 12 O 6
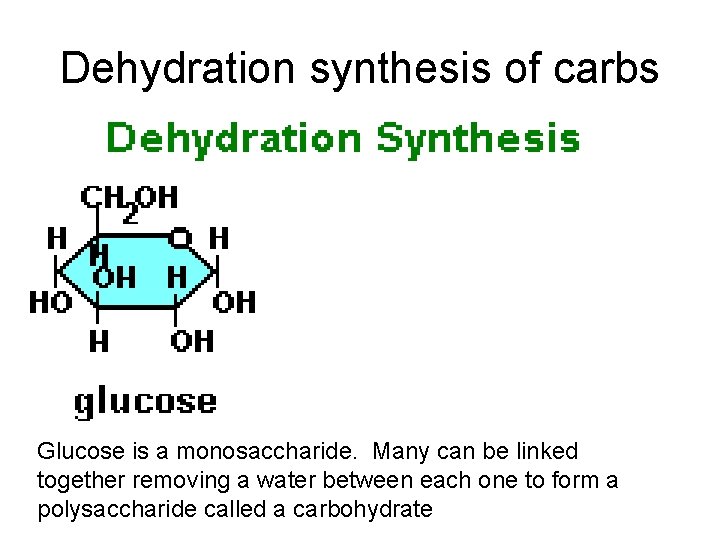
Dehydration synthesis of carbs Glucose is a monosaccharide. Many can be linked together removing a water between each one to form a polysaccharide called a carbohydrate

• Carbohydrates are called polymers. They come in two forms: 1. Disaccharides: two monomers: ex: sucrose, maltose 2. Polysaccharides: many monomers: ex: starch, glycogen, cellulose • http: //bcs. whfreeman. com/thelifewire/ content/chp 03/0302002. html
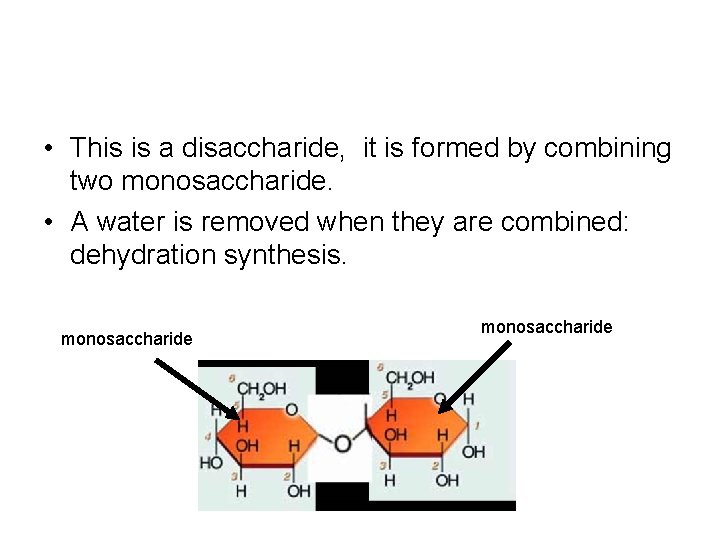
• This is a disaccharide, it is formed by combining two monosaccharide. • A water is removed when they are combined: dehydration synthesis. monosaccharide
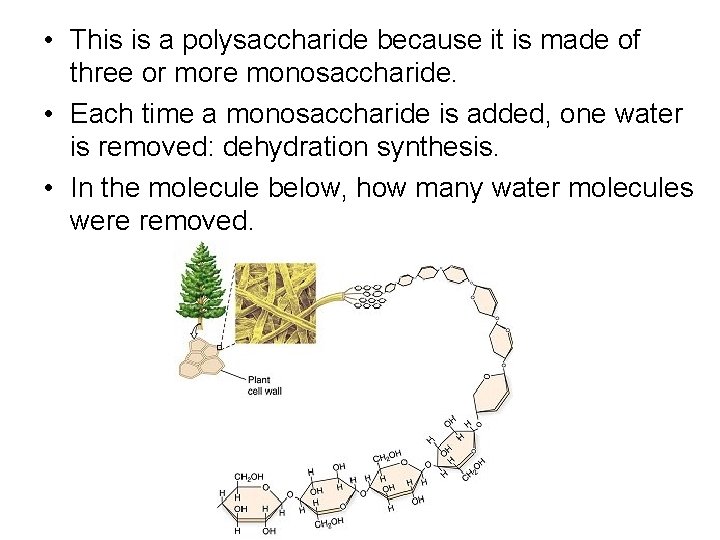
• This is a polysaccharide because it is made of three or more monosaccharide. • Each time a monosaccharide is added, one water is removed: dehydration synthesis. • In the molecule below, how many water molecules were removed.

2. Lipids • Elements of lipids: C, H, O • (Mostly C & H); no ratio between H and O • Examples: Fats, Waxes and Oils
Function of lipids – Stored energy: extra calories are stores as fat in the body. – Structural components (cell membrane)
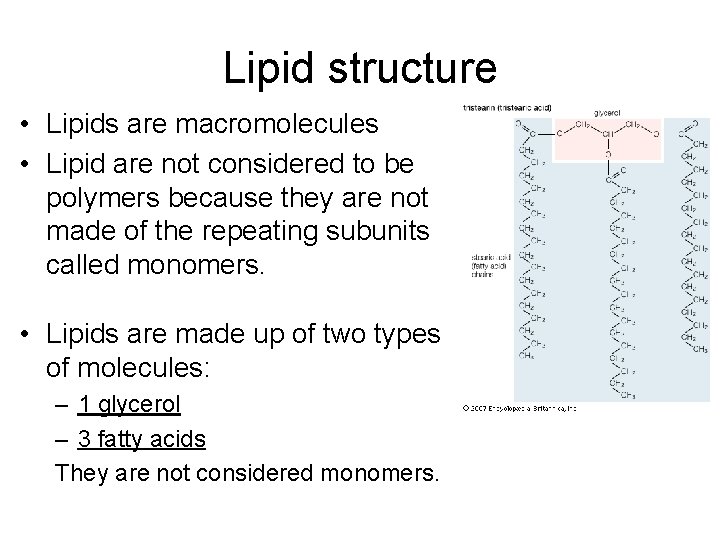
Lipid structure • Lipids are macromolecules • Lipid are not considered to be polymers because they are not made of the repeating subunits called monomers. • Lipids are made up of two types of molecules: – 1 glycerol – 3 fatty acids They are not considered monomers.

