Organic Compounds Importance of Macromolecules Molecules and atoms













- Slides: 75

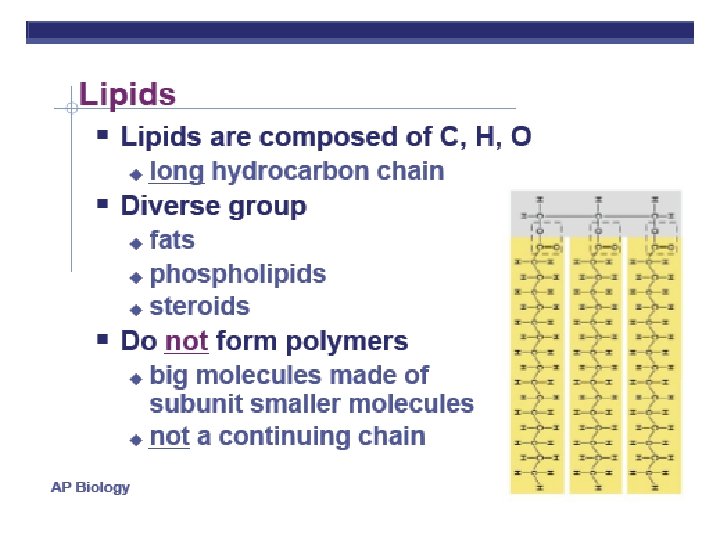
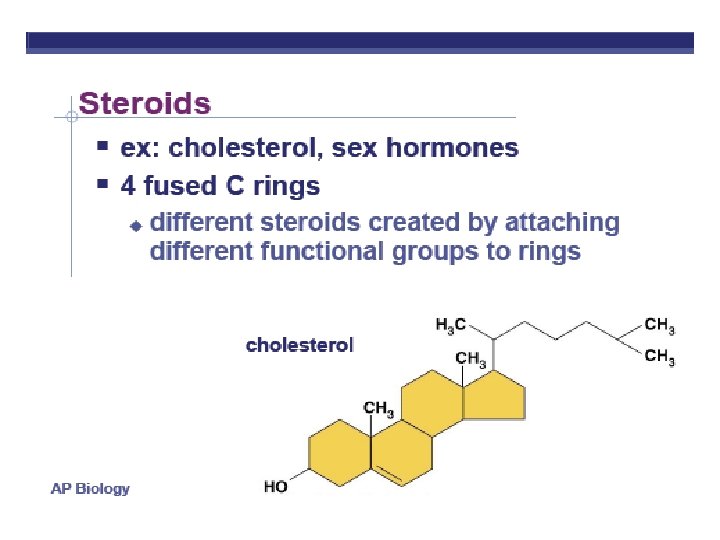
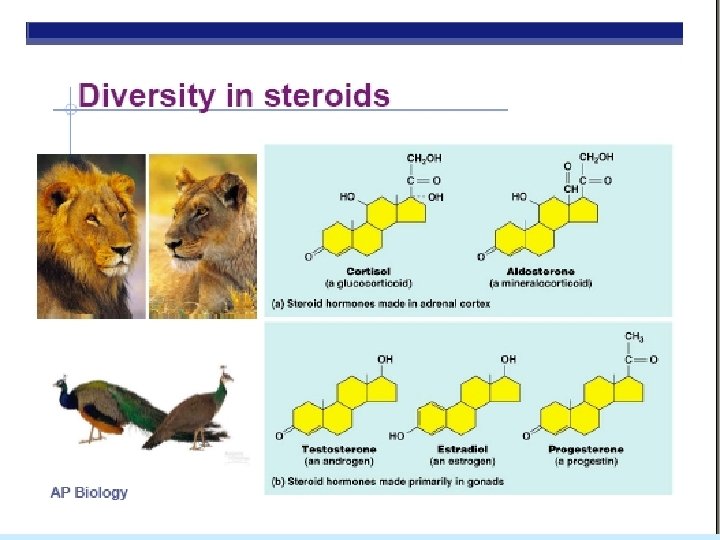
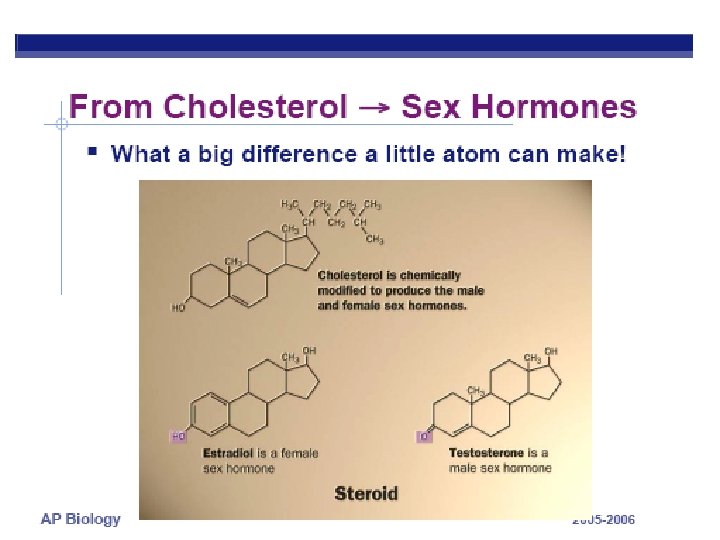
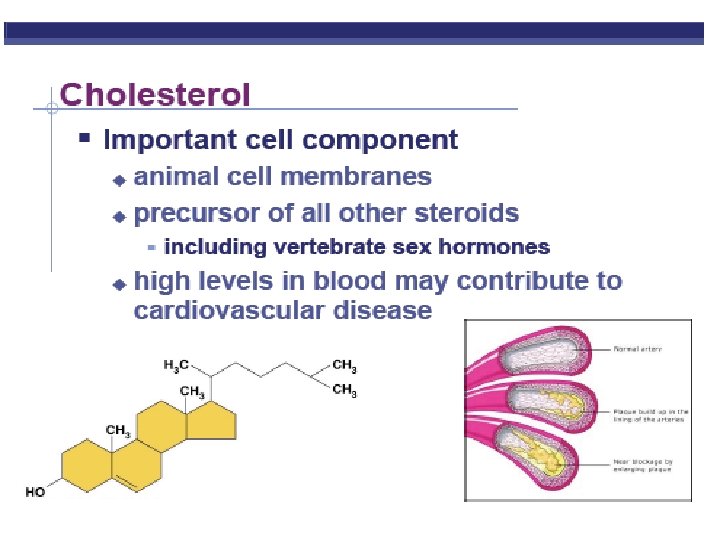
Organic Compounds

Importance of Macromolecules • Molecules and atoms from the environment are necessary to build new molecules within the cells of living things – Carbon moves from the environment to organisms where it is used to create macromolecules that are used in storage compounds and the formation of new cells

4 MAJOR MACROMOLECULES • Carbohydrates • Lipids • Proteins • Nucleic acids

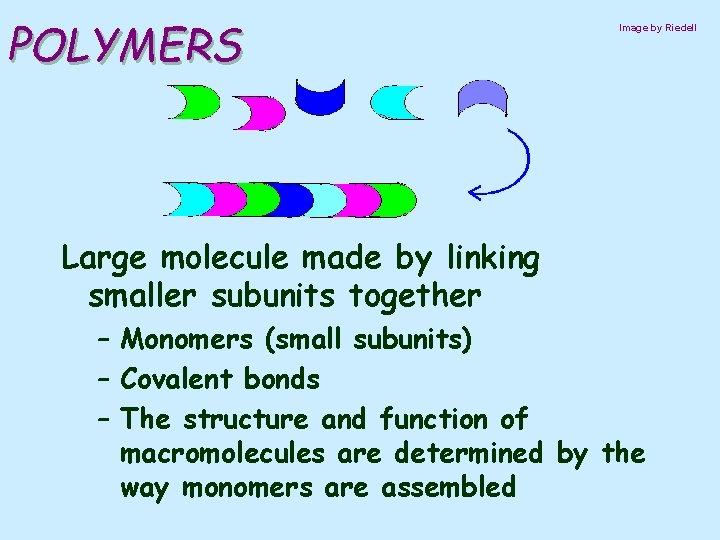
POLYMERS Image by Riedell Large molecule made by linking smaller subunits together – Monomers (small subunits) – Covalent bonds – The structure and function of macromolecules are determined by the way monomers are assembled

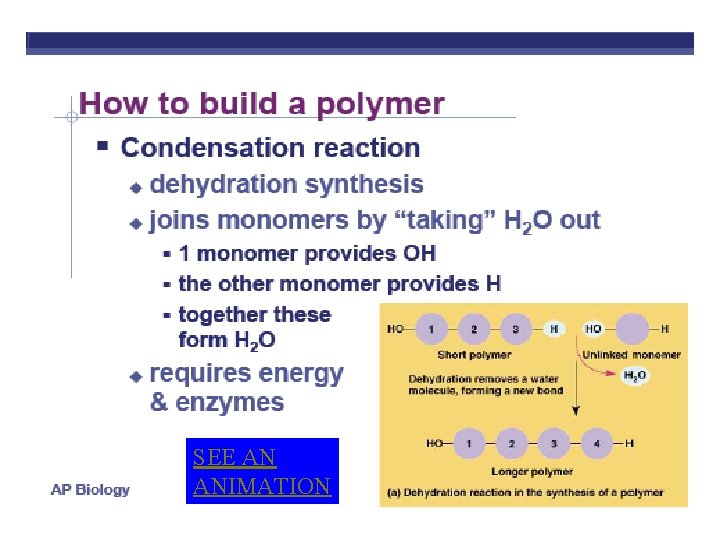
SEE AN ANIMATION

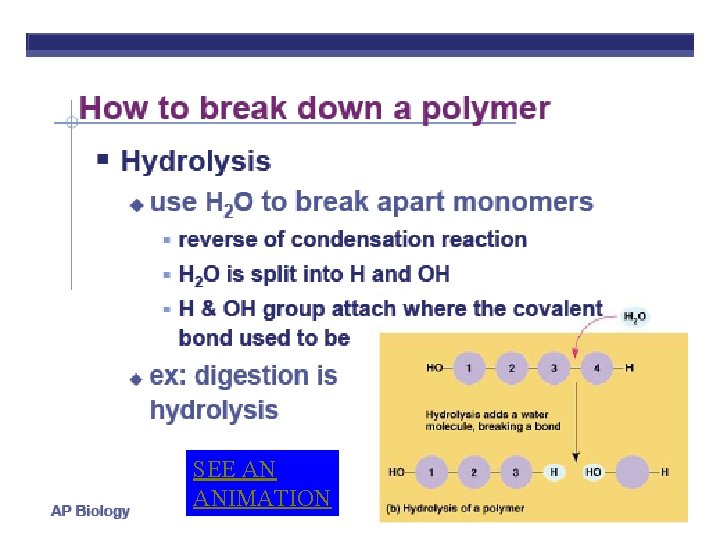
SEE AN ANIMATION

CARBOHYDRATES http: //www. graphic-design. com/Type/sugar/index. html http: //www. ifr. ac. uk/SPM/images/Starch%20 products. jpg

