Organic Geochemistry III The Carbon Cycle Lecture 48

Organic Geochemistry III; The Carbon Cycle Lecture 48
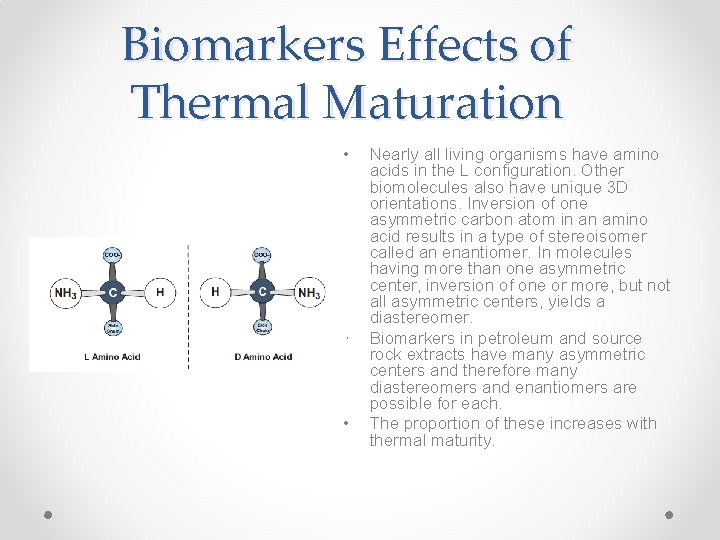
Biomarkers Effects of Thermal Maturation • • • Nearly all living organisms have amino acids in the L configuration. Other biomolecules also have unique 3 D orientations. Inversion of one asymmetric carbon atom in an amino acid results in a type of stereoisomer called an enantiomer. In molecules having more than one asymmetric center, inversion of one or more, but not all asymmetric centers, yields a diastereomer. Biomarkers in petroleum and source rock extracts have many asymmetric centers and therefore many diastereomers and enantiomers are possible for each. The proportion of these increases with thermal maturity.
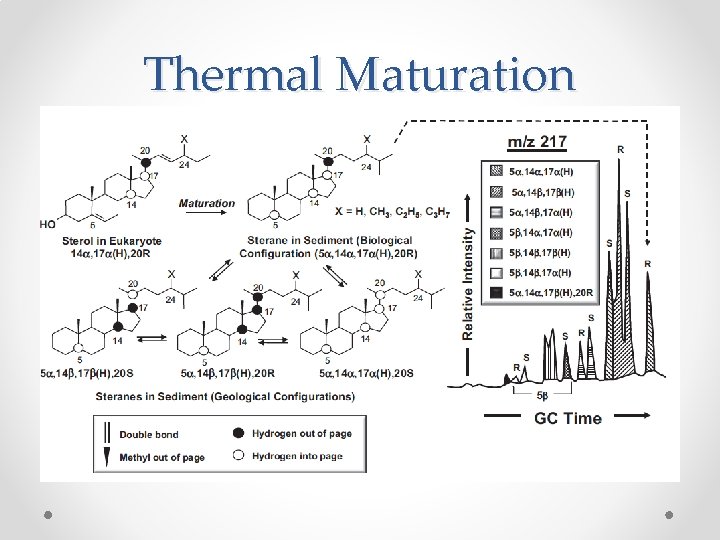
Thermal Maturation
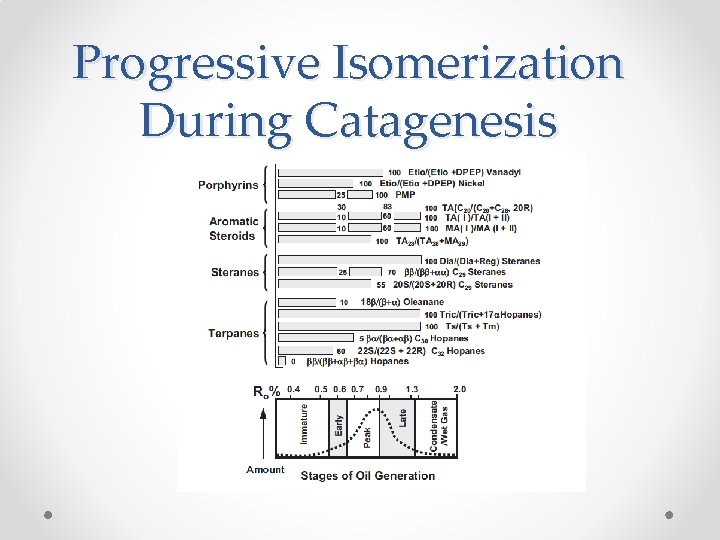
Progressive Isomerization During Catagenesis
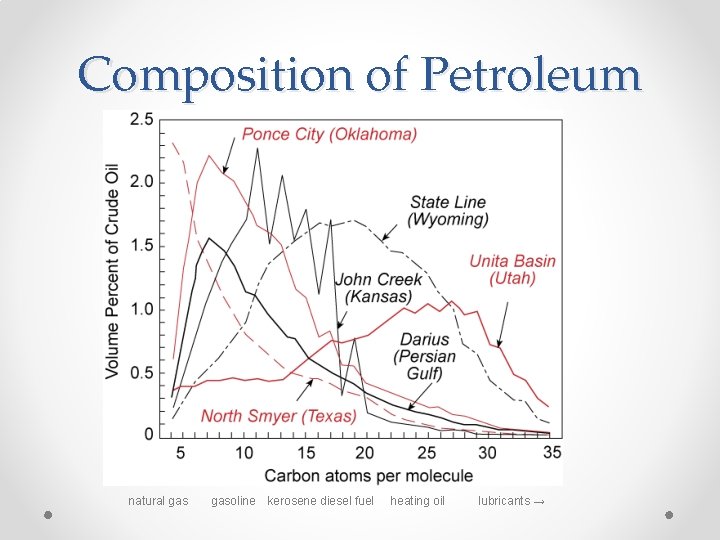
Composition of Petroleum natural gasoline kerosene diesel fuel heating oil lubricants →

