Organic Compounds Proteins are large molecules that contain

- Slides: 15

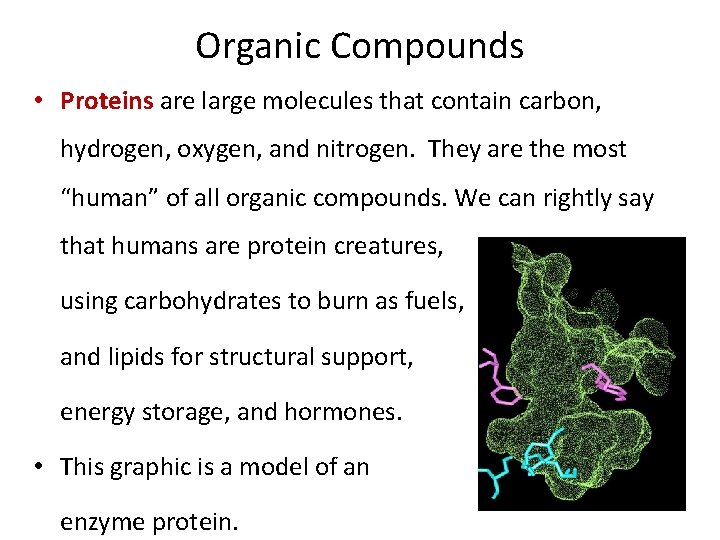
Organic Compounds • Proteins are large molecules that contain carbon, hydrogen, oxygen, and nitrogen. They are the most “human” of all organic compounds. We can rightly say that humans are protein creatures, using carbohydrates to burn as fuels, and lipids for structural support, energy storage, and hormones. • This graphic is a model of an enzyme protein.

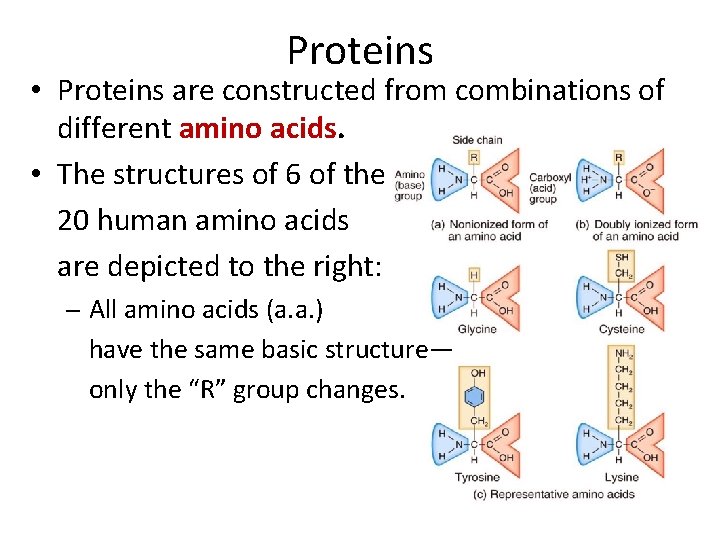
Proteins • Proteins are constructed from combinations of different amino acids. • The structures of 6 of the 20 human amino acids are depicted to the right: – All amino acids (a. a. ) have the same basic structure— only the “R” group changes.

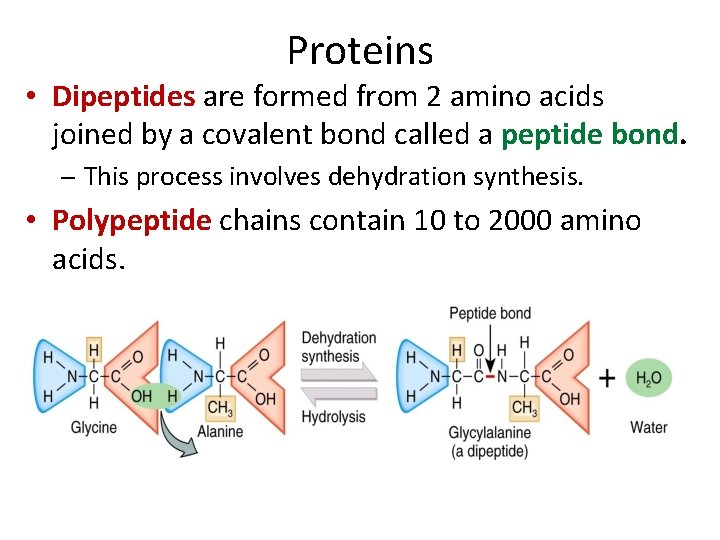
Proteins • Dipeptides are formed from 2 amino acids joined by a covalent bond called a peptide bond. – This process involves dehydration synthesis. • Polypeptide chains contain 10 to 2000 amino acids.