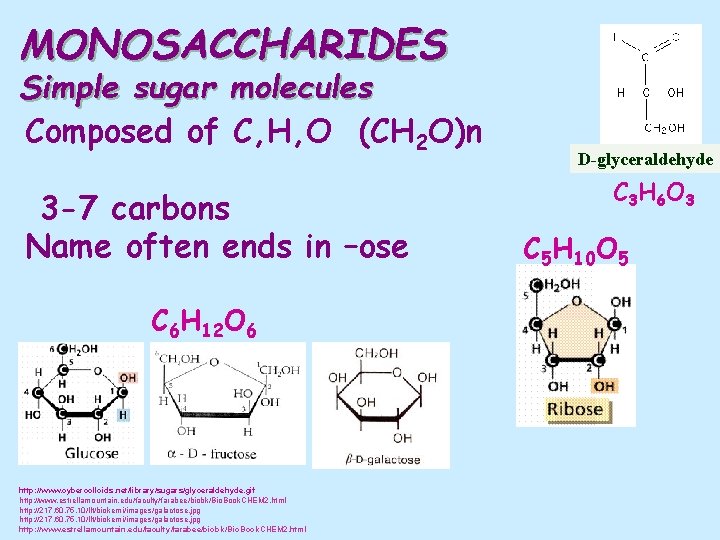
MONOSACCHARIDES Simple sugar molecules Composed of C, H, O (CH 2 O)n 3 -7 carbons Name often ends in –ose C 6 H 12 O 6 http: //www. cybercolloids. net/library/sugars/glyceraldehyde. gif http: //www. estrellamountain. edu/faculty/farabee/biobk/Bio. Book. CHEM 2. html http: //217. 60. 75. 10/llt/biokemi/images/galactose. jpg http: //www. estrellamountain. edu/faculty/farabee/biobk/Bio. Book. CHEM 2. html D-glyceraldehyde C 3 H 6 O 3 C 5 H 10 O 5

CARBOHYDRATES SUPPLY ENERGY Cells burn glucose and store the energy released as ATP Images from: http: //www. miranda. com/library. en/Images/Pictures/girls-runners. jpg http: //www. estrellamountain. edu/faculty/farabee/biobk/Bio. Book. CHEM 2. html

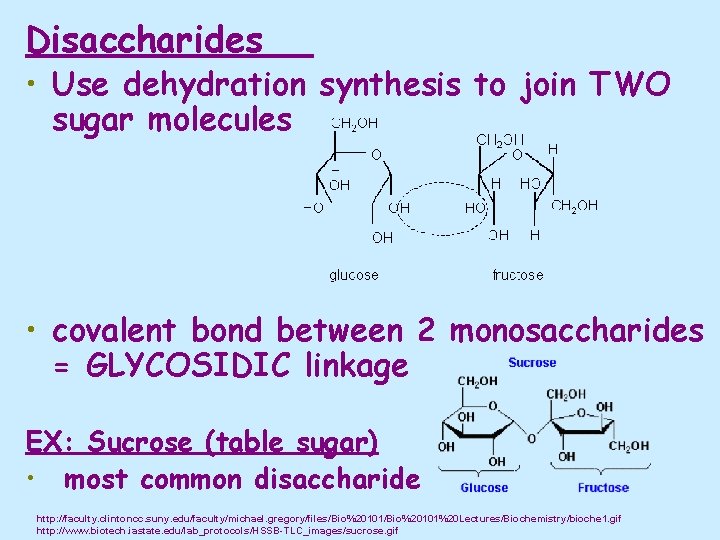
Disaccharides • Use dehydration synthesis to join TWO sugar molecules • covalent bond between 2 monosaccharides = GLYCOSIDIC linkage EX: Sucrose (table sugar) • most common disaccharide http: //faculty. clintoncc. suny. edu/faculty/michael. gregory/files/Bio%20101%20 Lectures/Biochemistry/bioche 1. gif http: //www. biotech. iastate. edu/lab_protocols/HSSB-TLC_images/sucrose. gif

DISACCHARIDES Glucose + Fructose → Sucrose + H 20 Glucose + Glucose → Maltose + H 20 Glucose + Galactose → Lactose + H 20

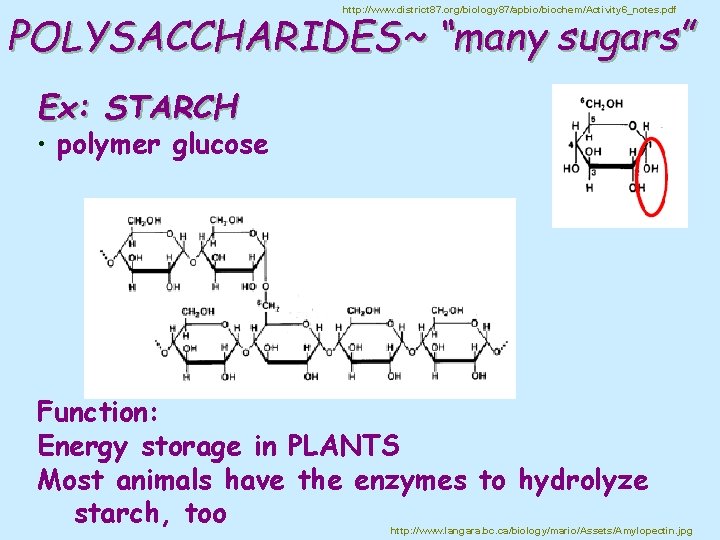
http: //www. district 87. org/biology 87/apbio/biochem/Activity 6_notes. pdf POLYSACCHARIDES~ “many sugars” Ex: STARCH • polymer glucose Function: Energy storage in PLANTS Most animals have the enzymes to hydrolyze starch, too http: //www. langara. bc. ca/biology/mario/Assets/Amylopectin. jpg

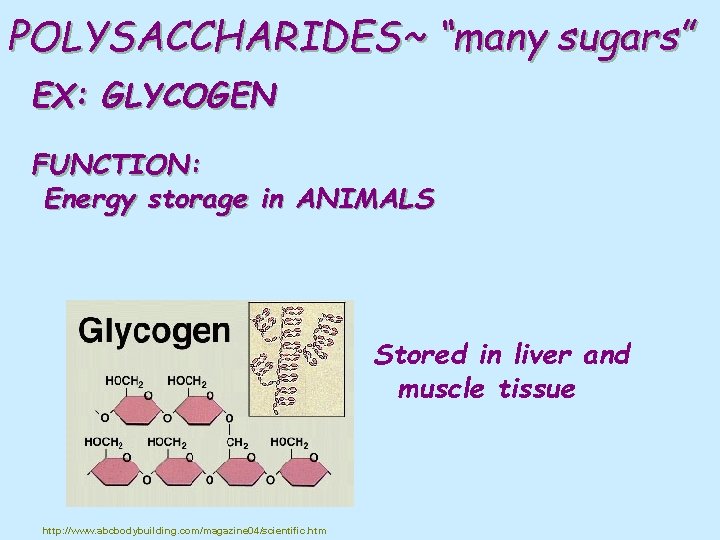
POLYSACCHARIDES~ “many sugars” EX: GLYCOGEN FUNCTION: Energy storage in ANIMALS Stored in liver and muscle tissue http: //www. abcbodybuilding. com/magazine 04/scientific. htm

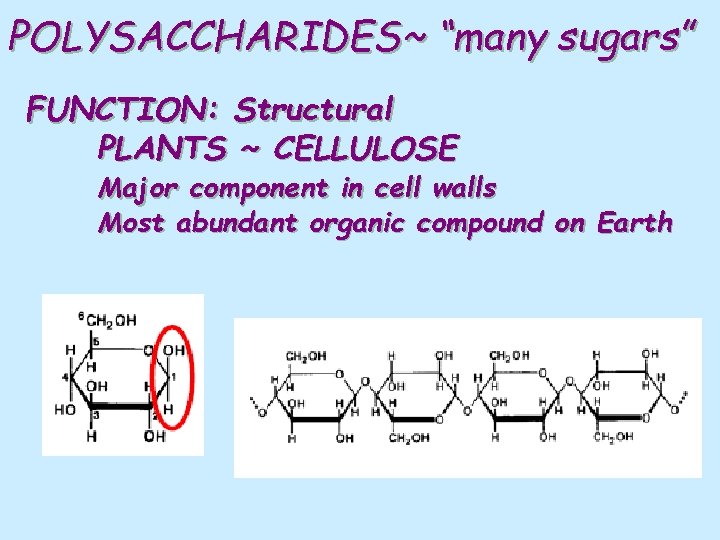
POLYSACCHARIDES~ “many sugars” FUNCTION: Structural PLANTS ~ CELLULOSE Major component in cell walls Most abundant organic compound on Earth

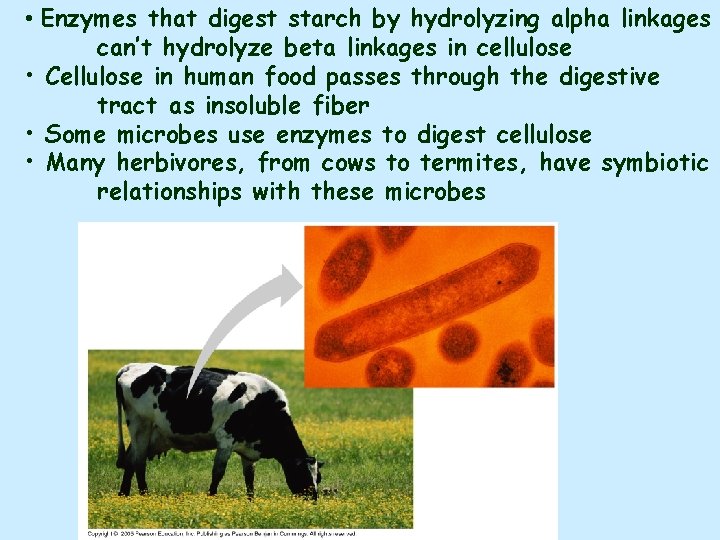
• Enzymes that digest starch by hydrolyzing alpha linkages can’t hydrolyze beta linkages in cellulose • Cellulose in human food passes through the digestive tract as insoluble fiber • Some microbes use enzymes to digest cellulose • Many herbivores, from cows to termites, have symbiotic relationships with these microbes