Biomarker Indicators of Paleo-environment
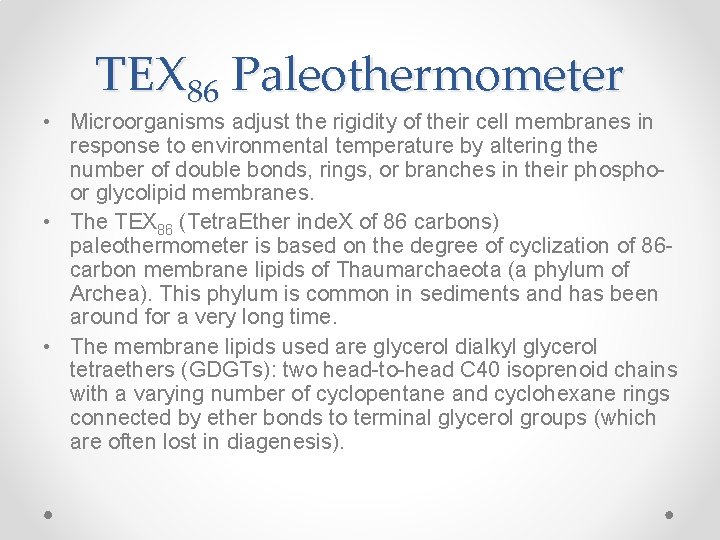
TEX 86 Paleothermometer • Microorganisms adjust the rigidity of their cell membranes in response to environmental temperature by altering the number of double bonds, rings, or branches in their phosphoor glycolipid membranes. • The TEX 86 (Tetra. Ether inde. X of 86 carbons) paleothermometer is based on the degree of cyclization of 86 carbon membrane lipids of Thaumarchaeota (a phylum of Archea). This phylum is common in sediments and has been around for a very long time. • The membrane lipids used are glycerol dialkyl glycerol tetraethers (GDGTs): two head-to-head C 40 isoprenoid chains with a varying number of cyclopentane and cyclohexane rings connected by ether bonds to terminal glycerol groups (which are often lost in diagenesis).
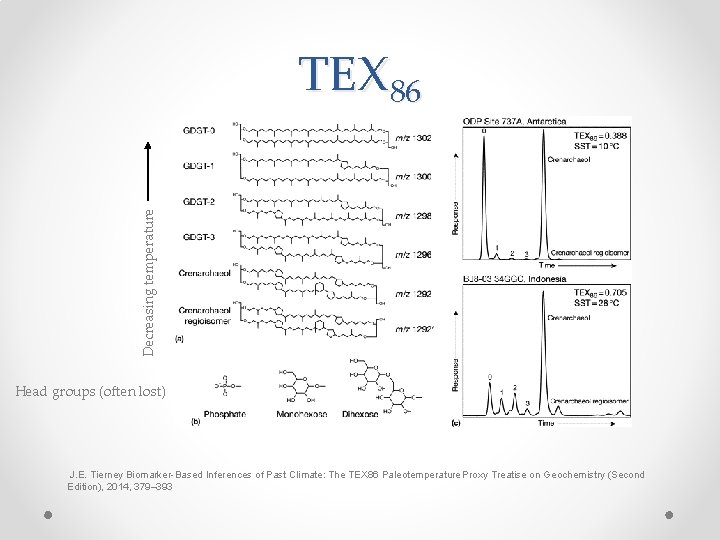
Decreasing temperature TEX 86 Head groups (often lost) J. E. Tierney Biomarker-Based Inferences of Past Climate: The TEX 86 Paleotemperature Proxy Treatise on Geochemistry (Second Edition), 2014, 379– 393