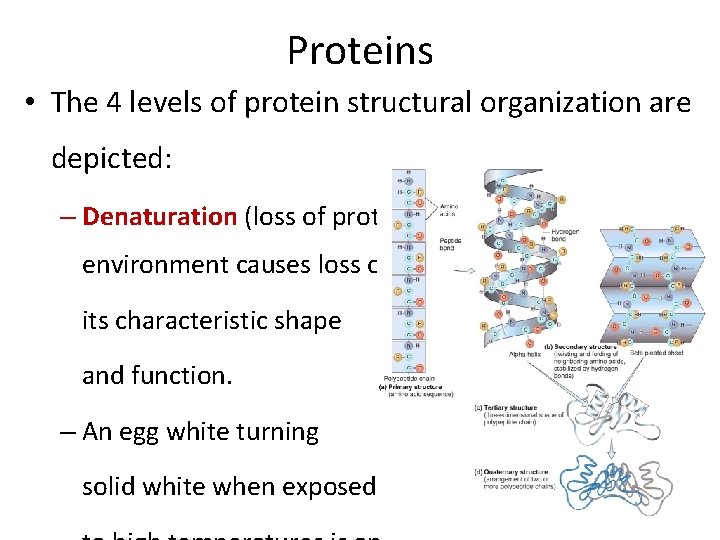
Proteins • There are 4 levels at which proteins are structurally organized : – Primary (10) – Secondary (20) – Tertiary (30) – Quaternary (40) • The resulting shape of the protein greatly influences its ability to recognize and bind to

Proteins • The 4 levels of protein structural organization are depicted: – Denaturation (loss of protein structure) by a hostile environment causes loss of its characteristic shape and function. – An egg white turning solid white when exposed

Proteins • Enzymes are special proteins that catalyze (speed up) metabolic reaction in all living cells. • The substrate is the substance upon which an enzyme has its effect. In this regard, enzymes are highly specific. • Enzymes are subject to a variety of cellular controls. • Enzymes speed up chemical reactions by increasing frequency of collisions, lowering activation energy, and properly orienting colliding molecules.

Enzymes Interactions Animation • Enzyme Functions and ATP You must be connected to the internet to run this animation.

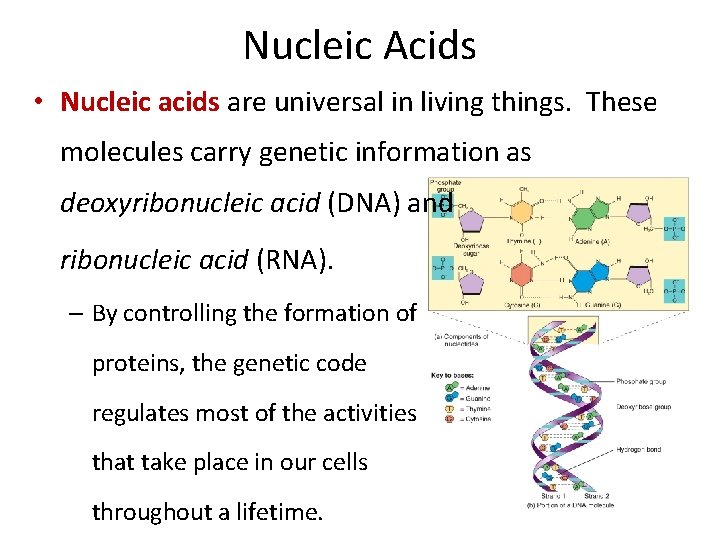
Nucleic Acids • Nucleic acids are universal in living things. These molecules carry genetic information as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). – By controlling the formation of proteins, the genetic code regulates most of the activities that take place in our cells throughout a lifetime.