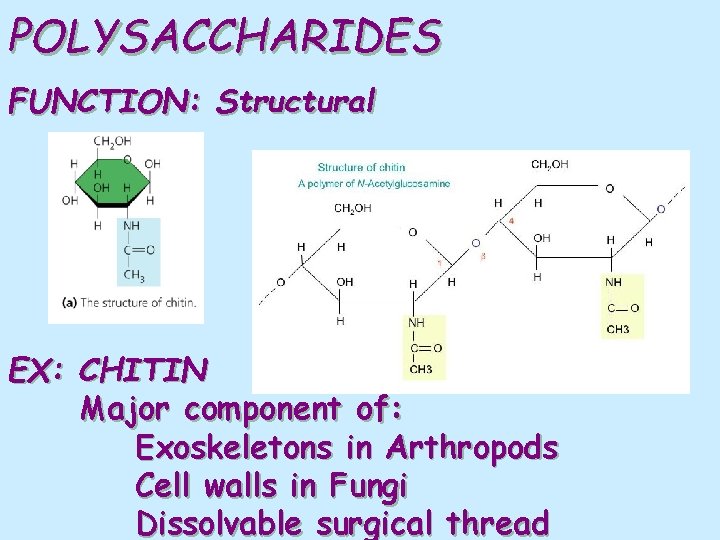
POLYSACCHARIDES FUNCTION: Structural EX: CHITIN Major component of: Exoskeletons in Arthropods Cell walls in Fungi Dissolvable surgical thread






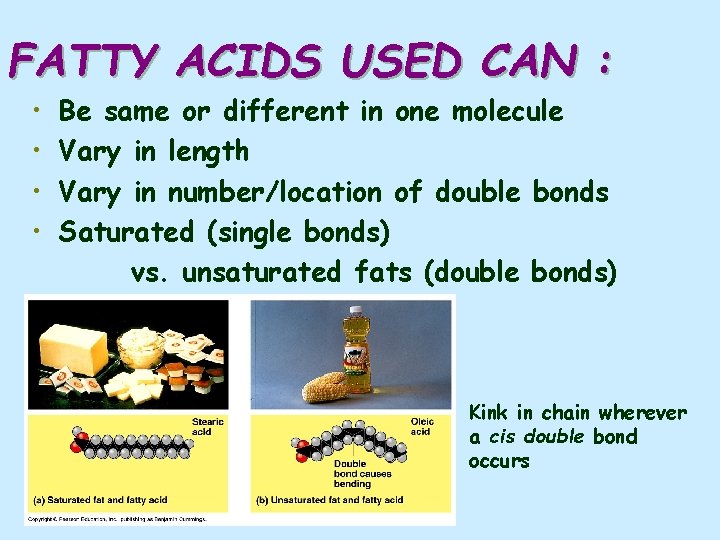
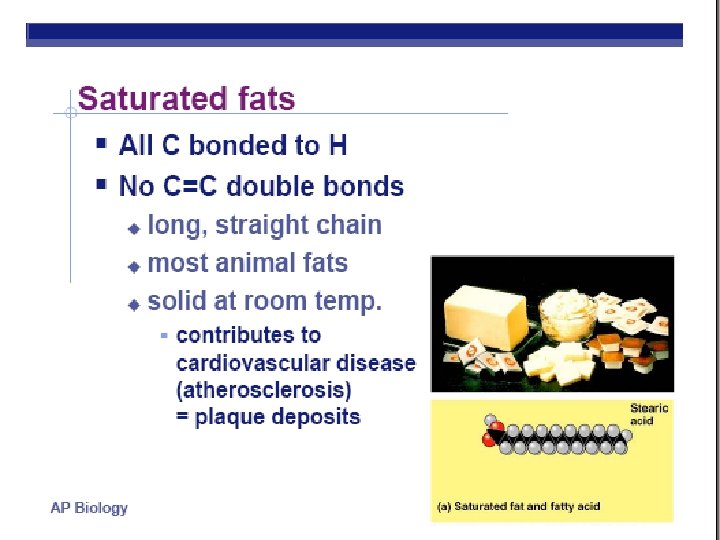
FATTY ACIDS USED CAN : • • Be same or different in one molecule Vary in length Vary in number/location of double bonds Saturated (single bonds) vs. unsaturated fats (double bonds) Kink in chain wherever a cis double bond occurs

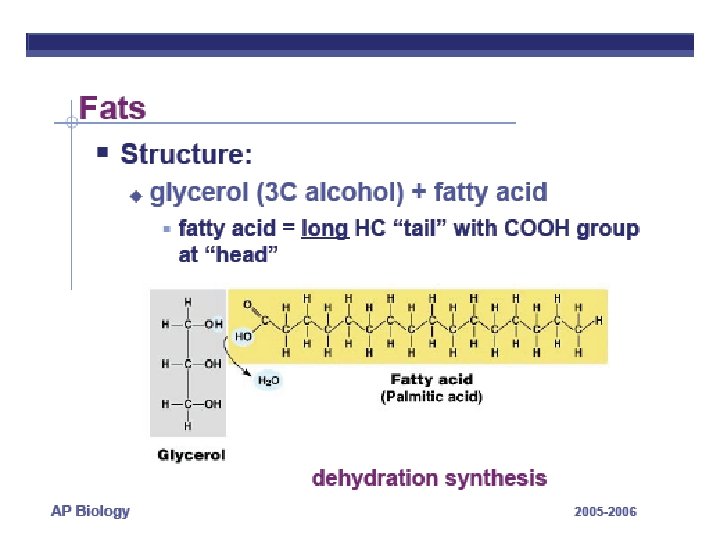
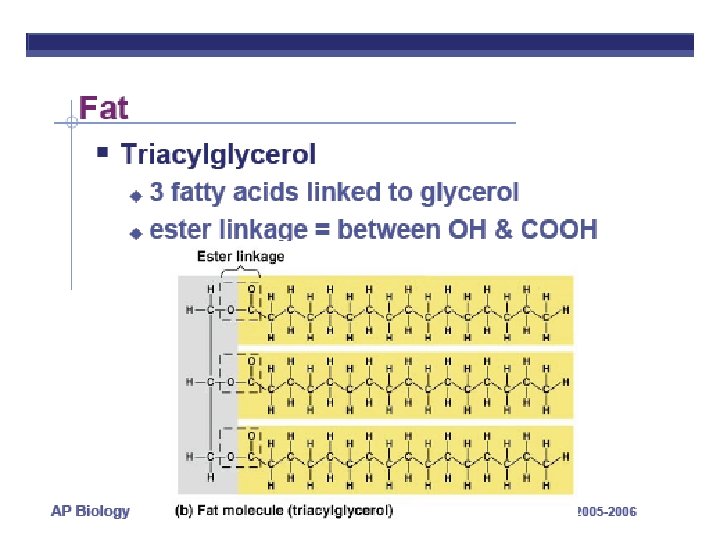
FATS LONG HC chain • NON-POLAR • HYDROPHOBIC FUNCTION: • Energy storage very rich 2 X energy in carbos • Cushions organs • Insulates body Think whale blubber!


Lipids, II

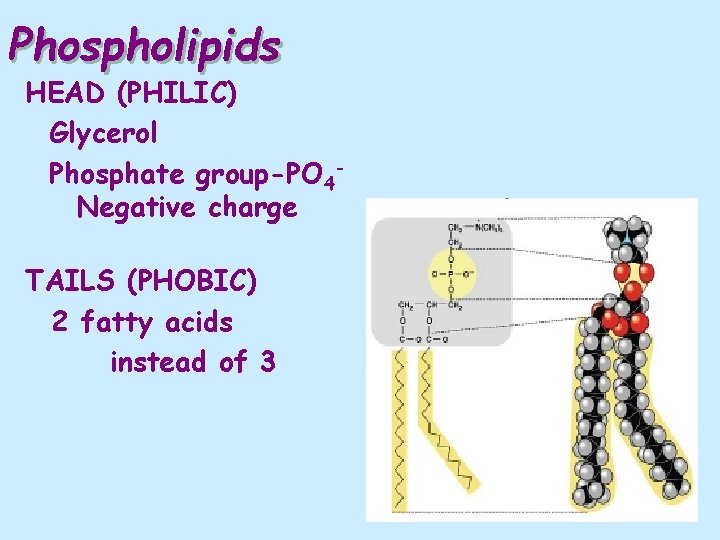
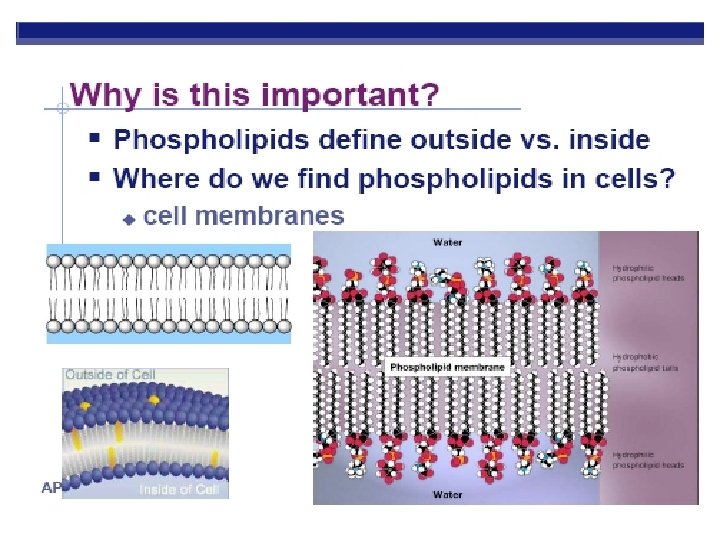
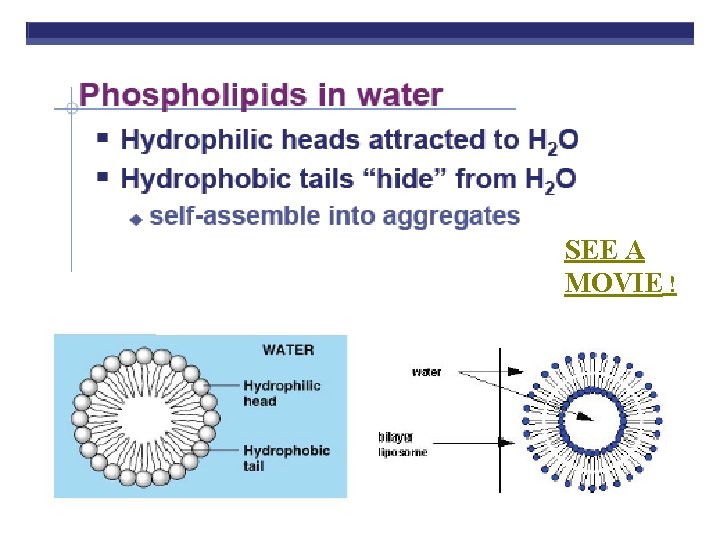
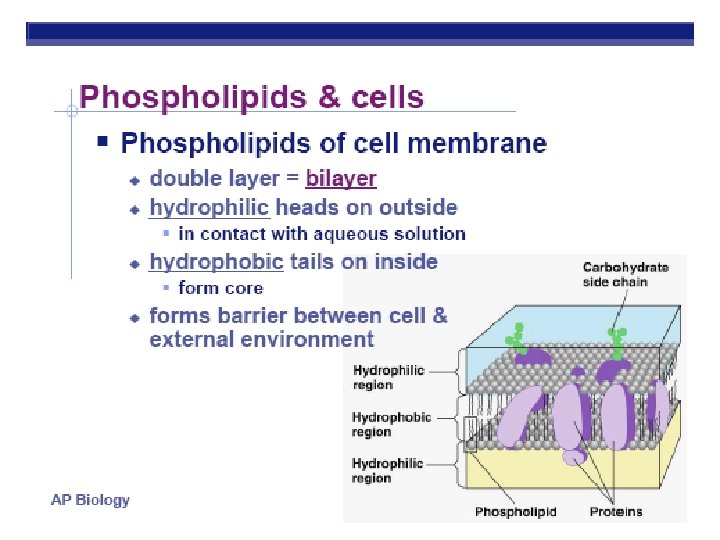
Phospholipids HEAD (PHILIC) Glycerol Phosphate group-PO 4 Negative charge TAILS (PHOBIC) 2 fatty acids instead of 3