Saturated vs. unsaturated Two types of lipids – Saturated: the fatty acid parts has the maximum number of hydrogen because there are no double bonds. – Unsaturated: contains one or more carbon to carbon double bonds so it has less hydrogen bonded. C=C
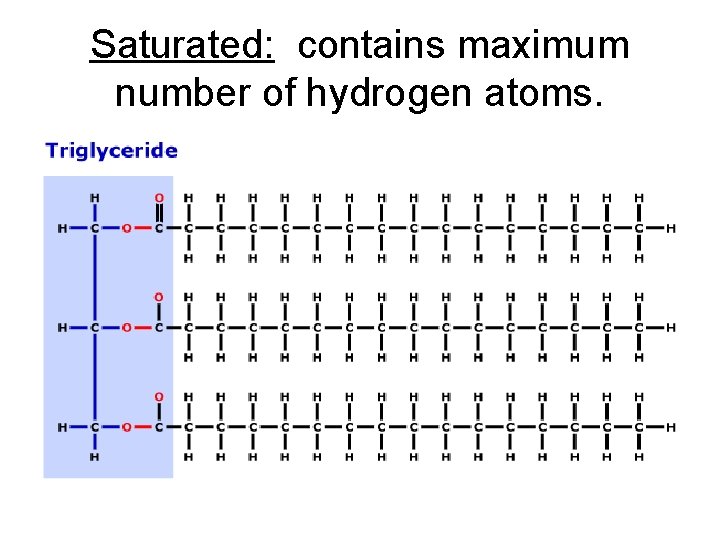
Saturated: contains maximum number of hydrogen atoms.
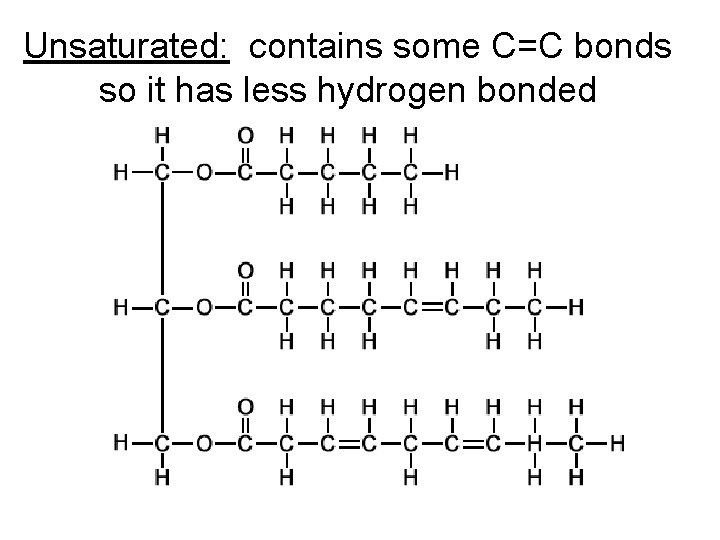
Unsaturated: contains some C=C bonds so it has less hydrogen bonded
3. Proteins • Elements of proteins: C, H, O, N Proteins are macromolecules and polymers. Monomers or building blocks of proteins are called amino acids. Many amino acids are linked together to form each protein
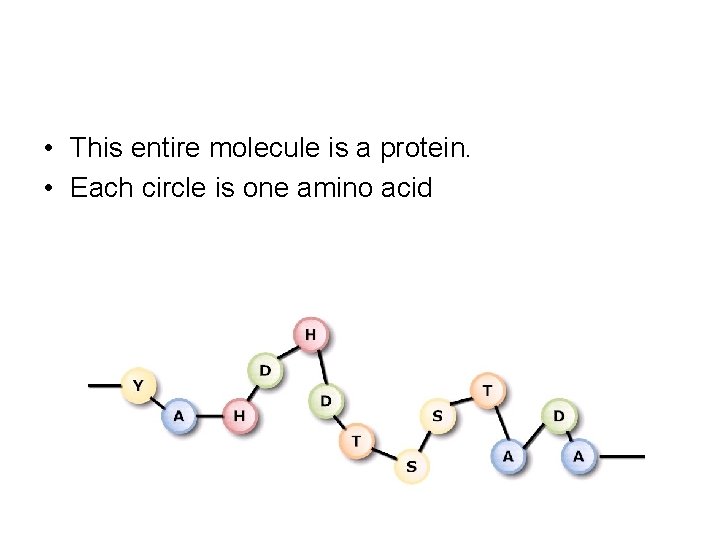
• This entire molecule is a protein. • Each circle is one amino acid
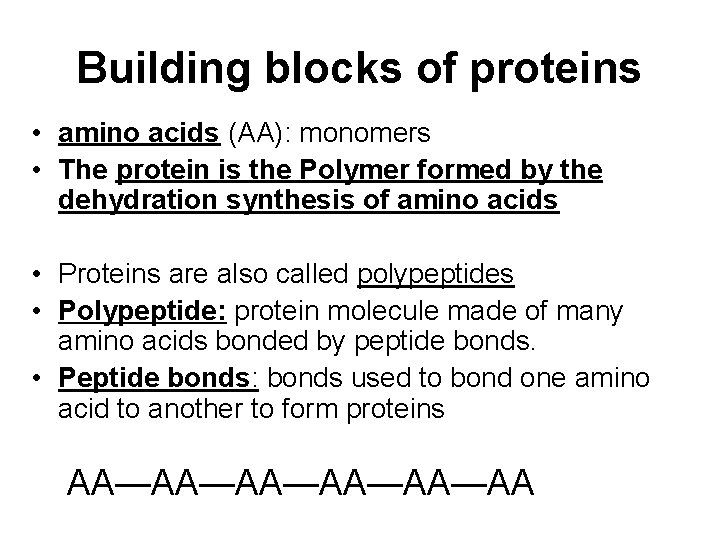
Building blocks of proteins • amino acids (AA): monomers • The protein is the Polymer formed by the dehydration synthesis of amino acids • Proteins are also called polypeptides • Polypeptide: protein molecule made of many amino acids bonded by peptide bonds. • Peptide bonds: bonds used to bond one amino acid to another to form proteins AA—AA—AA—AA
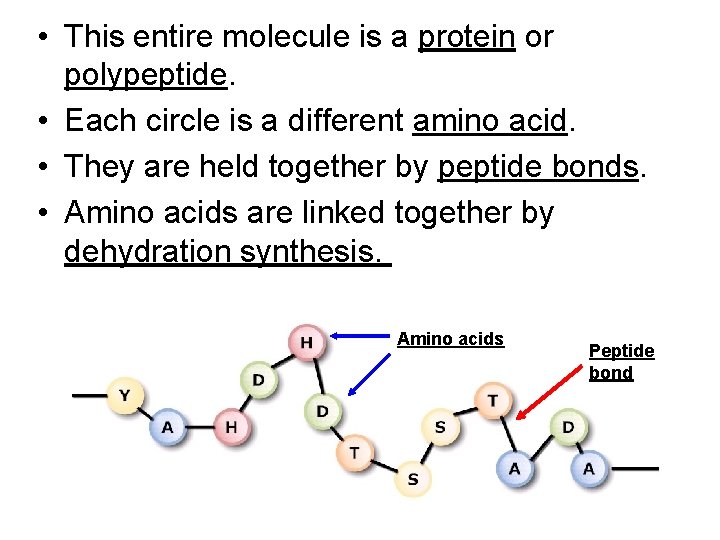
• This entire molecule is a protein or polypeptide. • Each circle is a different amino acid. • They are held together by peptide bonds. • Amino acids are linked together by dehydration synthesis. Amino acids Peptide bond
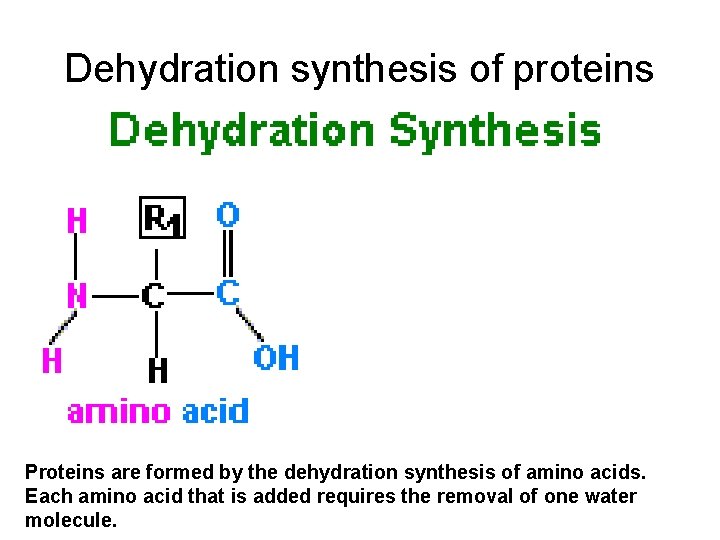