Compound-Specific Isotope Analysis • Further information can be obtained from biomarkers by analyzing their isotopic composition, something known as compound-specific isotope analysis. • Individual compounds are first separated by high-performance liquid chromatography then analyzed for isotopic composition in a mass spectrometer. • We have already discussed the C-37 alkenone d 13 C paleo. CO 2 proxy as one example. • d 13 C fractionation during photosynthesis is sensitive to moisture in the environment – under dry conditions, stomata open less, hence a greater fraction of internal CO 2 is consumed in photosynthesis (more efficient use of the CO 2), hence the isotopic fractionation is smaller.
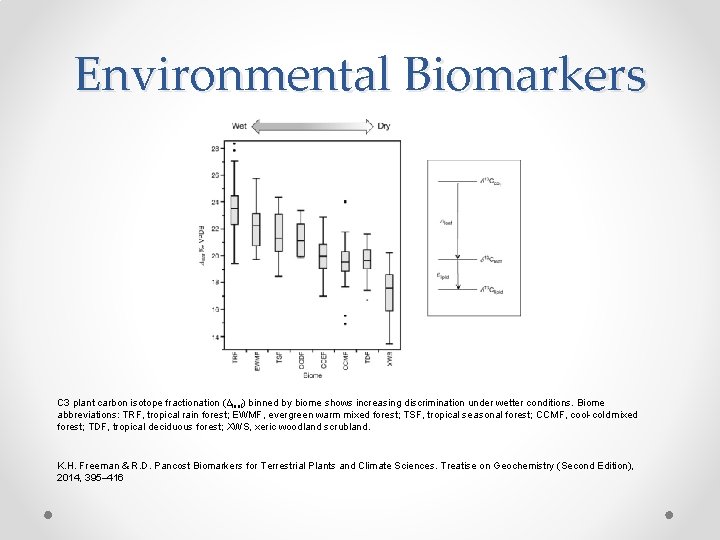
Environmental Biomarkers C 3 plant carbon isotope fractionation (Δleaf) binned by biome shows increasing discrimination under wetter conditions. Biome abbreviations: TRF, tropical rain forest; EWMF, evergreen warm mixed forest; TSF, tropical seasonal forest; CCMF, cool-cold mixed forest; TDF, tropical deciduous forest; XWS, xeric woodland scrubland. K. H. Freeman & R. D. Pancost Biomarkers for Terrestrial Plants and Climate Sciences. Treatise on Geochemistry (Second Edition), 2014, 395– 416
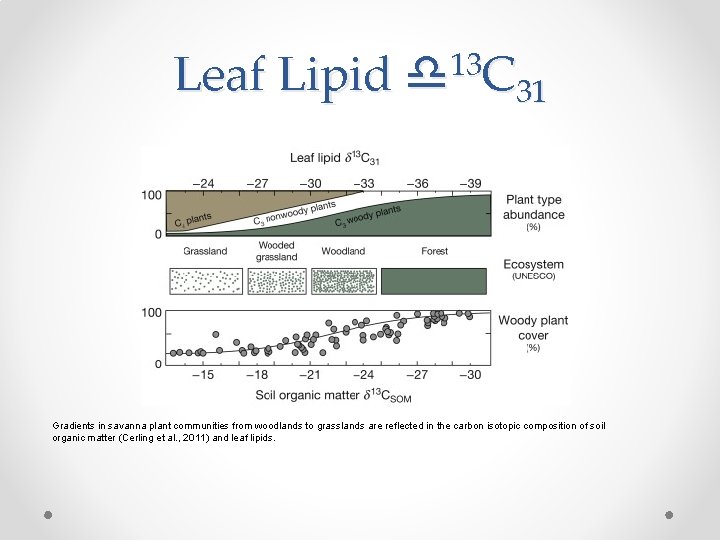
Leaf Lipid 13 d C 31 Gradients in savanna plant communities from woodlands to grasslands are reflected in the carbon isotopic composition of soil organic matter (Cerling et al. , 2011) and leaf lipids.

The Carbon Cycle and Climate
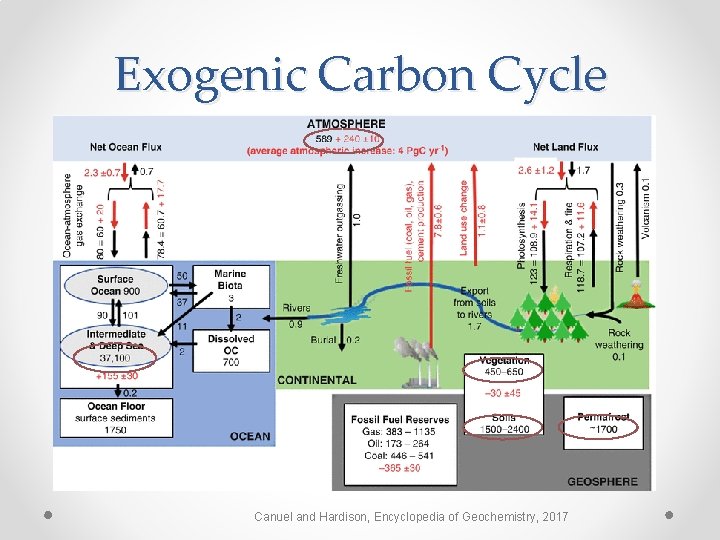
Exogenic Carbon Cycle Canuel and Hardison, Encyclopedia of Geochemistry, 2017
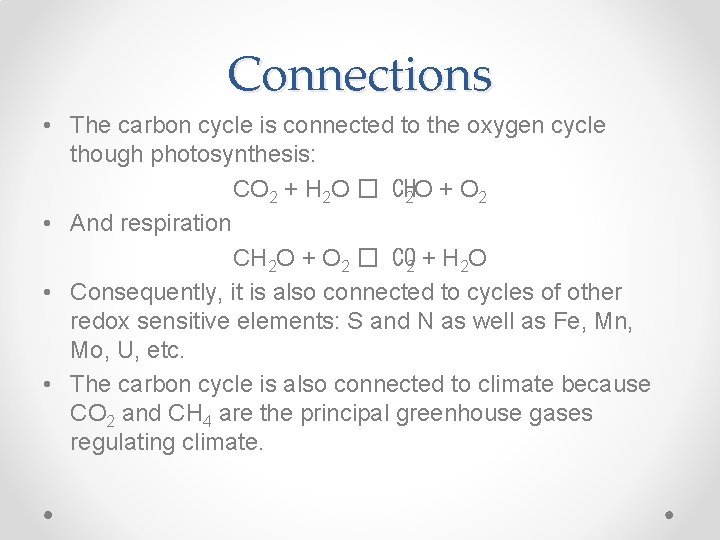
Connections • The carbon cycle is connected to the oxygen cycle though photosynthesis: CO 2 + H 2 O � CH 2 O + O 2 • And respiration CH 2 O + O 2 � CO 2 + H 2 O • Consequently, it is also connected to cycles of other redox sensitive elements: S and N as well as Fe, Mn, Mo, U, etc. • The carbon cycle is also connected to climate because CO 2 and CH 4 are the principal greenhouse gases regulating climate.
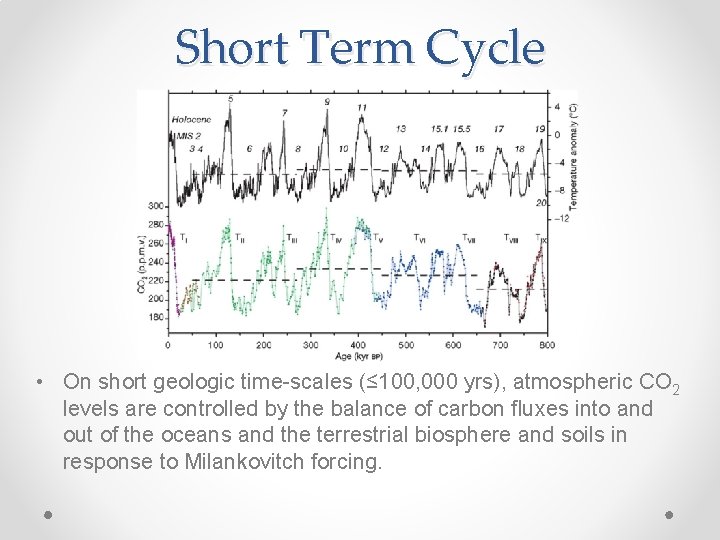
Short Term Cycle • On short geologic time-scales (≤ 100, 000 yrs), atmospheric CO 2 levels are controlled by the balance of carbon fluxes into and out of the oceans and the terrestrial biosphere and soils in response to Milankovitch forcing.