SEE A MOVIE !







PROTEINS http: //images. foodnetwork. com/webfood/images/gethealthy/nutritionalallstars/Lean. Proteins_header. jpg

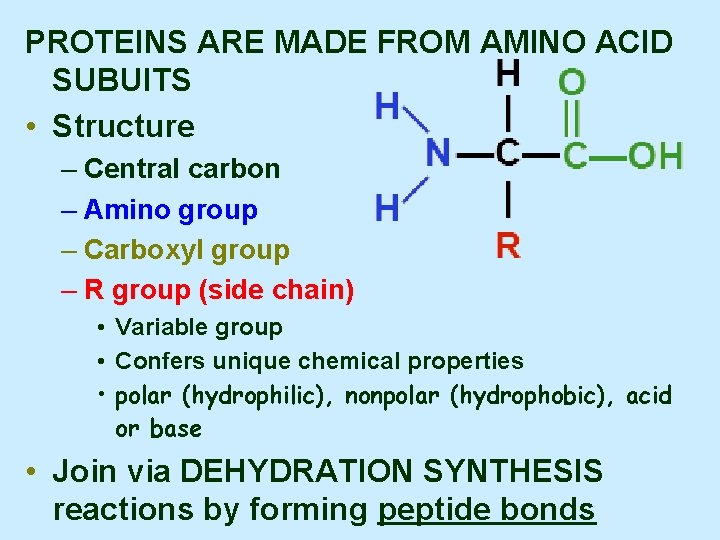
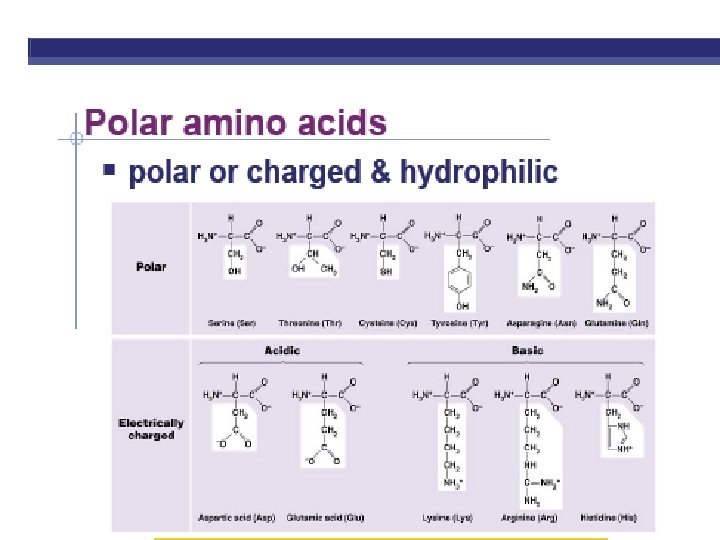
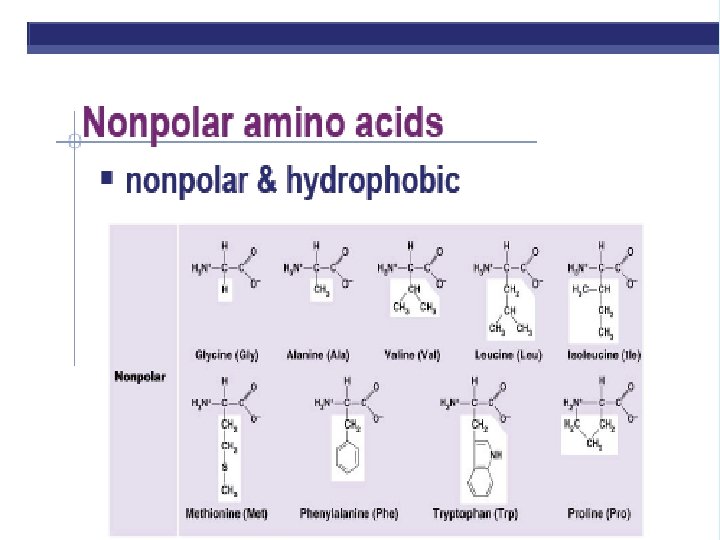
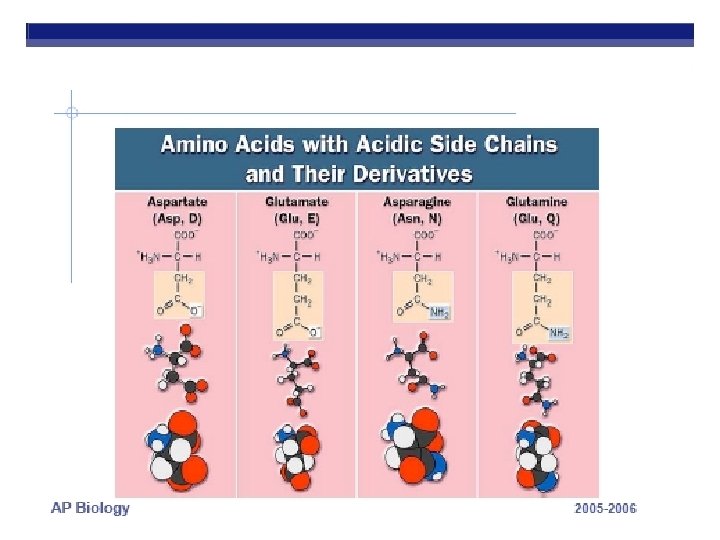
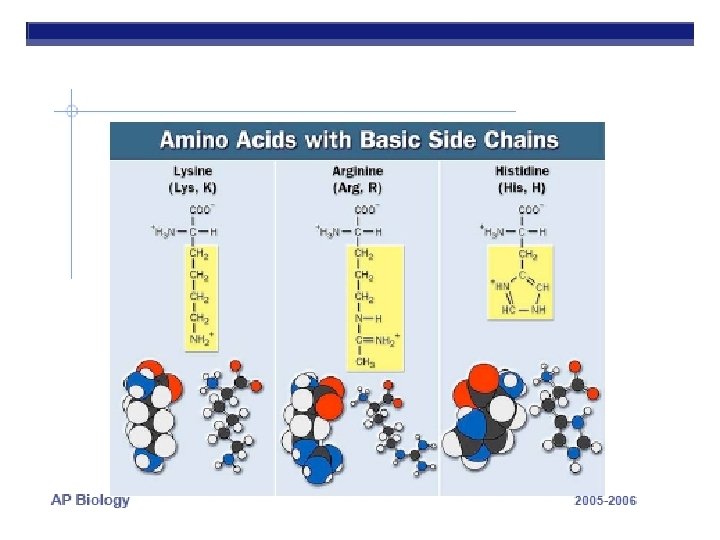
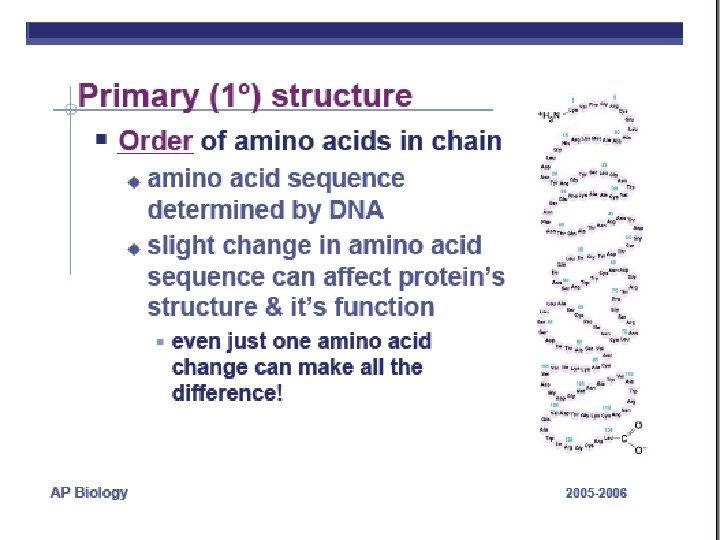
PROTEINS ARE MADE FROM AMINO ACID SUBUITS • Structure – Central carbon – Amino group – Carboxyl group – R group (side chain) • Variable group • Confers unique chemical properties • polar (hydrophilic), nonpolar (hydrophobic), acid or base • Join via DEHYDRATION SYNTHESIS reactions by forming peptide bonds