Dehydration synthesis of proteins Proteins are formed by the dehydration synthesis of amino acids. Each amino acid that is added requires the removal of one water molecule.
• Functions of Proteins (polypeptides) – Control rate of reactions such as enzymes – Building materials: muscles, pigments, cell membrane – Fight disease (antibodies)
Structure of amino acid • All of the 22 amino acids have the same basic structure. • What differs is the R group. There are 22 different R groups.
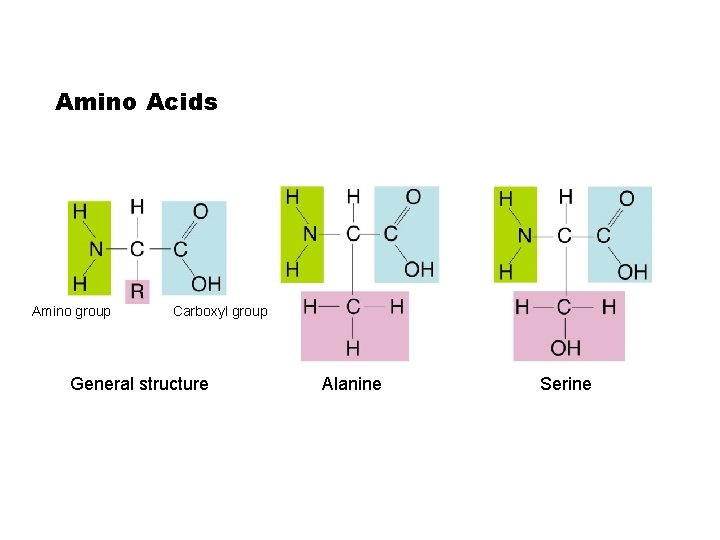
Amino Acids Amino group Carboxyl group General structure Go to Section: Alanine Serine

What makes one protein different from another? 1. Number of amino acids: some have two amino acids, some have thousands. 2. Types of amino acids: 22 different amino acids in all. . 3. Order of amino acids: many combinations of the 22 amino acids If any one of these factors changes, then the resulting protein changes too!!!!

• The following three polypeptides would all produce different proteins: – A. His – Ser – Phe – Val – B. Ala – Gly – Phe – Leu – Tyro – C. Ala – Gly – Phe – Leu – Ala Examples of proteins include: insulin, hemoglobin, melanin and all enzymes http: //bcs. whfreeman. com/thelifewire/content/chp 03/030 2002. html
Enzymes • Enzymes are organic catalysts that control chemical reactions in the living organisms • They either speed up or slow down the Rate of the reaction. • The reaction would happen anyway but enzymes make them happen quicker. • When your body builds proteins, specific enzymes help. • When your body digest foods specific enzymes help.
