Organic Compounds What is organic chemistry Biochemistry The

Organic Compounds
What is organic chemistry? Biochemistry! § The study of all compounds containing the element CARBON §Atomic number = 6 § 4 valence electrons §Can easily bond with H, O, N, P, and other C (can create carbon chains)

§ Natural elements: make up 96% of the mass of a human: CARBON, HYDROGEN, OXYGEN, NITROGEN (CHON) § Trace elements: only needed in small amounts, called “minerals”:
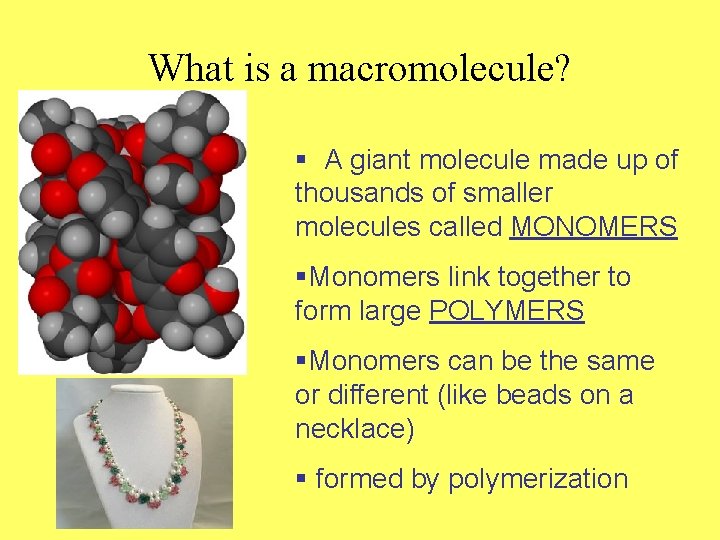
What is a macromolecule? § A giant molecule made up of thousands of smaller molecules called MONOMERS §Monomers link together to form large POLYMERS §Monomers can be the same or different (like beads on a necklace) § formed by polymerization
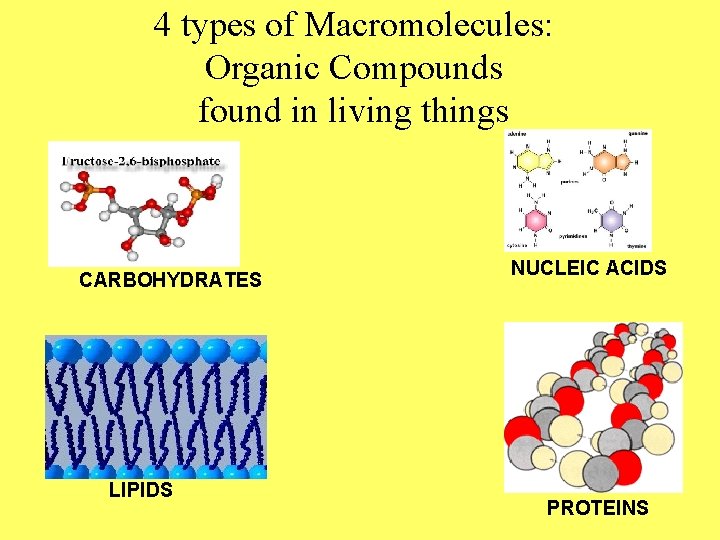
4 types of Macromolecules: Organic Compounds found in living things CARBOHYDRATES LIPIDS NUCLEIC ACIDS PROTEINS


Carbohydrates • • Carbon/Hydrogen/Oxygen 1: 2: 1 CHO Main source of E for living things Breakdown of sugar (e. g. glucose) supplies E for cell’s activities • Extra sugar stored as Complex Carbs (starches)(polysaccharide) • Single sugar molecules = monosaccharide (glucose, galactose, fructose) • Excess sugar stored as glycogen

CARBOHYDRATES §Monomer: simple sugar (glucose) §Polymer: Starches (cellulose) § Function: main source of energy for cellular respiration §Examples: Monosaccharide, disaccharide, polysaccharide, glucose, lactose, fructose, potatoes, pasta, bread, grains