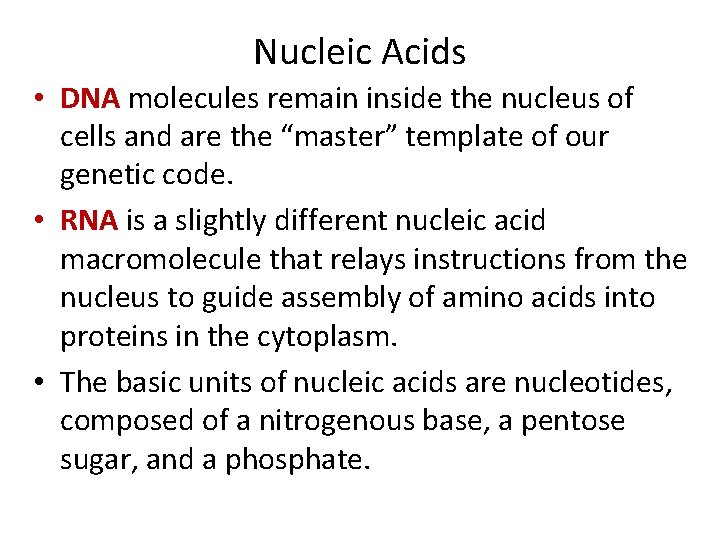
Nucleic Acids • DNA molecules remain inside the nucleus of cells and are the “master” template of our genetic code. • RNA is a slightly different nucleic acid macromolecule that relays instructions from the nucleus to guide assembly of amino acids into proteins in the cytoplasm. • The basic units of nucleic acids are nucleotides, composed of a nitrogenous base, a pentose sugar, and a phosphate.

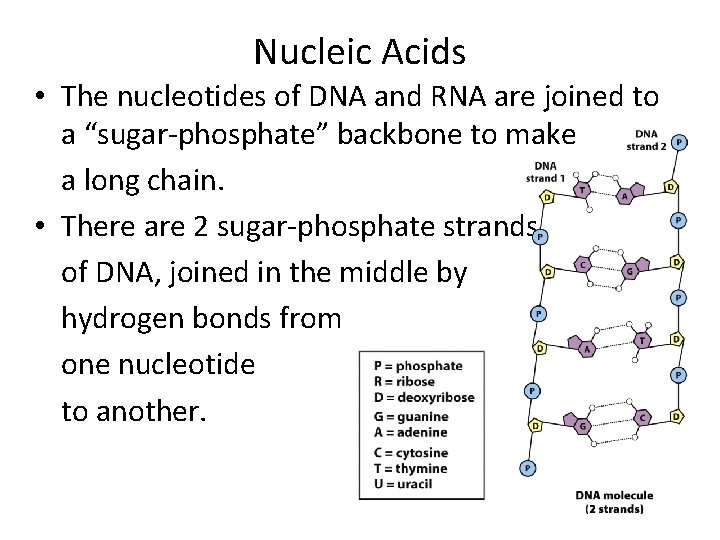
Nucleic Acids • The nucleotides of DNA and RNA are joined to a “sugar-phosphate” backbone to make a long chain. • There are 2 sugar-phosphate strands of DNA, joined in the middle by hydrogen bonds from one nucleotide to another.

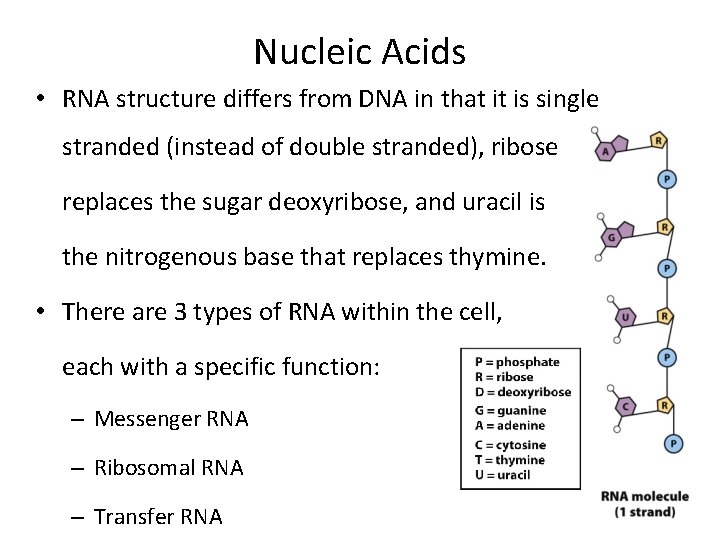
Nucleic Acids • RNA structure differs from DNA in that it is single stranded (instead of double stranded), ribose replaces the sugar deoxyribose, and uracil is the nitrogenous base that replaces thymine. • There are 3 types of RNA within the cell, each with a specific function: – Messenger RNA – Ribosomal RNA – Transfer RNA