Glacial Cycling • Glacial cycles affect CO 2 fluxes by: o Changing the volume and temperature of the oceans: The smaller the volume of the ocean, the less CO 2 it can hold; CO 2 is more soluble in water at lower temperature. The effect of glacial cycles on these two factors is thus opposite. o Glacial cycles affect the terrestrial biota, but, again, with opposing effects: sea-level drops and the land expands, but expansion of glaciers also reduces this area. Precipitation patterns also change. This affects both the biomass the mass of dead organic matter in soils, etc. o The most important changes in CO 2 fluxes to climate-driven changes in ocean circulation and hence the storage of CO 2 in the deep ocean. The key ocean circulation changes appears to be a climate-driven migration of the westerly winds in the Southern Ocean. In the present interglacial climate, the most intense westerly winds are located south of the Antarctic polar front. As a result of a phenomenon called Ekman transport, these winds drive water away from Antarctica, and as a result, water rises, or “upwells” from depth, allowing CO 2 built-up in the deep ocean to vent to the atmosphere, keeping atmospheric CO 2 concentrations high. During glacial times, these westerlies shifted equatorward allowing for build-up of CO 2 in circum-Antarctic deep water. In addition, changes in the efficiency of the biologic pump affect the balance of CO 2 between ocean and atmosphere.
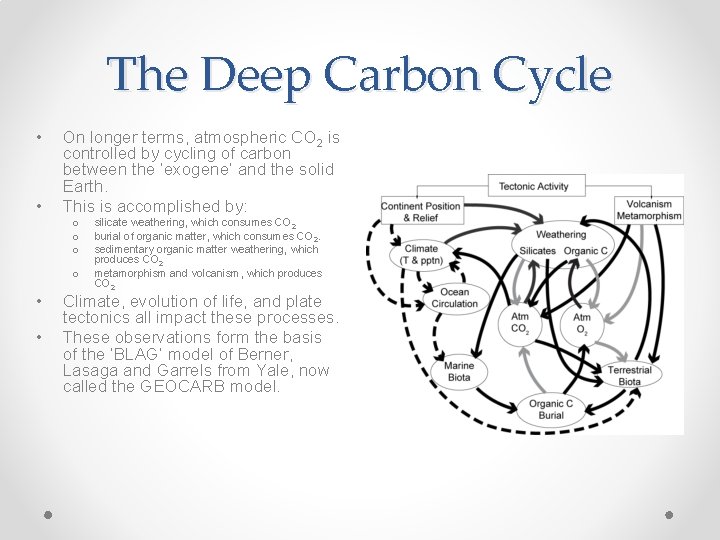
The Deep Carbon Cycle • • On longer terms, atmospheric CO 2 is controlled by cycling of carbon between the ‘exogene’ and the solid Earth. This is accomplished by: o o • • silicate weathering, which consumes CO 2 burial of organic matter, which consumes CO 2. sedimentary organic matter weathering, which produces CO 2 metamorphism and volcanism, which produces CO 2 Climate, evolution of life, and plate tectonics all impact these processes. These observations form the basis of the ‘BLAG’ model of Berner, Lasaga and Garrels from Yale, now called the GEOCARB model.
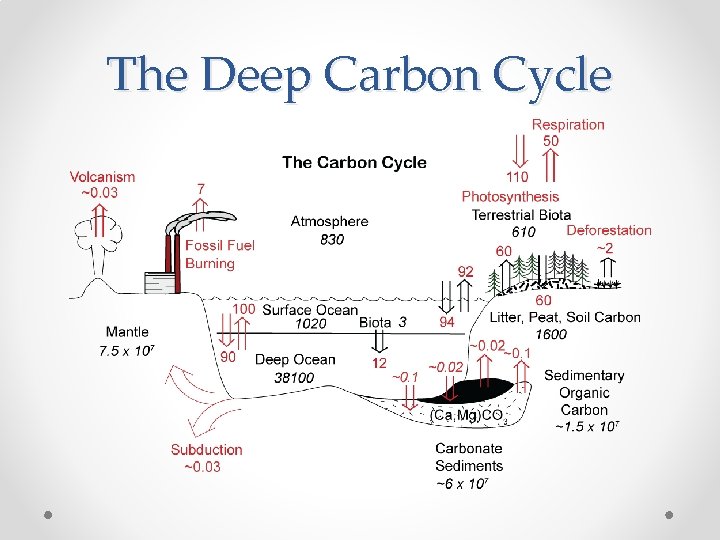
The Deep Carbon Cycle

Role of Silicate Weathering • When dissolved in water, CO 2 forms carbonic acid and dissociates (Chapter 6), providing hydrogen ions that then attack silicate minerals: CO 2 + H 2 O ⇌ H+ + HCO 32 H+ + Ca. Al 2 Si 3 O 8 ⇌ Al 2 Si 2 O 5(OH)4 + Ca 2+ • Calcium released in this way is carried by rivers to the sea along with bicarbonate ions where they precipitate as calcite: • Ca 2+ + HCO 3 - ⇌ H+ + Ca. CO 3 • Much of the calcite redissolves in the deep water or sediment, but some is buried as part of the carbonate sediment. • Metamorphism tends to release CO 2, as will volcanism with CO 2 subducted into the mantle.