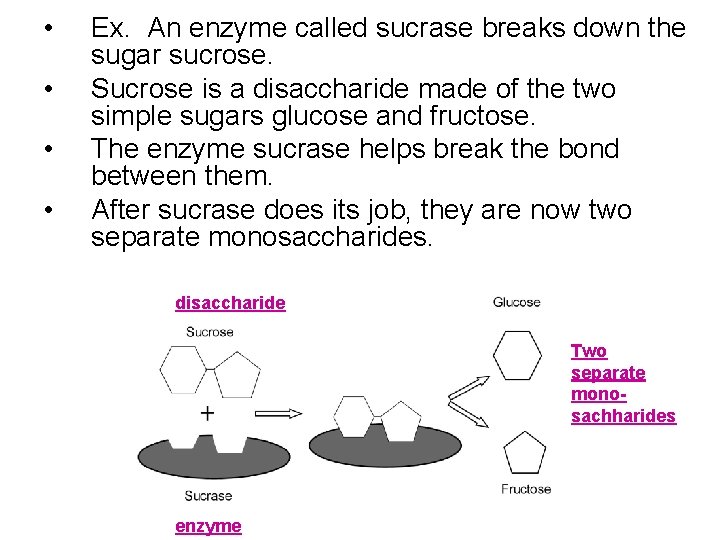
• • Ex. An enzyme called sucrase breaks down the sugar sucrose. Sucrose is a disaccharide made of the two simple sugars glucose and fructose. The enzyme sucrase helps break the bond between them. After sucrase does its job, they are now two separate monosaccharides. disaccharide Two separate monosachharides enzyme
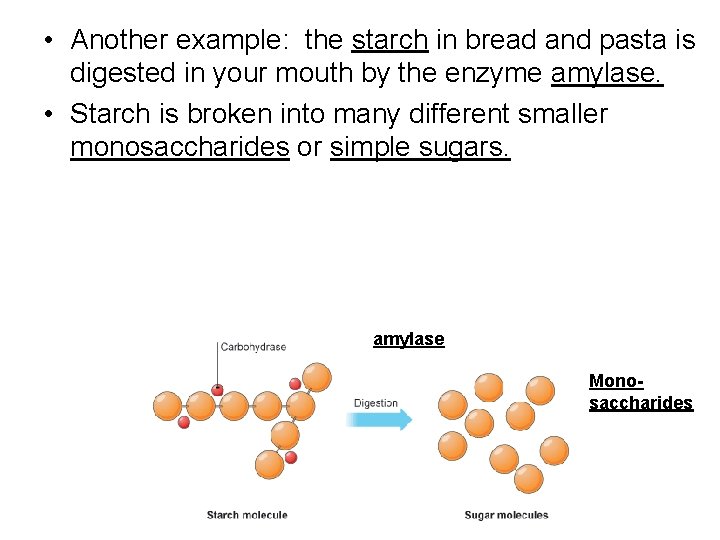
• Another example: the starch in bread and pasta is digested in your mouth by the enzyme amylase. • Starch is broken into many different smaller monosaccharides or simple sugars. amylase Monosaccharides

• Notice that enzymes always end with the suffix “-ase” • Example: sucrase and amylase • Sugars always end in “-ose” • Example: sucrose

• Remember an enzyme is a catalyst – Catalyst – a substance that speeds up the rate of a reaction. – Enzymes work by lowering a reaction’s activation energy – Activation Energy- the energy that is needed to get a reaction started – Enzymes help to conserve energy by lowering the activation energy.
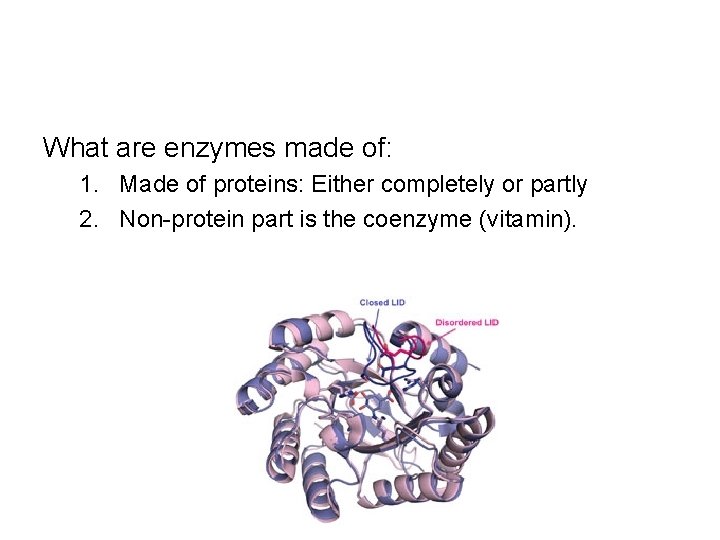
What are enzymes made of: 1. Made of proteins: Either completely or partly 2. Non-protein part is the coenzyme (vitamin).