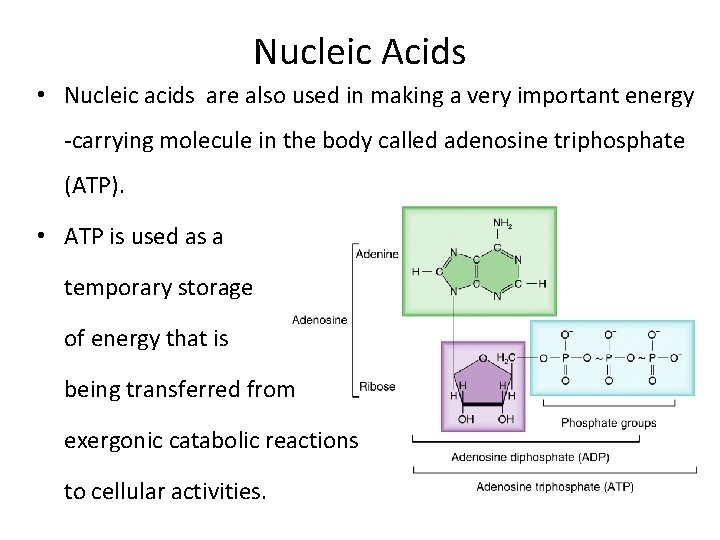
Nucleic Acids • Nucleic acids are also used in making a very important energy -carrying molecule in the body called adenosine triphosphate (ATP). • ATP is used as a temporary storage of energy that is being transferred from exergonic catabolic reactions to cellular activities.

Nucleic Acids • ATP is often called the “molecular unit of currency” of intracellular energy transfer. [1]. • Synthesis of ATP is catalyzed by the ATP synthase enzyme which adds the terminal high energy phosphate bond (often depicted as ~P as opposed to a regular –P bond) to ADP. – Energy from 1 glucose molecule is used during both anaerobic and aerobic respiration to create 36 to 38 molecules of ATP.

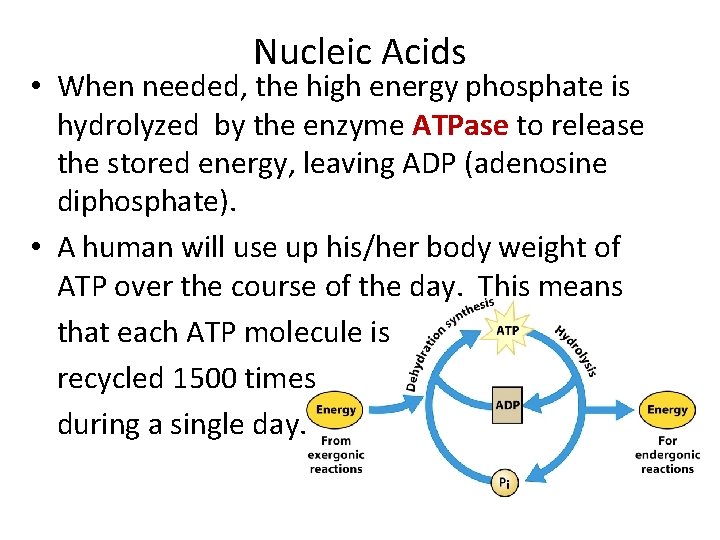
Nucleic Acids • When needed, the high energy phosphate is hydrolyzed by the enzyme ATPase to release the stored energy, leaving ADP (adenosine diphosphate). • A human will use up his/her body weight of ATP over the course of the day. This means that each ATP molecule is recycled 1500 times during a single day.

End of Chapter 2 Copyright 2012 John Wiley & Sons, Inc. All rights reserved. Reproduction or translation of this work beyond that permitted in section 117 of the 1976 United States Copyright Act without express permission of the copyright owner is unlawful. Request for further information should be addressed to the Permission Department, John Wiley & Sons, Inc. The purchaser may make back-up copies for his/her own use only and not for distribution or resale. The Publishers assumes no responsibility for errors, omissions, or damages caused by the use of these programs or from the use of the information herein. .