Connection to Climate

Greenhouse Theory Development • 1824 French mathematician Jean-Baptiste Joseph Fourier speculates that the Earth’s atmosphere acts as an insulator to outgoing heat: the greenhouse effect. • 1860 Irish physicist John Tyndall demonstrates that H 2 O and CO 2 absorb IR radiation and concludes they are responsible for Fourier’s greenhouse effect. Jean-Baptiste Joseph Fourier John Tyndall

Greenhouse Theory & Climate Change • 1895 Swedish chemist Svante Arrhenius publishes paper “On the influence of carbonic acid in the air upon temperature of the ground” predicting that burning fossil fuels will lead to global warming. • In 1957 oceanographer Roger Revelle and physicist Hans Suess revive the greenhouse/climate change question. • 1958 Charles Keeling begins measuring CO 2 on Mauna Loa and at the South Pole. Svante Arrhenius

Greenhouse Gases • The principal gases in the modern Earth’s atmosphere, N 2, O 2, and Ar; they do not absorb in the infrared part of the spectrum. • Certain trace gases in the atmosphere, notably H 2 O, CO 2, CH 4, and N 2 O absorb infrared radiation at different wavelengths. • Notice any systematic differences between these?
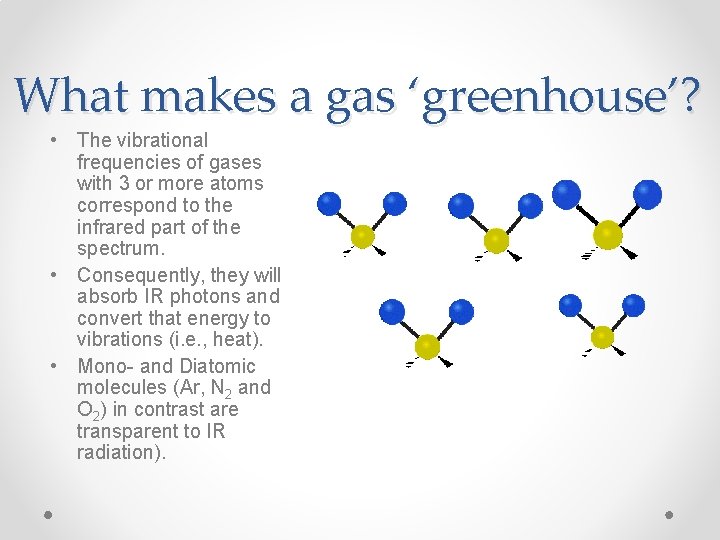
What makes a gas ‘greenhouse’? • The vibrational frequencies of gases with 3 or more atoms correspond to the infrared part of the spectrum. • Consequently, they will absorb IR photons and convert that energy to vibrations (i. e. , heat). • Mono- and Diatomic molecules (Ar, N 2 and O 2) in contrast are transparent to IR radiation).