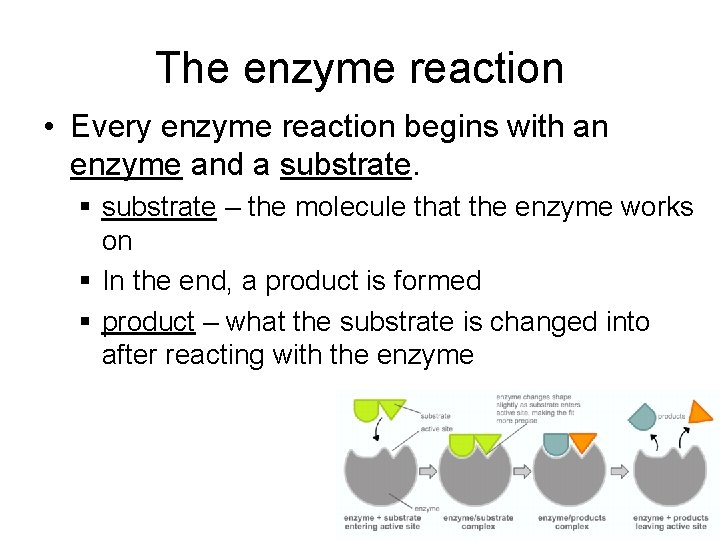
The enzyme reaction • Every enzyme reaction begins with an enzyme and a substrate. § substrate – the molecule that the enzyme works on § In the end, a product is formed § product – what the substrate is changed into after reacting with the enzyme
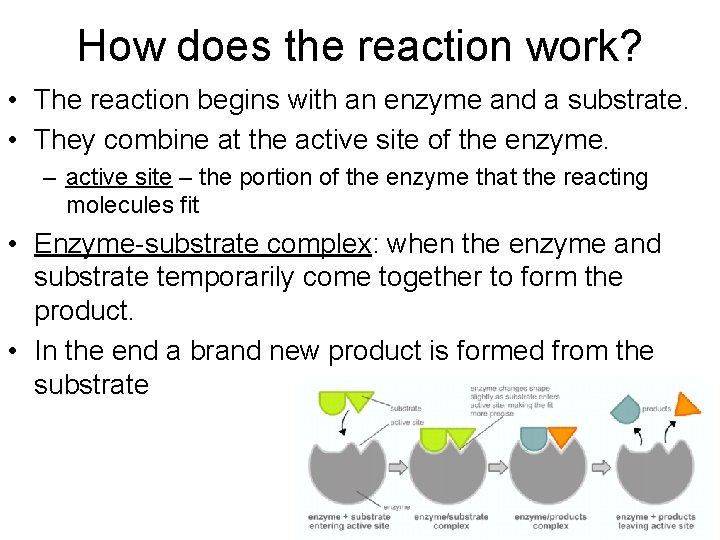
How does the reaction work? • The reaction begins with an enzyme and a substrate. • They combine at the active site of the enzyme. – active site – the portion of the enzyme that the reacting molecules fit • Enzyme-substrate complex: when the enzyme and substrate temporarily come together to form the product. • In the end a brand new product is formed from the substrate
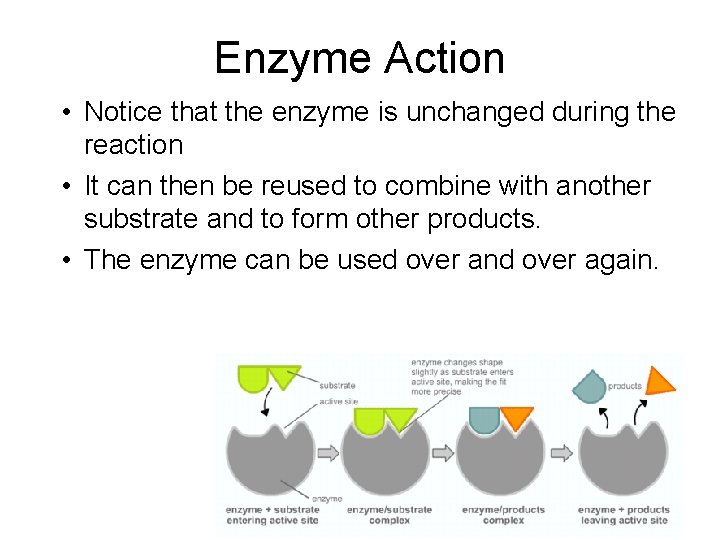
Enzyme Action • Notice that the enzyme is unchanged during the reaction • It can then be reused to combine with another substrate and to form other products. • The enzyme can be used over and over again.
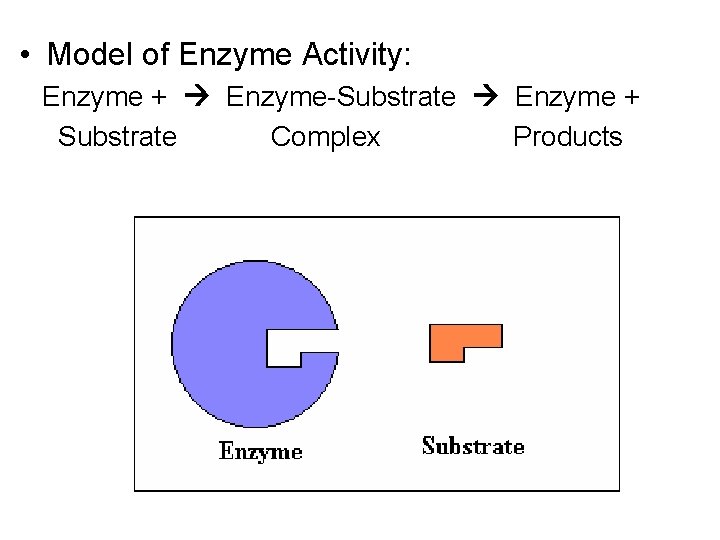
• Model of Enzyme Activity: Enzyme + Enzyme-Substrate Enzyme + Substrate Complex Products

• Enzymes are highly specific! This means that they do not react with just any substrates. They react with specific substrates. Ex: Sucrase + sucrose glucose + fructose (enzyme) + (substrate) (Product) • The enzyme sucrase will only break down the sugar sucrose. • It is specific to sucrose and will not work with any other substrate. • Other substrates will not fit the active site of sucrase.