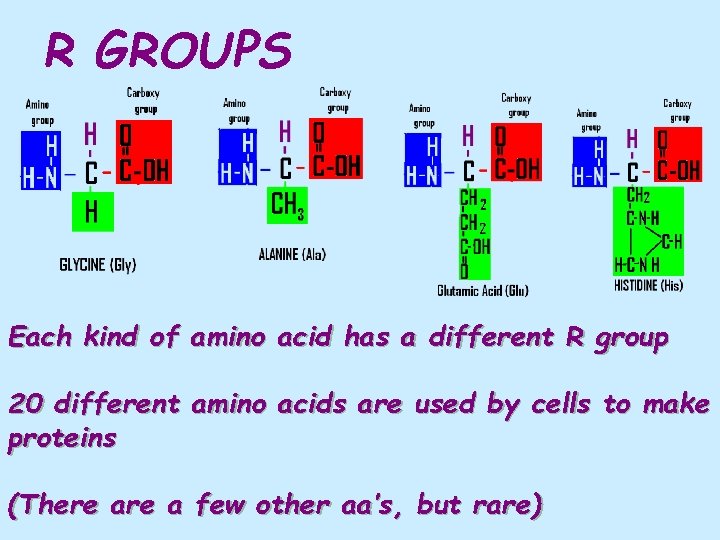
R GROUPS Each kind of amino acid has a different R group 20 different amino acids are used by cells to make proteins (There a few other aa’s, but rare)

See an animation

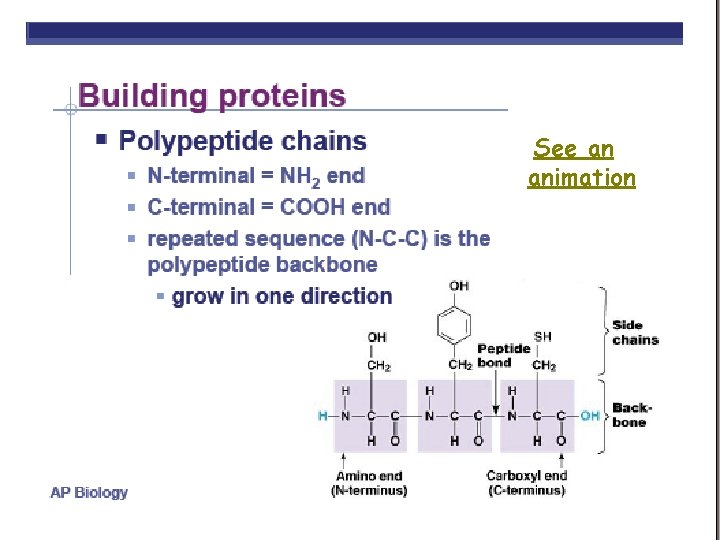
POLYPEPTIDES • POLYMERS OF AMINO ACIDS ARE CALLED POLYPEPTIDES • DNA determines the amino acid sequence http: //www. emc. maricopa. edu/faculty/farabee/BIOBK/Bio. Book. CHEM 2. html http: //www. cherishedtimedesigns. com/images/Bali. Charm. Bracelet. Graduation 500. jpg

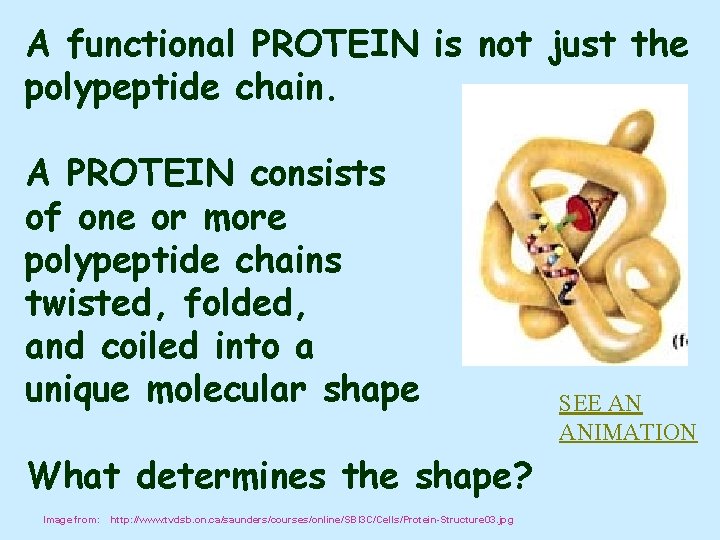
A functional PROTEIN is not just the polypeptide chain. A PROTEIN consists of one or more polypeptide chains twisted, folded, and coiled into a unique molecular shape What determines the shape? Image from: http: //www. tvdsb. on. ca/saunders/courses/online/SBI 3 C/Cells/Protein-Structure 03. jpg SEE AN ANIMATION






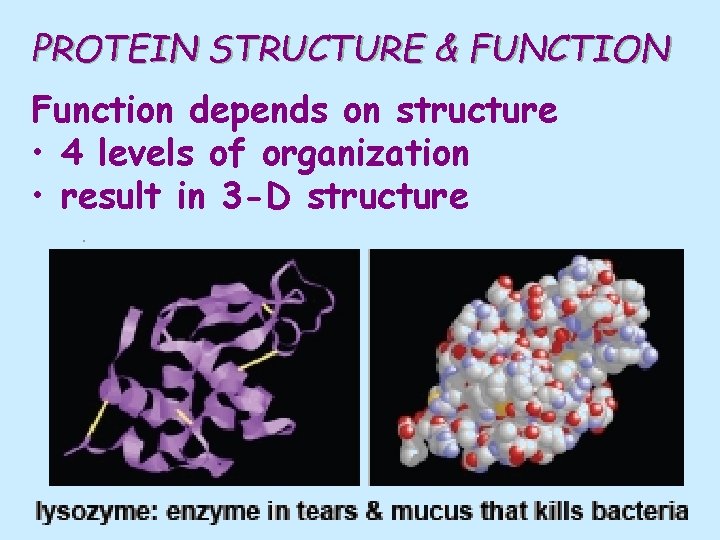
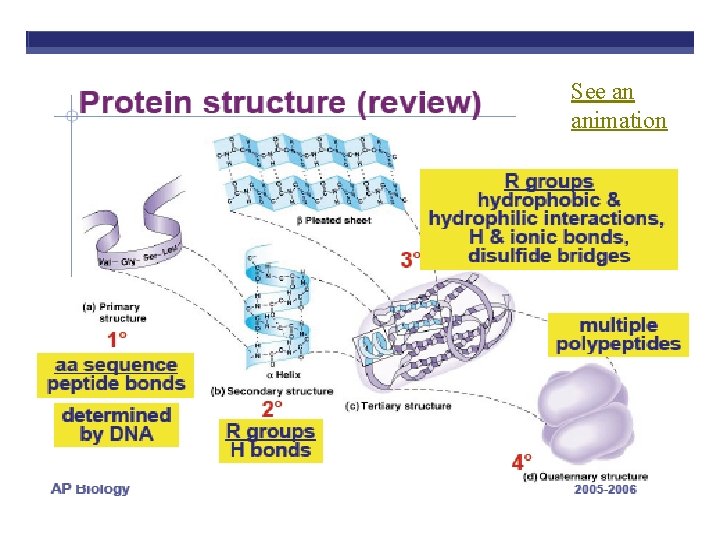
PROTEIN STRUCTURE & FUNCTION Function depends on structure • 4 levels of organization • result in 3 -D structure


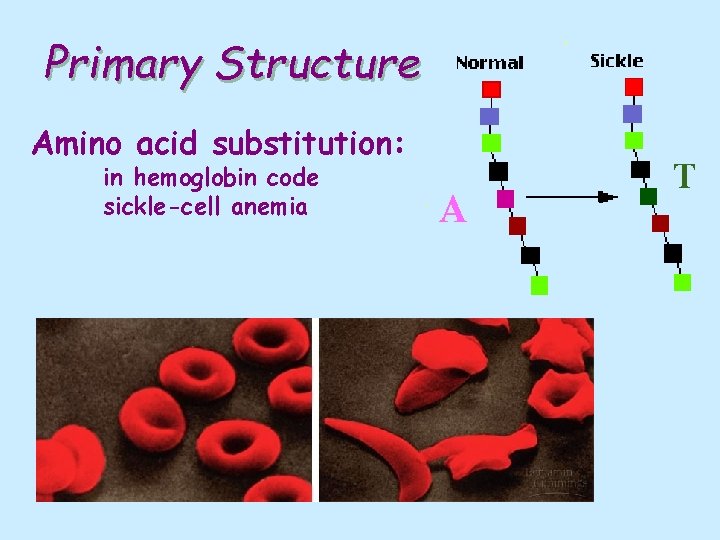
Primary Structure Amino acid substitution: in hemoglobin code sickle-cell anemia A T

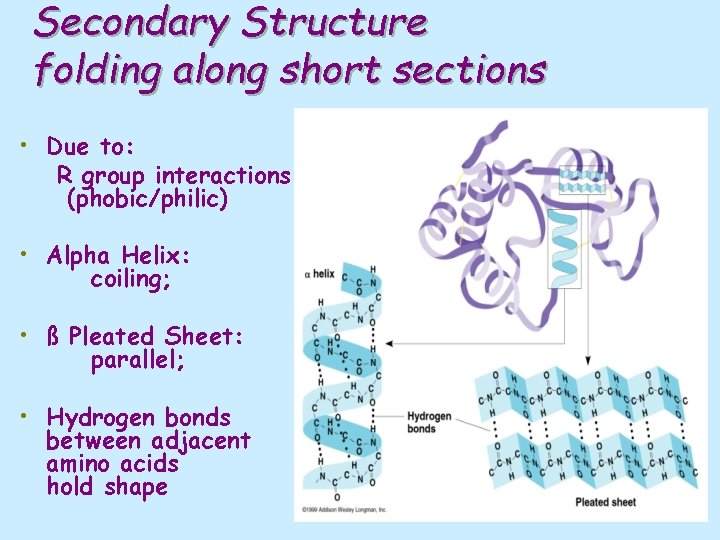
Secondary Structure folding along short sections • Due to: R group interactions (phobic/philic) • Alpha Helix: coiling; • ß Pleated Sheet: parallel; • Hydrogen bonds between adjacent amino acids hold shape