Greenhouse Effect • • Absorption scales with the log of concentration. Thus, for example, small changes in the abundance of CH 4 have a greater relative effect on the energy balance than do small changes in more abundant CO 2, even though CO 2 absorbs at frequencies close to the Earth’s maximum spectral emittance and is thus inherently a more effective greenhouse gas. The combined effect of these gases is to absorb much of the infrared radiated from the Earth’s surface and to raise the average temperature of the Earth’s surface from 254 K (-19°C) to 286 K (+13°C). H 2 O is the most powerful of the greenhouse gases, because it absorbs over a relatively wide range of frequencies and because its concentration is relatively high (its atmospheric concentration can be up to 4% on a very hot, humid day). However, the residence time of water in the atmosphere is quite short, so that its effect alone can only be limited. On long time-scales variations in the concentration of CO 2 and the other greenhouse gases control climate. Variations in atmospheric greenhouse gas concentrations are a result of how carbon is cycled between the atmosphere and other reservoirs and how the Earth and life have evolved over the last 4. 5 Ga.
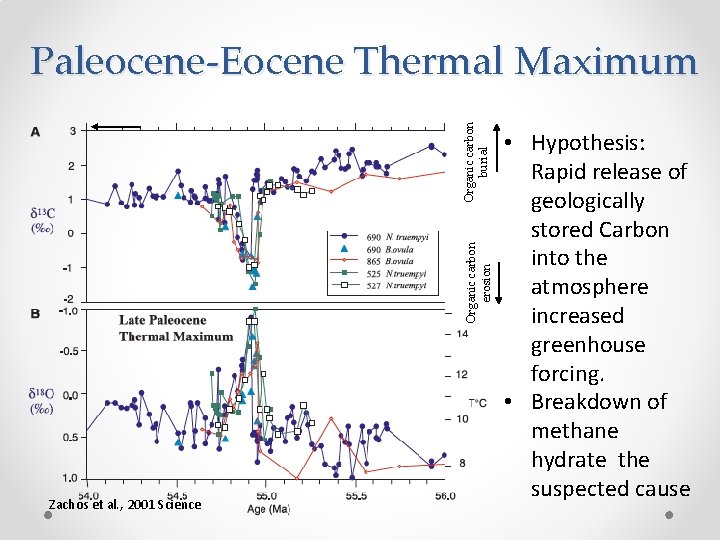
Organic carbon erosion Organic carbon burial Paleocene-Eocene Thermal Maximum Zachos et al. , 2001 Science • Hypothesis: Rapid release of geologically stored Carbon into the atmosphere increased greenhouse forcing. • Breakdown of methane hydrate the suspected cause
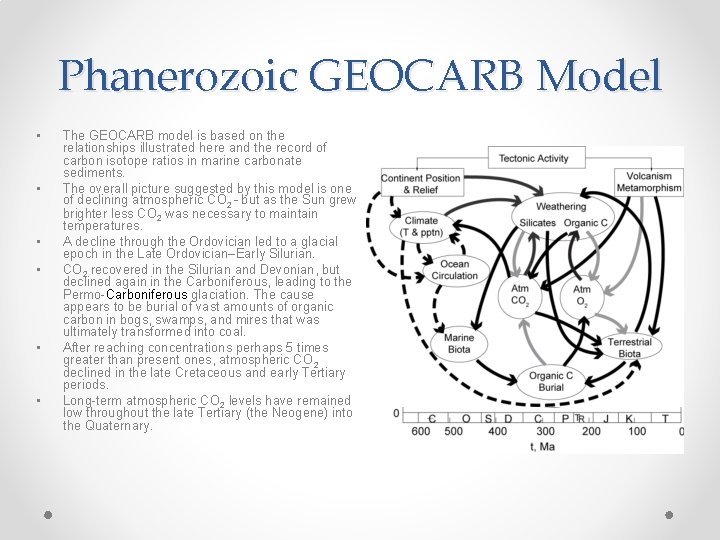
Phanerozoic GEOCARB Model • • • The GEOCARB model is based on the relationships illustrated here and the record of carbon isotope ratios in marine carbonate sediments. The overall picture suggested by this model is one of declining atmospheric CO 2 – but as the Sun grew brighter less CO 2 was necessary to maintain temperatures. A decline through the Ordovician led to a glacial epoch in the Late Ordovician–Early Silurian. CO 2 recovered in the Silurian and Devonian, but declined again in the Carboniferous, leading to the Permo-Carboniferous glaciation. The cause appears to be burial of vast amounts of organic carbon in bogs, swamps, and mires that was ultimately transformed into coal. After reaching concentrations perhaps 5 times greater than present ones, atmospheric CO 2 declined in the late Cretaceous and early Tertiary periods. Long-term atmospheric CO 2 levels have remained low throughout the late Tertiary (the Neogene) into the Quaternary.

The Oxygen Connection Deep, deep time: The Precambrian
Evolution of the Carbon Cycle • A steady increase in brightness of the Sun. The Sun is now about 30% brighter than it was 4. 5 billion years ago when it first became a main sequence star. This increase in insolation would result in a surface temperature increase of nearly 22°C (the present mean global surface temperature is now about 13°C) if greenhouse forcing were constant. o (at the same time, tectonic activity driven by energy from radioactive decay of U, Th, and K, and initial heat has declined). • Evolution of life has had a profound effect on the nature of the atmosphere, and, as a result, on climate. The Earth’s atmosphere in Hadean and early Archean times would have had no oxygen and CO 2 would have been the dominant component (e. g. , Mars and Venus). It may well have been modestly reducing, with some CH 4 present. o Since CO 2 and CH 4 are greenhouse gases, the greenhouse effect would have been much greater – perhaps resolving the faint young Sun paradox.
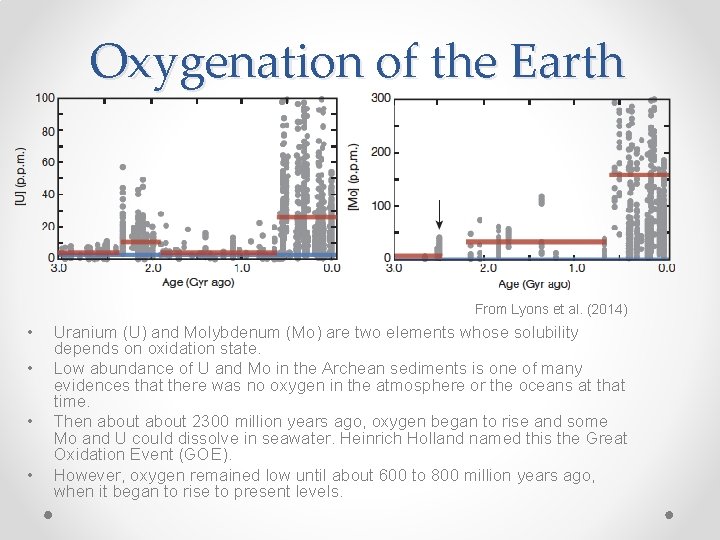
Oxygenation of the Earth From Lyons et al. (2014) • • Uranium (U) and Molybdenum (Mo) are two elements whose solubility depends on oxidation state. Low abundance of U and Mo in the Archean sediments is one of many evidences that there was no oxygen in the atmosphere or the oceans at that time. Then about 2300 million years ago, oxygen began to rise and some Mo and U could dissolve in seawater. Heinrich Holland named this the Great Oxidation Event (GOE). However, oxygen remained low until about 600 to 800 million years ago, when it began to rise to present levels.
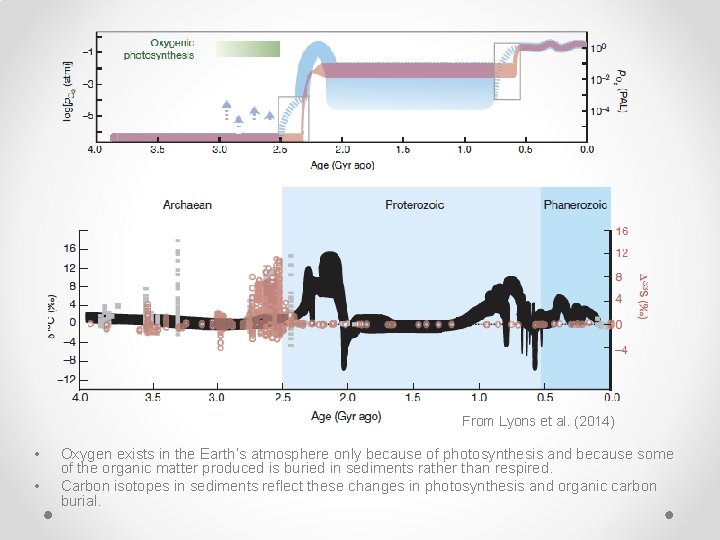
From Lyons et al. (2014) • • Oxygen exists in the Earth’s atmosphere only because of photosynthesis and because some of the organic matter produced is buried in sediments rather than respired. Carbon isotopes in sediments reflect these changes in photosynthesis and organic carbon burial.