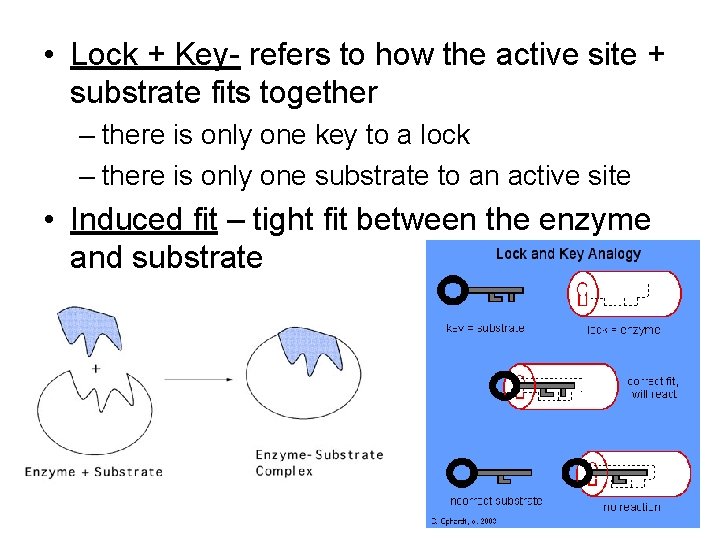
• Lock + Key- refers to how the active site + substrate fits together – there is only one key to a lock – there is only one substrate to an active site • Induced fit – tight fit between the enzyme and substrate
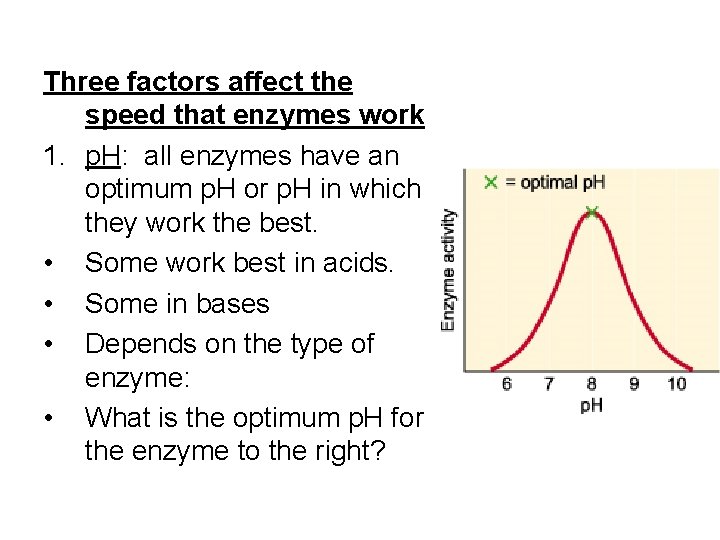
Three factors affect the speed that enzymes work 1. p. H: all enzymes have an optimum p. H or p. H in which they work the best. • Some work best in acids. • Some in bases • Depends on the type of enzyme: • What is the optimum p. H for the enzyme to the right?
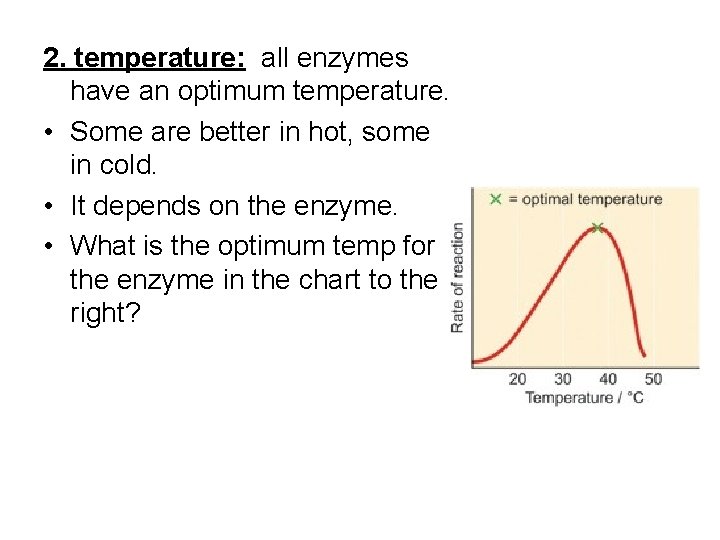
2. temperature: all enzymes have an optimum temperature. • Some are better in hot, some in cold. • It depends on the enzyme. • What is the optimum temp for the enzyme in the chart to the right?
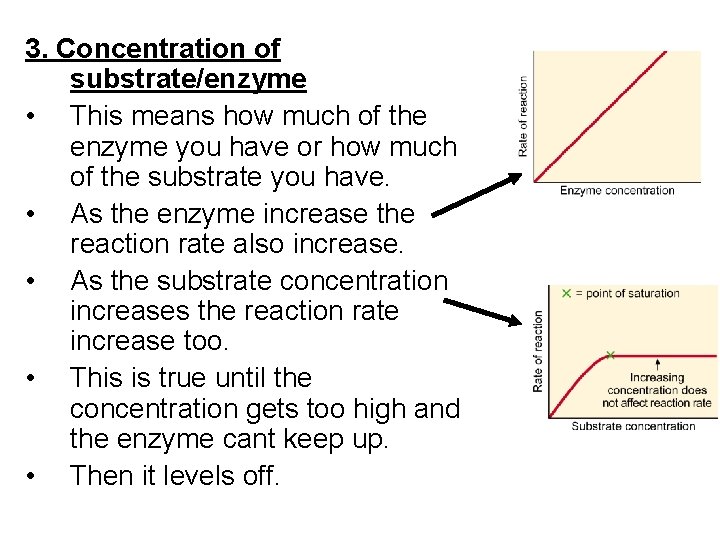
3. Concentration of substrate/enzyme • This means how much of the enzyme you have or how much of the substrate you have. • As the enzyme increase the reaction rate also increase. • As the substrate concentration increases the reaction rate increase too. • This is true until the concentration gets too high and the enzyme cant keep up. • Then it levels off.