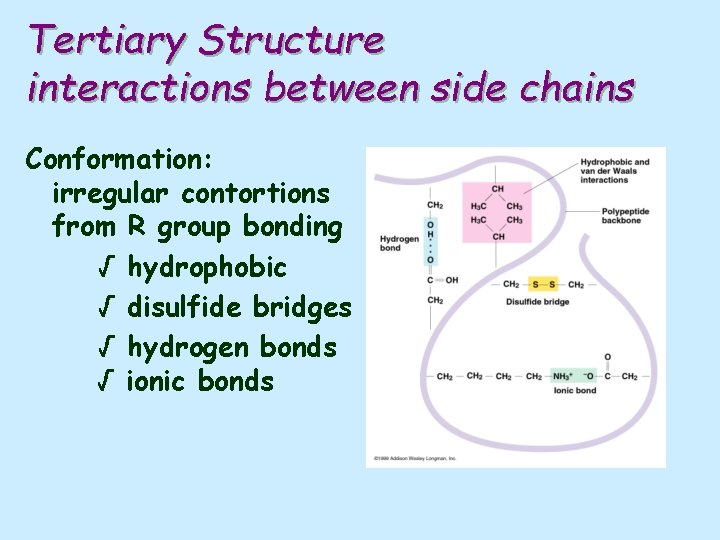
Tertiary Structure interactions between side chains Conformation: irregular contortions from R group bonding √ hydrophobic √ disulfide bridges √ hydrogen bonds √ ionic bonds

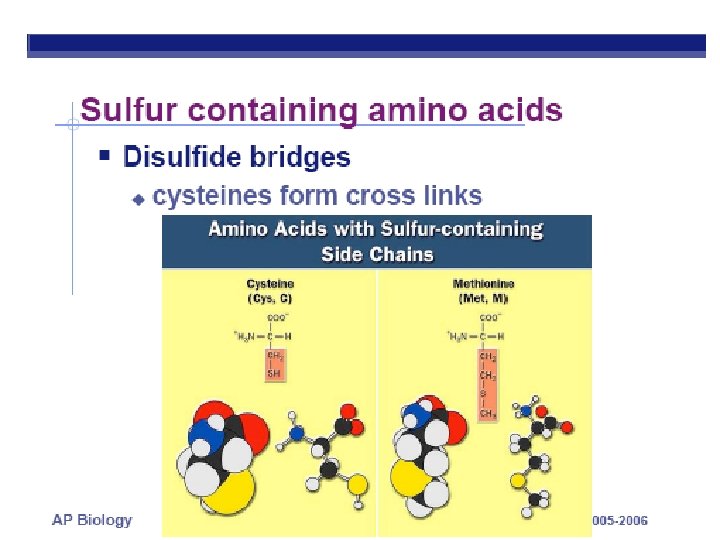
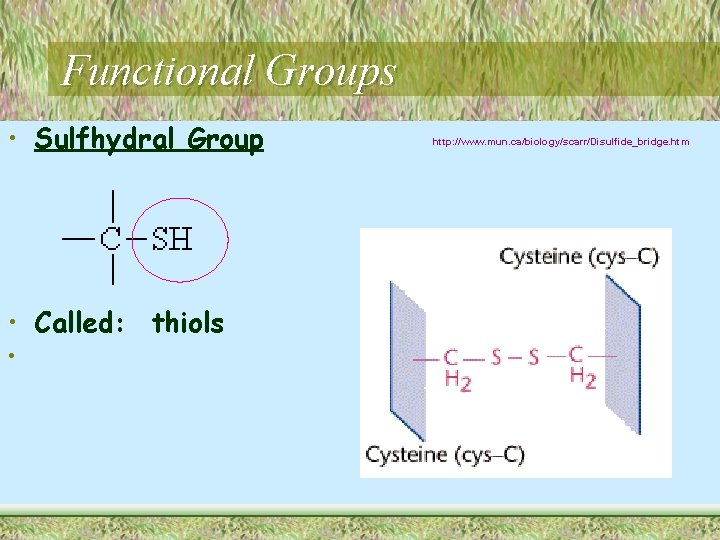
Functional Groups • Sulfhydral Group • Called: thiols • http: //www. mun. ca/biology/scarr/Disulfide_bridge. htm

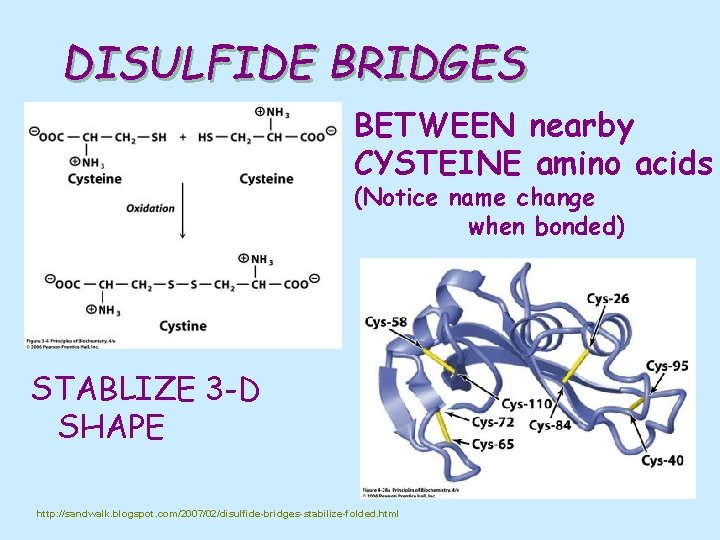
DISULFIDE BRIDGES BETWEEN nearby CYSTEINE amino acids (Notice name change when bonded) STABLIZE 3 -D SHAPE http: //sandwalk. blogspot. com/2007/02/disulfide-bridges-stabilize-folded. html

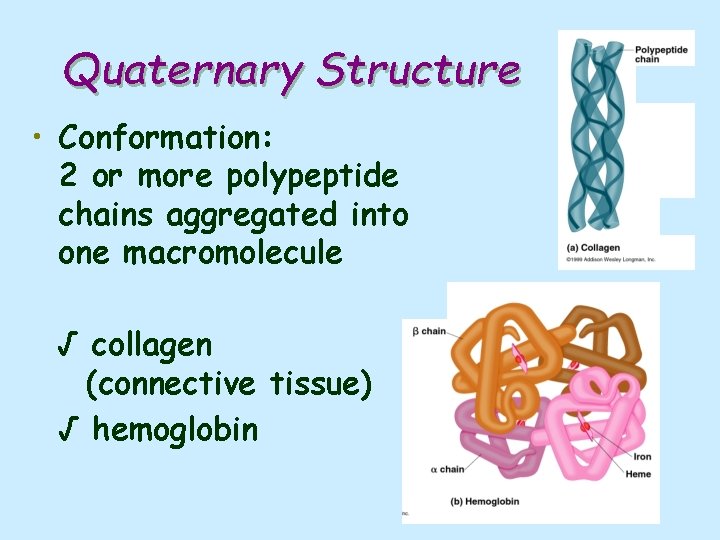
Quaternary Structure • Conformation: 2 or more polypeptide chains aggregated into one macromolecule √ collagen (connective tissue) √ hemoglobin

See an animation

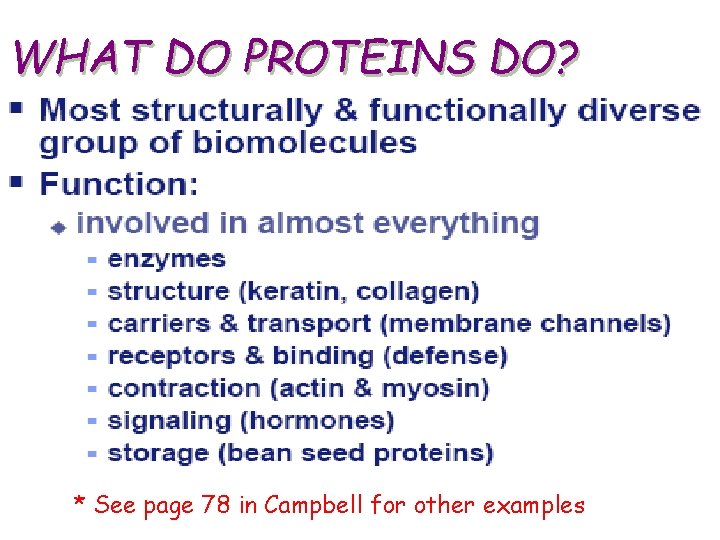
WHAT DO PROTEINS DO? * See page 78 in Campbell for other examples

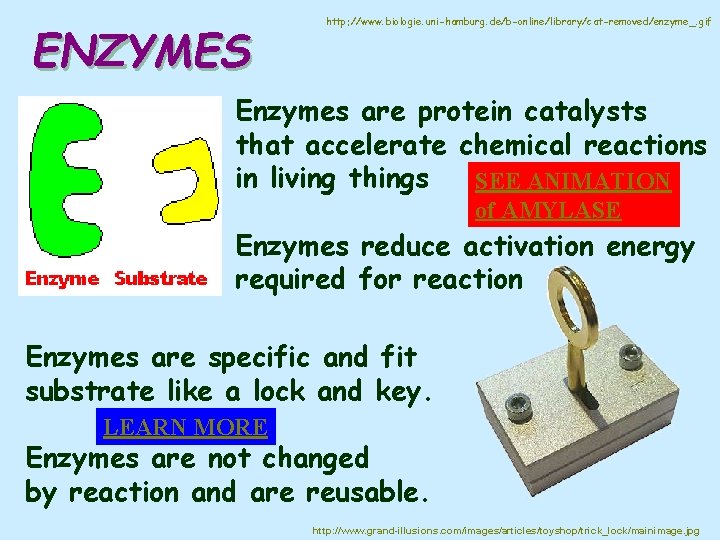
ENZYMES http: //www. biologie. uni-hamburg. de/b-online/library/cat-removed/enzyme_. gif Enzymes are protein catalysts that accelerate chemical reactions in living things SEE ANIMATION of AMYLASE Enzymes reduce activation energy required for reaction Enzymes are specific and fit substrate like a lock and key. LEARN MORE Enzymes are not changed by reaction and are reusable. http: //www. grand-illusions. com/images/articles/toyshop/trick_lock/mainimage. jpg

PROTEIN CONFORMATION ALSO DEPENDS ON PHYSICAL ENVIRONMENT LEARN MORE • p. H • Salt concentration • Temperature See a movie Choose narrated http: //www. nealbrownstudio. com/adm/photo/163_nb_fried_egg. jpg http: //www. desktopfotos. de/Downloads/melt_cd. jpg