Climate and the GOE • Knowing how atmospheric CO 2 and climate varied in the Precambrian is a much more difficult proposition. • There is some sparse and equivocal evidence for glaciation around 2. 8 Ga. • There is strong evidence of glaciations in roughly between 2. 4 to 2. 2 Ga and roughly between 0. 8 to 0. 6 Ga. o In both the Proterozoic events, glacial sediments (diamictites) appear to have been deposited at tropical, rather than polar, latitudes and at low elevation. • The Paleoproterozoic diamictites in the Huronian formation occur stratigraphically above an older conglomerate containing abundant detrital pyrite (Fe. S 2) and uraninite (UO 2). That reduced minerals could survive erosion, transport, and deposition suggests they were deposited in an oxygen-free atmosphere. They are overlain by redbeds indicative of an oxidizing atmosphere. This period is known as the Great Oxidation Event (GOE). Oxygen levels in the atmosphere before the GOE were rose from 10 -5 lower than present to 5– 15% of present atmospheric levels. o Huronian glaciation might have resulted from a decrease in atmospheric CH 4 at this time.
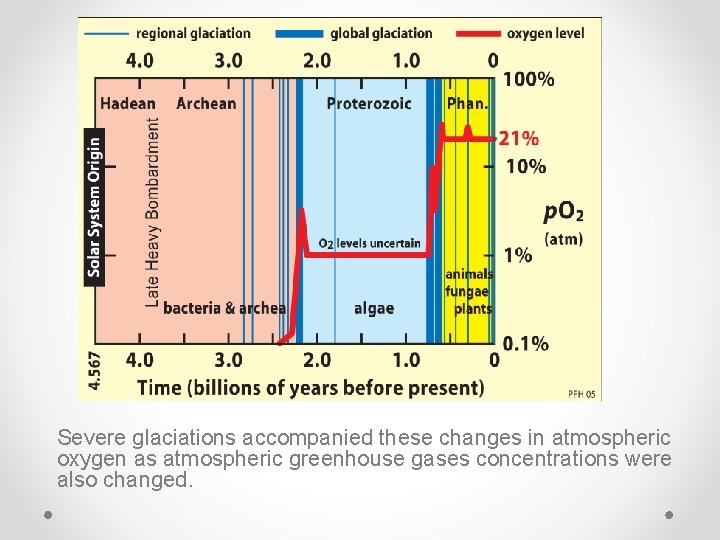
Severe glaciations accompanied these changes in atmospheric oxygen as atmospheric greenhouse gases concentrations were also changed.

Paleoproterozoic (Cryogenian) Glaciations • Followed the break-up of the Rodinia supercontinent around 800 Ma. Rodinia was located at low latitude at the time and the fragments remained at low latitude. • All models involve significant disturbances to the carbon cycle and a consequent crash in greenhouse gas inventory. • Models: o Rising oxygen levels around this time greatly reduced the methane flux from oxygenpoor oceans. That in turn reduced the CO 2 levels in the atmosphere (because the methane eventually oxidizes to CO 2. o High rates of tropical weathering as Rodinia broke up led to enhanced ocean nutrient levels and productivity, and efficient burial of organic carbon. • In all the models, once glaciation begins, the Earth “whitens” and the much greater albedo provides a powerful feedback driving further cooling.

Darwin’s Dilemma • “There is another…difficulty, which is much more serious. I allude to the manner in which species belonging to several* of the main divisions of the animal kingdom suddenly appear in the lowest known fossiliferous rocks…. But to the question why we do not find rich fossiliferous deposits belonging to these assumed earliest periods before the Cambrian system, I can give no satisfactory answer. The case at present must remain inexplicable; and may be truly urged as a valid argument against the views [Evolution] here entertained. ” Darwin, Origin of Species, 1859 *actually, nearly all