Lipids • • Carbon/Hydrogen/Oxygen CHO Usually not soluble in H 2 O Fats, oils, waxes, steroids (chemical messengers) • Store E • Cell membranes
§ Monomer: Fatty Acid §Polymer: Triglycerides, oil, waxes §Function: store energy, insulation, protection, waterproofing, form cell membranes §Examples: red meat, cheese, dairy §Usually not soluble in water §Saturated: has maximum number of H bonds, usually solid at room temperature §Unsaturated: at least one double bond, causes “kinks”, usually liquid LIPIDS
Proteins • • Carbon/Hydrogen/Oxygen/Nitrogen CHON Amino acids (20 different a. a. ) Enzymes Regulate cell processes Form body tissues Transport substances in/out cells Fight disease
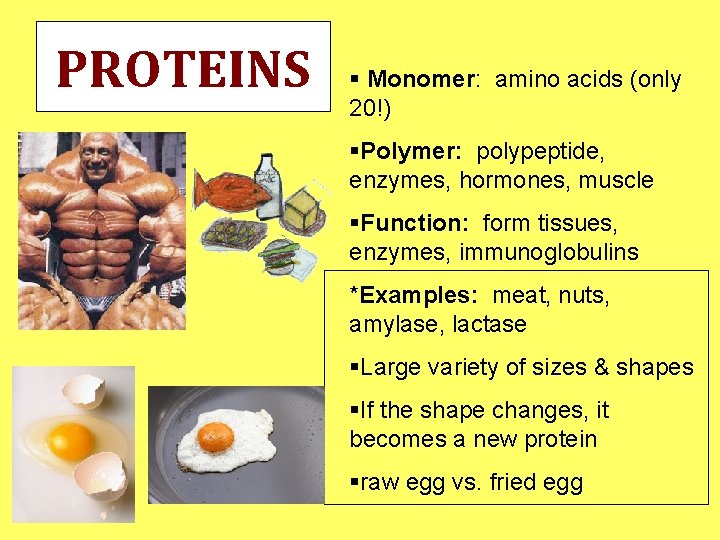
PROTEINS § Monomer: amino acids (only 20!) §Polymer: polypeptide, enzymes, hormones, muscle §Function: form tissues, enzymes, immunoglobulins *Examples: meat, nuts, amylase, lactase §Large variety of sizes & shapes §If the shape changes, it becomes a new protein §raw egg vs. fried egg

Nucleic Acids • Carbon, Hydrogen, Oxygen, Nitrogen, Phosporus • CHONP • Nucleotides – 5 carbon sugar – Phosphate group – Nitrogenous base • Polynucleotide = Nucleic Acid • DNA or RNA
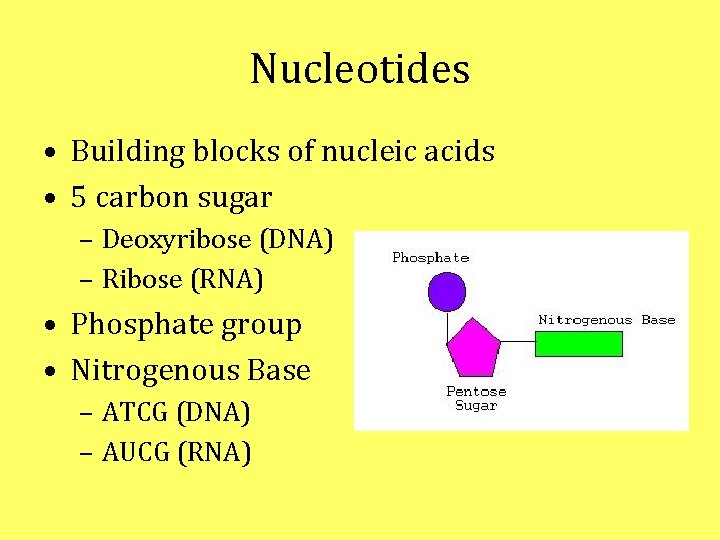
Nucleotides • Building blocks of nucleic acids • 5 carbon sugar – Deoxyribose (DNA) – Ribose (RNA) • Phosphate group • Nitrogenous Base – ATCG (DNA) – AUCG (RNA)
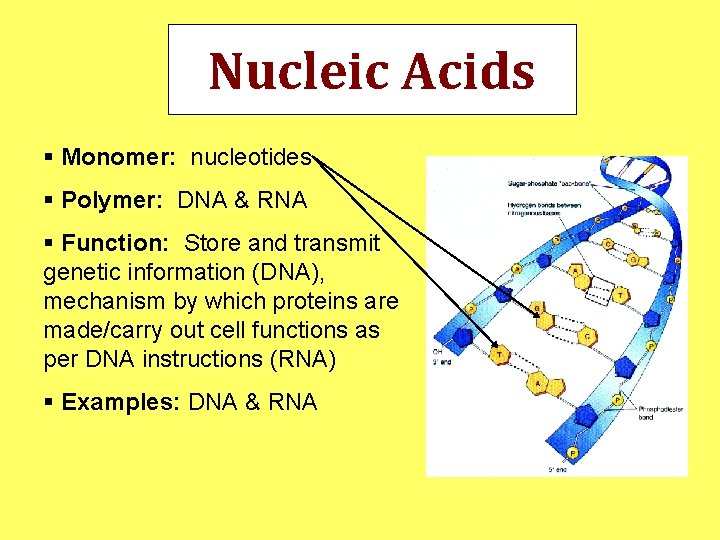
Nucleic Acids § Monomer: nucleotides § Polymer: DNA & RNA § Function: Store and transmit genetic information (DNA), mechanism by which proteins are made/carry out cell functions as per DNA instructions (RNA) § Examples: DNA & RNA

Organic Compounds • • CHO CHONP
- Slides: 16