Proteins that have denatured are biologically inactive Once conditions change, protein may need help returning to its functional shape. Facilitation of folding


NUCLEIC ACIDS

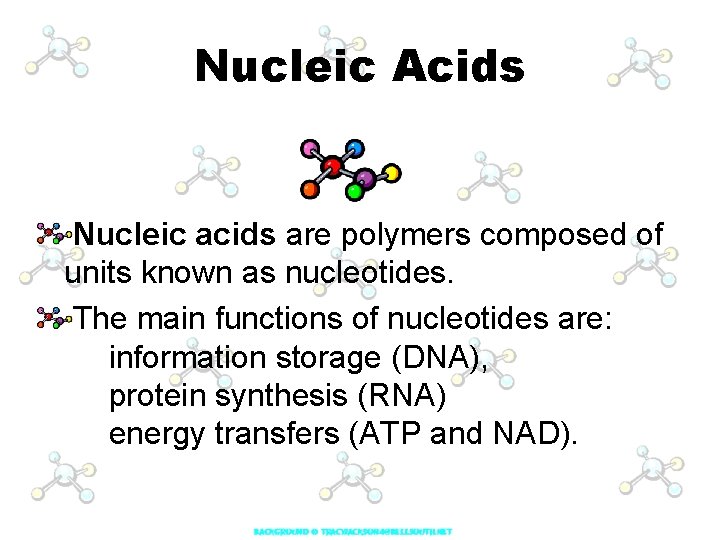
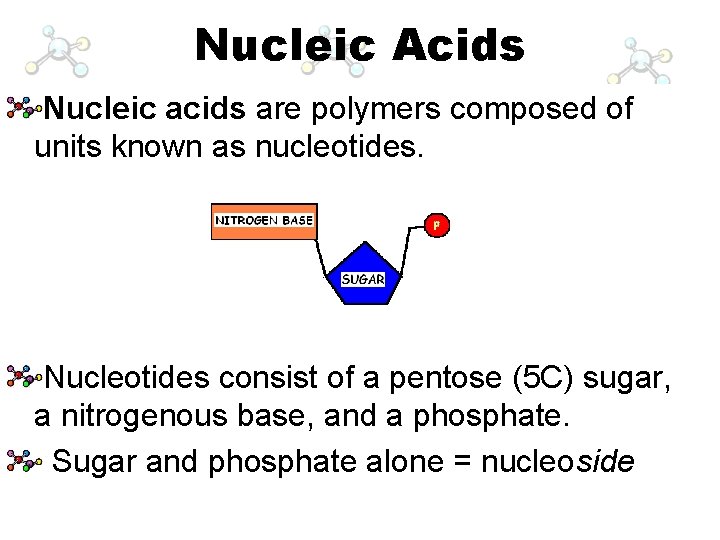
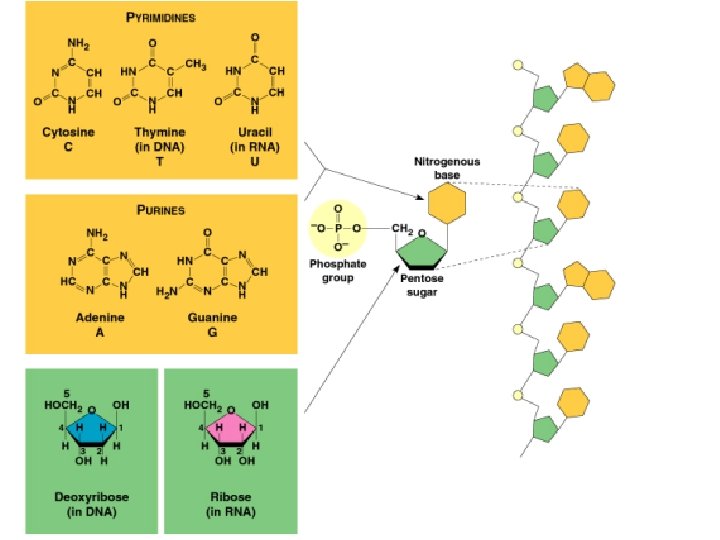
Nucleic Acids Nucleic acids are polymers composed of units known as nucleotides. The main functions of nucleotides are: information storage (DNA), protein synthesis (RNA) energy transfers (ATP and NAD).

Nucleic Acids Nucleic acids are polymers composed of units known as nucleotides. Nucleotides consist of a pentose (5 C) sugar, a nitrogenous base, and a phosphate. Sugar and phosphate alone = nucleoside

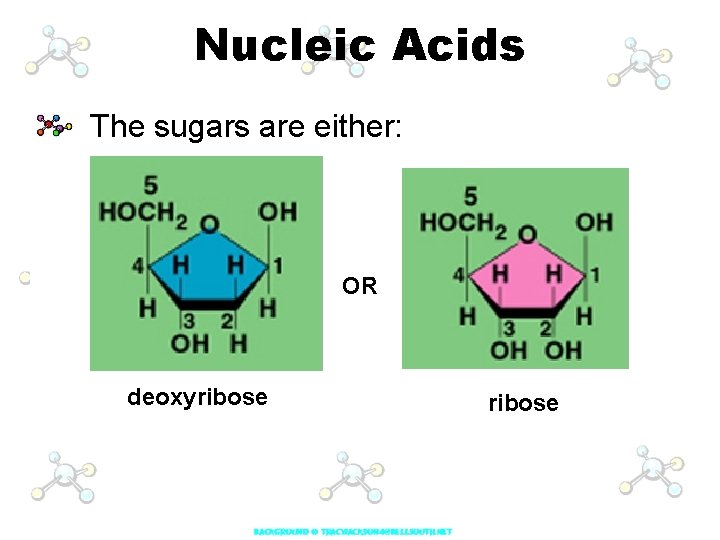
Nucleic Acids The sugars are either: OR deoxyribose

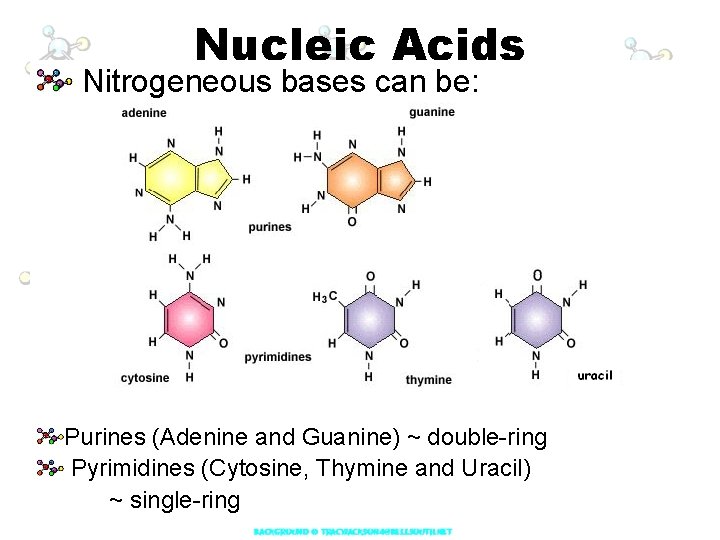
Nucleic Acids Nitrogeneous bases can be: Purines (Adenine and Guanine) ~ double-ring Pyrimidines (Cytosine, Thymine and Uracil) ~ single-ring

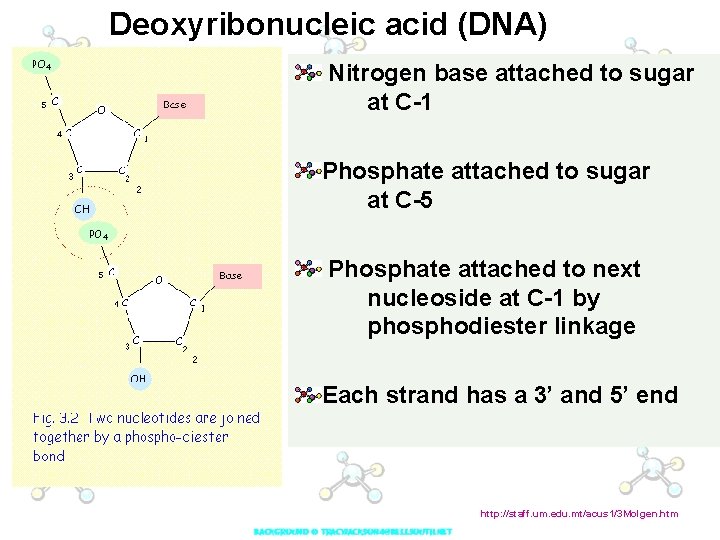
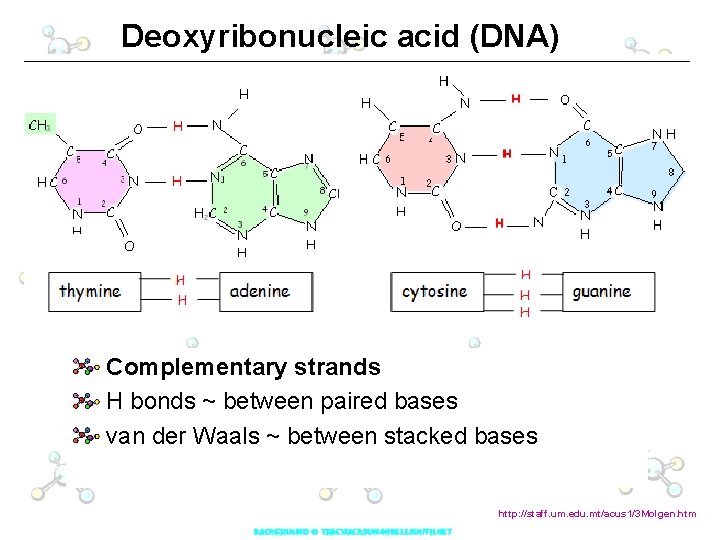
Deoxyribonucleic acid (DNA) Nitrogen base attached to sugar at C-1 Phosphate attached to sugar at C-5 Phosphate attached to next nucleoside at C-1 by phosphodiester linkage Each strand has a 3’ and 5’ end http: //staff. um. edu. mt/acus 1/3 Molgen. htm