Guess what happened just as atmospheric O 2 began to rise again?
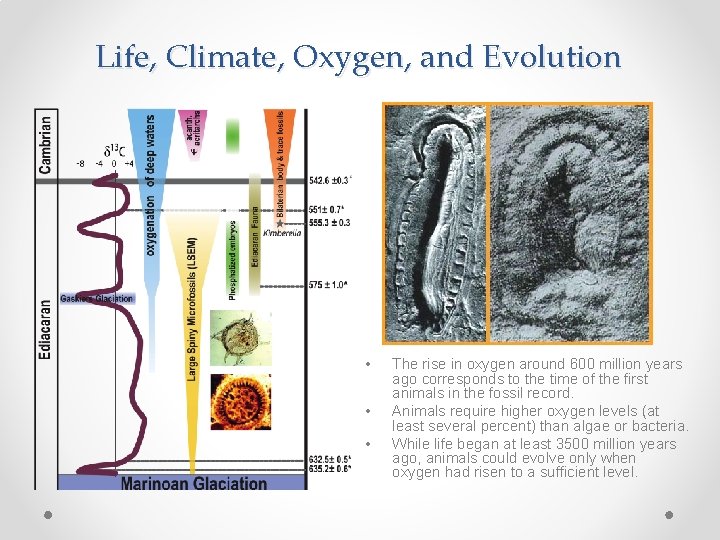
Life, Climate, Oxygen, and Evolution • • • The rise in oxygen around 600 million years ago corresponds to the time of the first animals in the fossil record. Animals require higher oxygen levels (at least several percent) than algae or bacteria. While life began at least 3500 million years ago, animals could evolve only when oxygen had risen to a sufficient level.
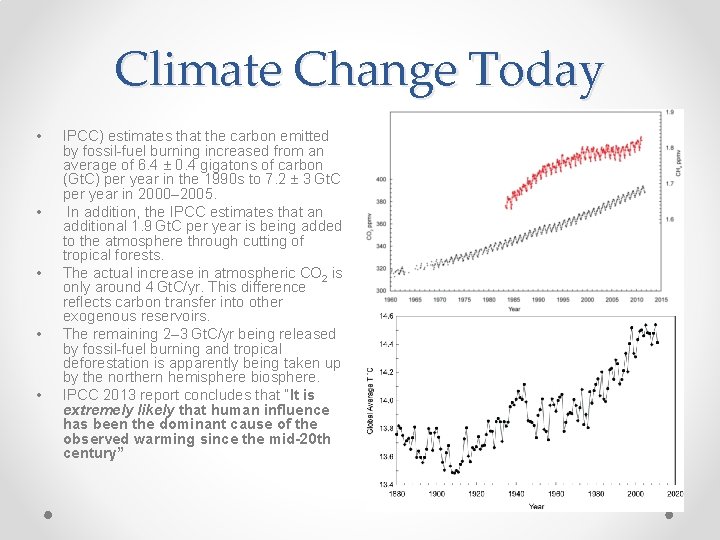
Climate Change Today • • • IPCC) estimates that the carbon emitted by fossil-fuel burning increased from an average of 6. 4 ± 0. 4 gigatons of carbon (Gt. C) per year in the 1990 s to 7. 2 ± 3 Gt. C per year in 2000– 2005. In addition, the IPCC estimates that an additional 1. 9 Gt. C per year is being added to the atmosphere through cutting of tropical forests. The actual increase in atmospheric CO 2 is only around 4 Gt. C/yr. This difference reflects carbon transfer into other exogenous reservoirs. The remaining 2– 3 Gt. C/yr being released by fossil-fuel burning and tropical deforestation is apparently being taken up by the northern hemisphere biosphere. IPCC 2013 report concludes that “It is extremely likely that human influence has been the dominant cause of the observed warming since the mid-20 th century”
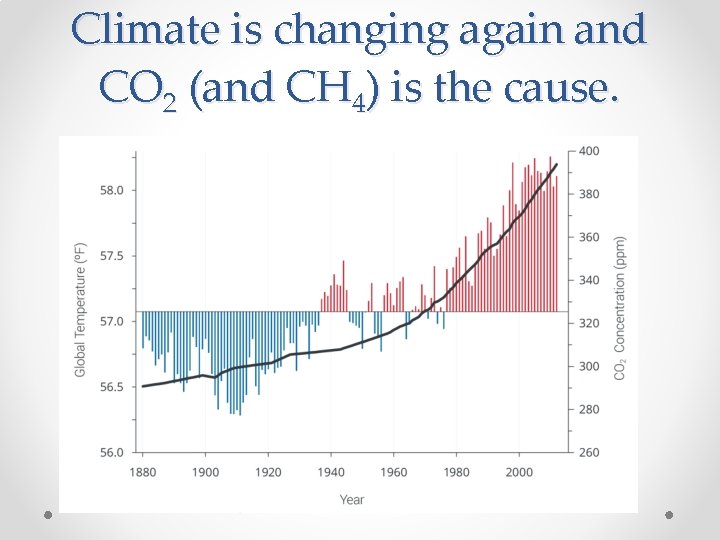
Climate is changing again and CO 2 (and CH 4) is the cause.
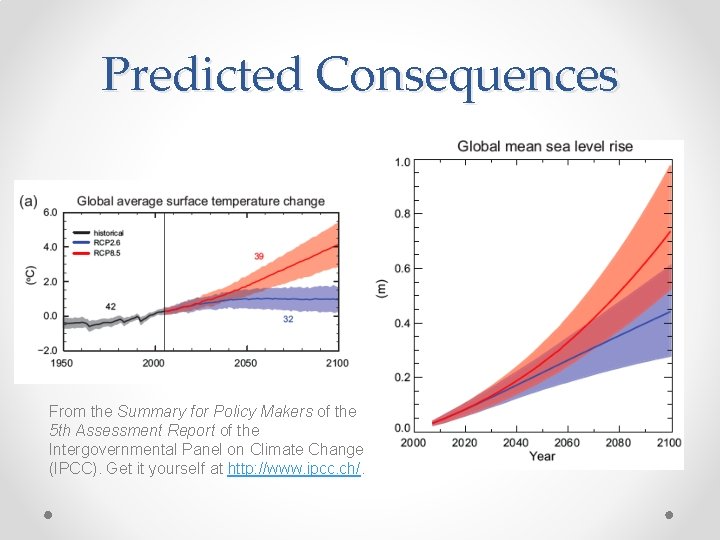
Predicted Consequences From the Summary for Policy Makers of the 5 th Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Get it yourself at http: //www. ipcc. ch/.
- Slides: 40