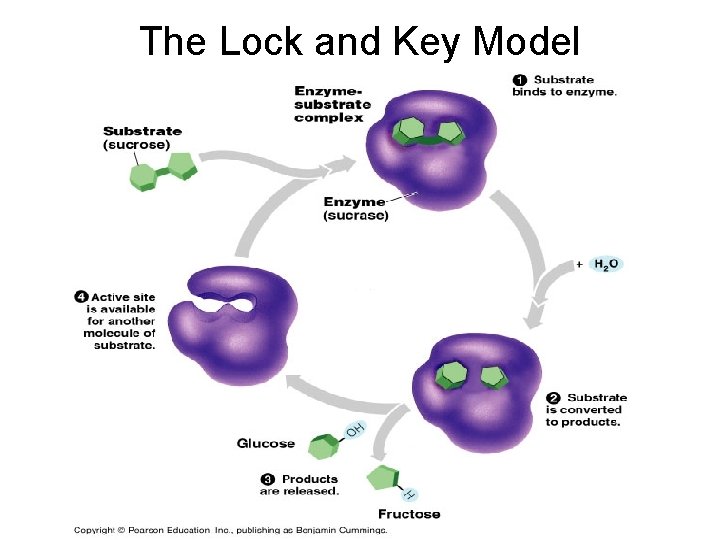
The Lock and Key Model
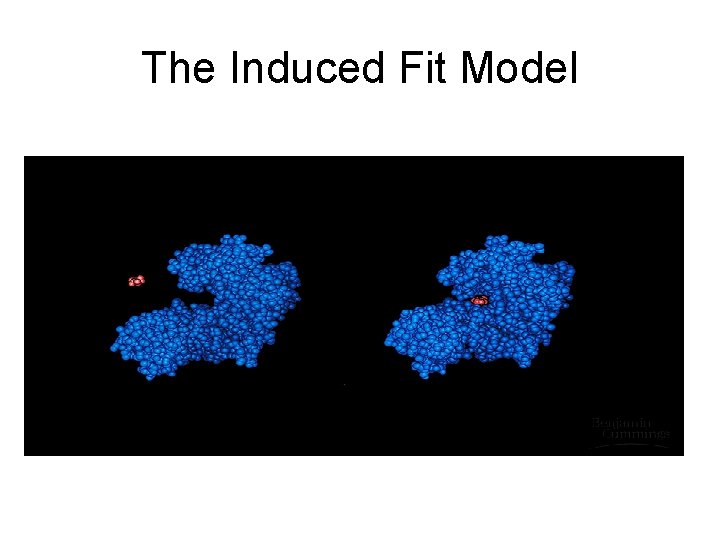
The Induced Fit Model

Enzyme video • http: //highered. mcgrawhill. com/sites/0072495855/student_view 0/ chapter 2/animation__how_enzymes_work. html • http: //bcs. whfreeman. com/thelifewire/conte nt/chp 06/0602001. html
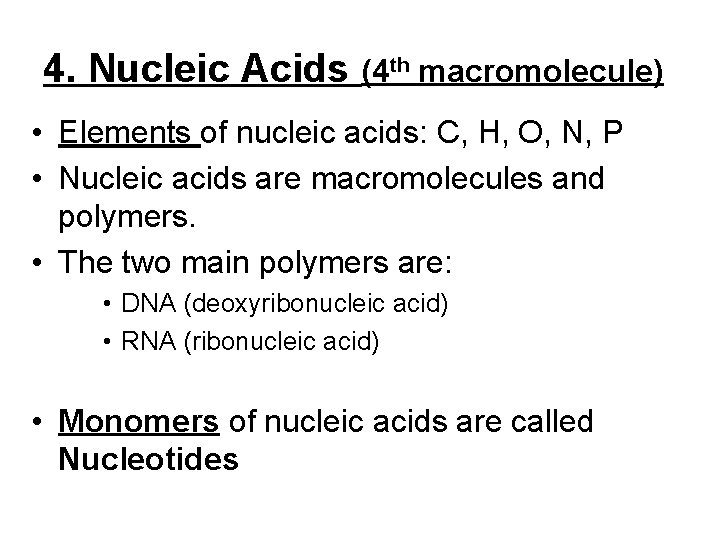
4. Nucleic Acids (4 th macromolecule) • Elements of nucleic acids: C, H, O, N, P • Nucleic acids are macromolecules and polymers. • The two main polymers are: • DNA (deoxyribonucleic acid) • RNA (ribonucleic acid) • Monomers of nucleic acids are called Nucleotides
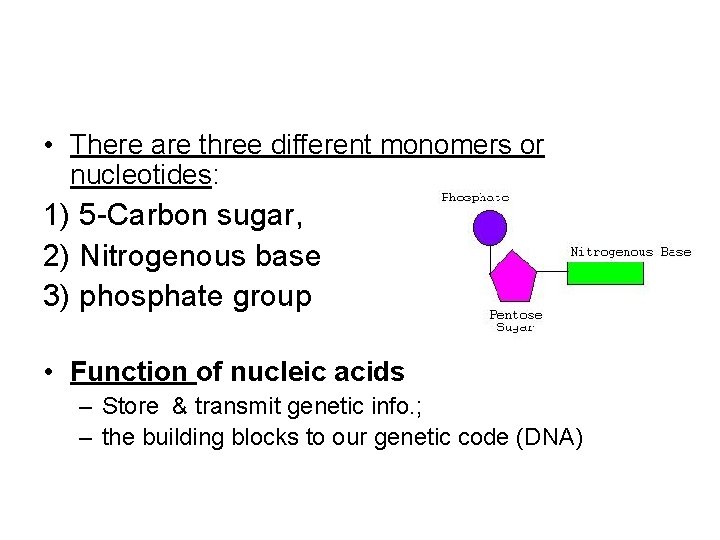
• There are three different monomers or nucleotides: 1) 5 -Carbon sugar, 2) Nitrogenous base 3) phosphate group • Function of nucleic acids – Store & transmit genetic info. ; – the building blocks to our genetic code (DNA)
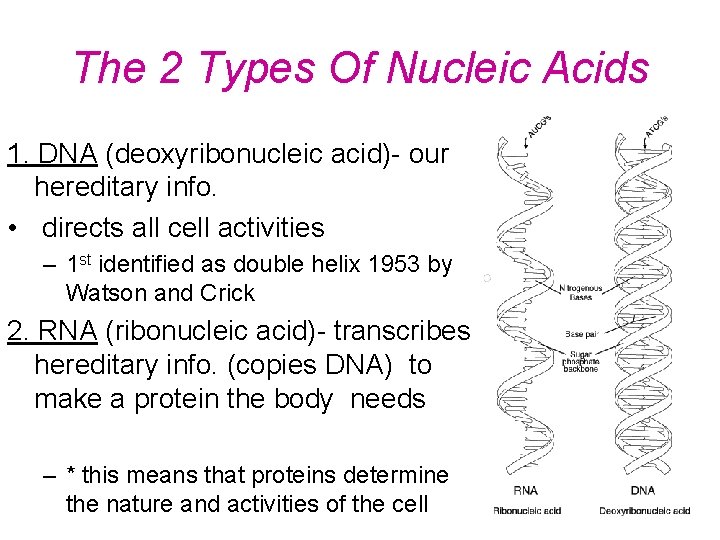
The 2 Types Of Nucleic Acids 1. DNA (deoxyribonucleic acid)- our hereditary info. • directs all cell activities – 1 st identified as double helix 1953 by Watson and Crick 2. RNA (ribonucleic acid)- transcribes hereditary info. (copies DNA) to make a protein the body needs – * this means that proteins determine the nature and activities of the cell