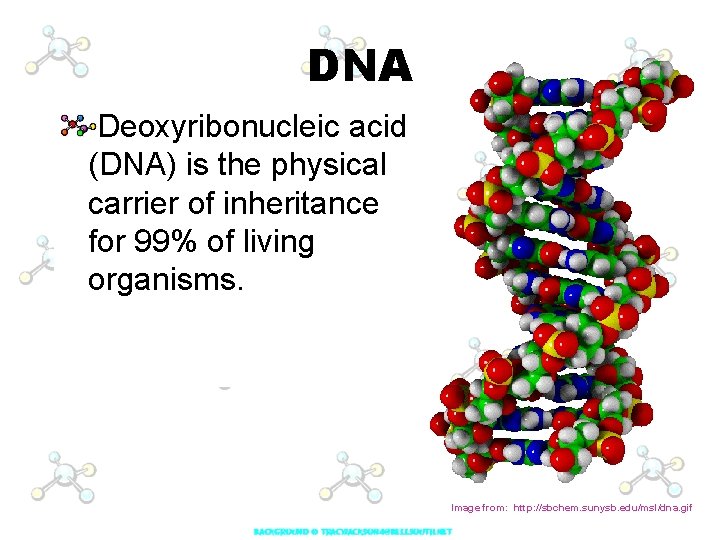
DNA Deoxyribonucleic acid (DNA) is the physical carrier of inheritance for 99% of living organisms. Image from: http: //sbchem. sunysb. edu/msl/dna. gif

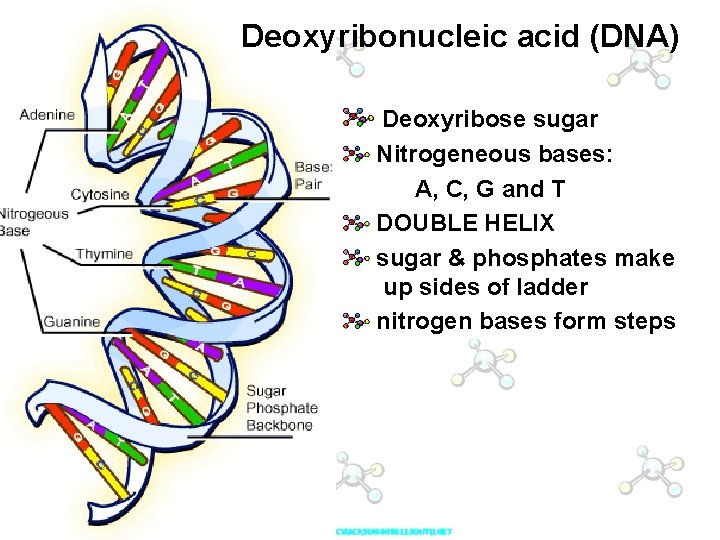
Deoxyribonucleic acid (DNA) Deoxyribose sugar Nitrogeneous bases: A, C, G and T DOUBLE HELIX sugar & phosphates make up sides of ladder nitrogen bases form steps

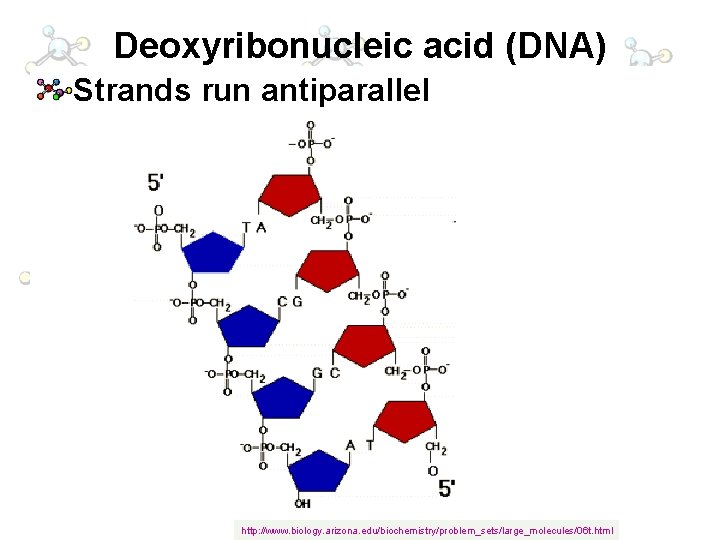
Deoxyribonucleic acid (DNA) Strands run antiparallel http: //www. biology. arizona. edu/biochemistry/problem_sets/large_molecules/06 t. html

Deoxyribonucleic acid (DNA) Complementary strands H bonds ~ between paired bases van der Waals ~ between stacked bases http: //staff. um. edu. mt/acus 1/3 Molgen. htm

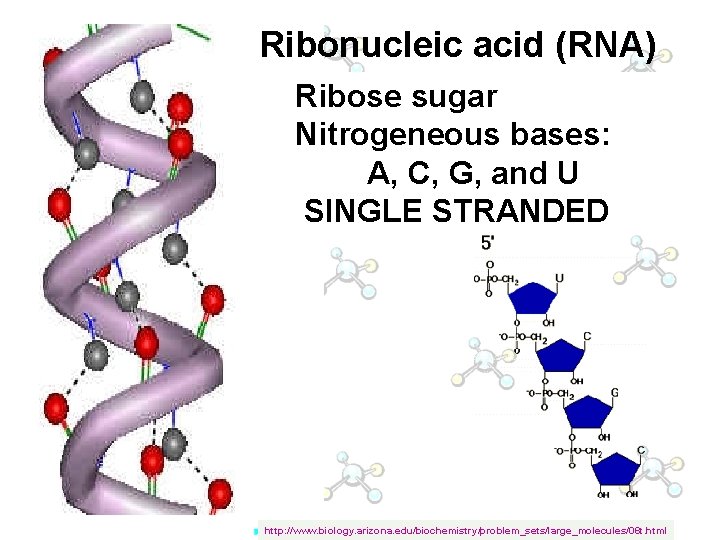
Ribonucleic acid (RNA) Ribose sugar Nitrogeneous bases: A, C, G, and U SINGLE STRANDED http: //www. biology. arizona. edu/biochemistry/problem_sets/large_molecules/06 t. html

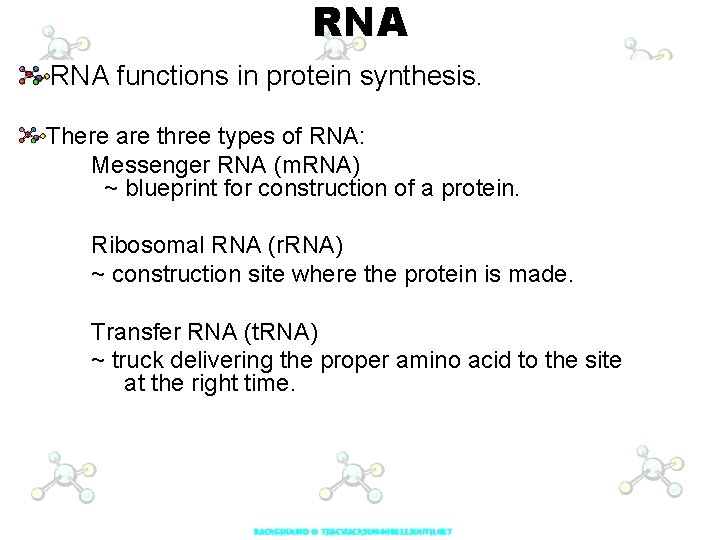
RNA functions in protein synthesis. There are three types of RNA: Messenger RNA (m. RNA) ~ blueprint for construction of a protein. Ribosomal RNA (r. RNA) ~ construction site where the protein is made. Transfer RNA (t. RNA) ~ truck delivering the proper amino acid to the site at the right time.

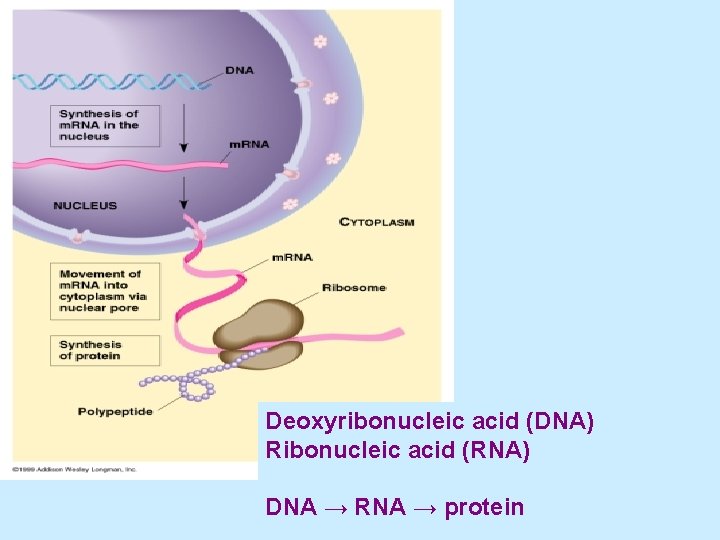
Deoxyribonucleic acid (DNA) Ribonucleic acid (RNA) DNA → RNA → protein

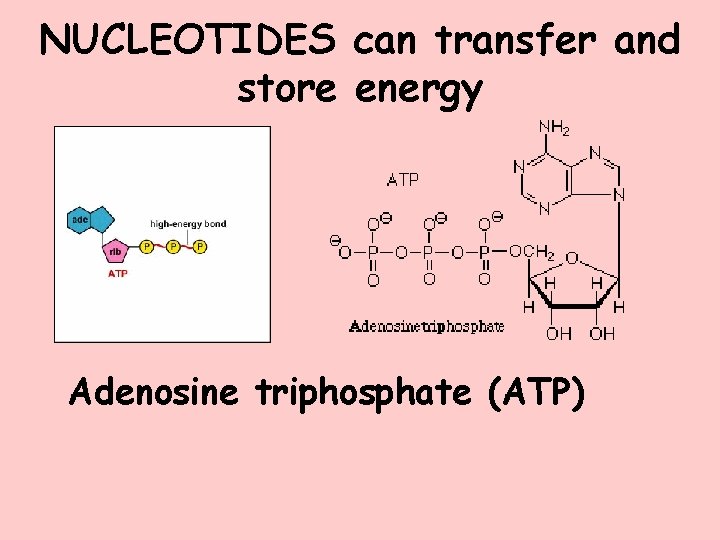
NUCLEOTIDES can transfer and store energy Adenosine triphosphate (ATP)

NUCLEOTIDES can transfer and store energy NAD+ NADP+ FAD Coenzyme A Energy and electron carriers used in photosynthesis and respiration More on this next unit!