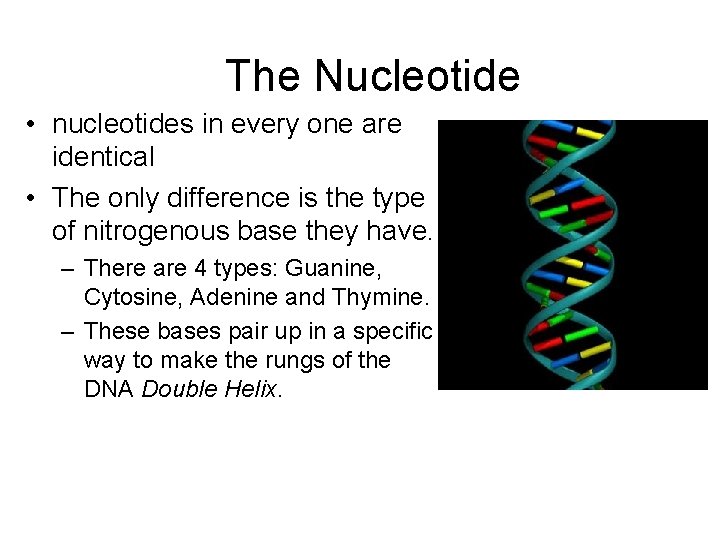
The Nucleotide • nucleotides in every one are identical • The only difference is the type of nitrogenous base they have. – There are 4 types: Guanine, Cytosine, Adenine and Thymine. – These bases pair up in a specific way to make the rungs of the DNA Double Helix.
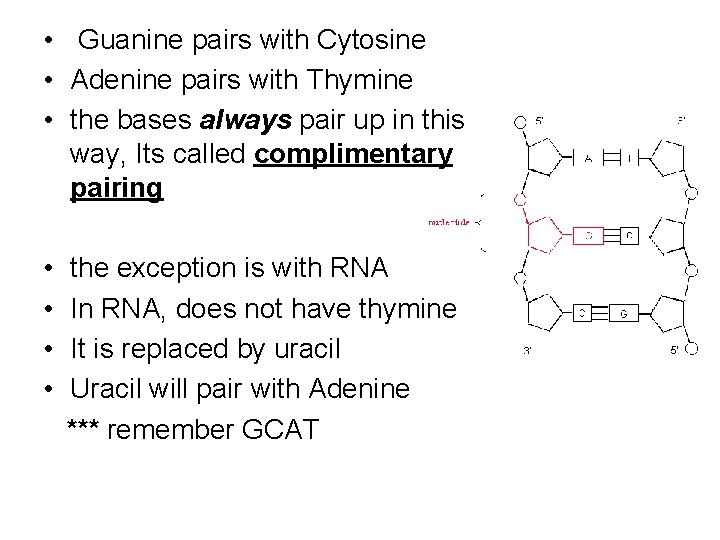
• Guanine pairs with Cytosine • Adenine pairs with Thymine • the bases always pair up in this way, Its called complimentary pairing • • the exception is with RNA In RNA, does not have thymine It is replaced by uracil Uracil will pair with Adenine *** remember GCAT

• http: //www. teachertube. com/view. Video. ph p? video_id=43705&title=Nucliec_Acids • http: //bcs. whfreeman. com/thelifewire/conte nt/chp 03/0302002. html
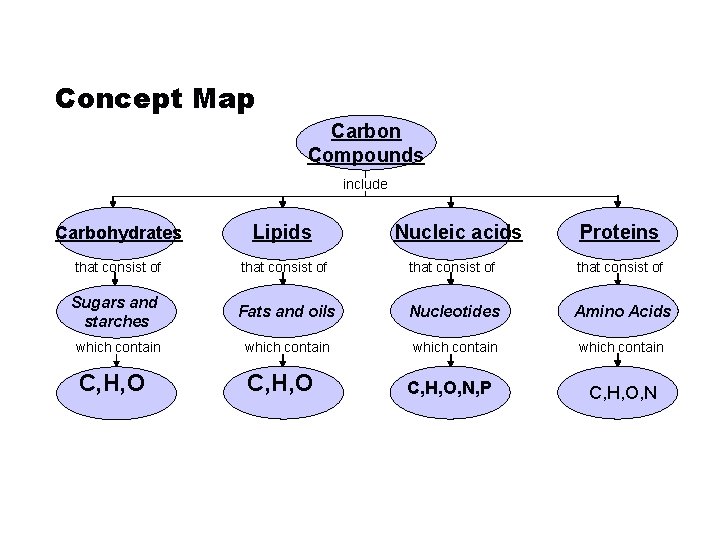
Concept Map Carbon Compounds include Carbohydrates Lipids that consist of Sugars and starches Fats and oils Nucleotides Amino Acids which contain C, H, O Go to Section: Nucleic acids C, H, O, N, P Proteins C, H, O, N
- Slides: 71