Organic compounds containing nitrogen and sulfur Alice Skoumalov



- Slides: 30

Organic compounds containing nitrogen and sulfur Alice Skoumalová

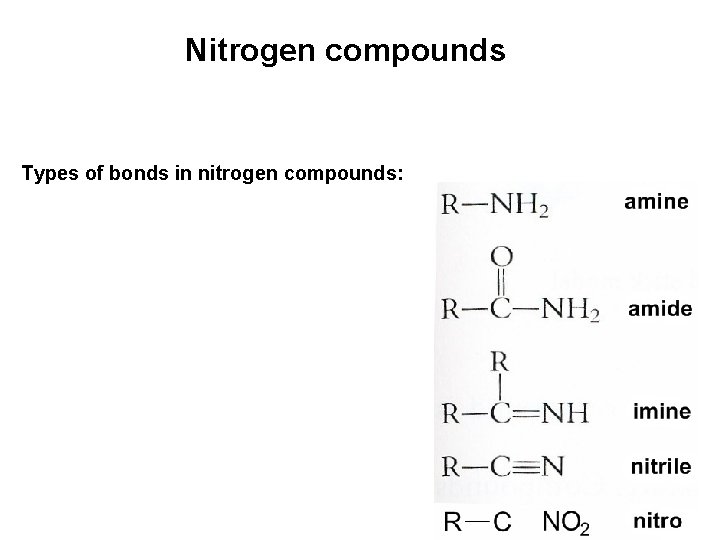
Nitrogen compounds Types of bonds in nitrogen compounds:

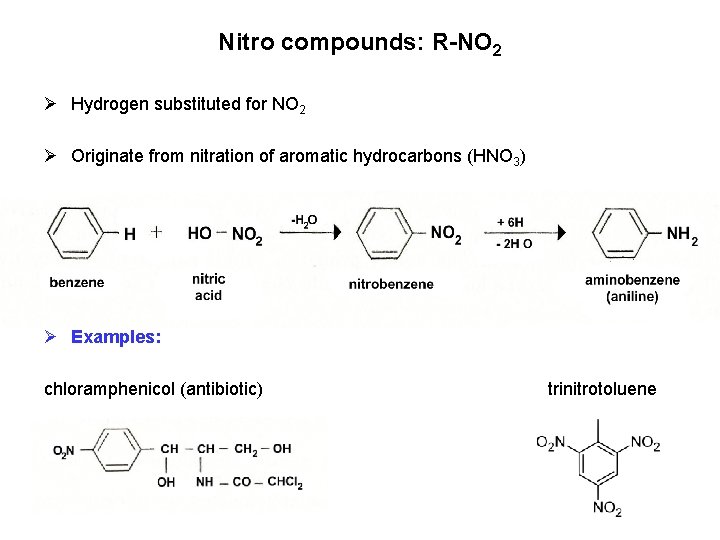
Nitro compounds: R-NO 2 Ø Hydrogen substituted for NO 2 Ø Originate from nitration of aromatic hydrocarbons (HNO 3) Ø Examples: chloramphenicol (antibiotic) trinitrotoluene

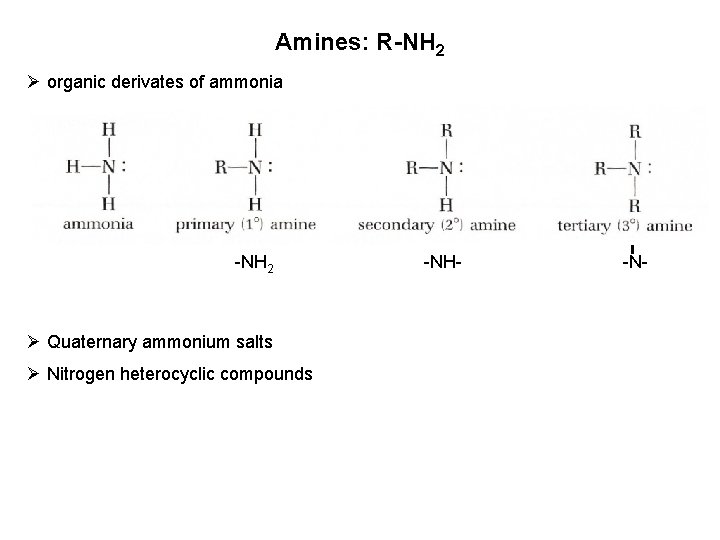
Amines: R-NH 2 Ø organic derivates of ammonia -NH 2 Ø Quaternary ammonium salts Ø Nitrogen heterocyclic compounds -NH- -N-

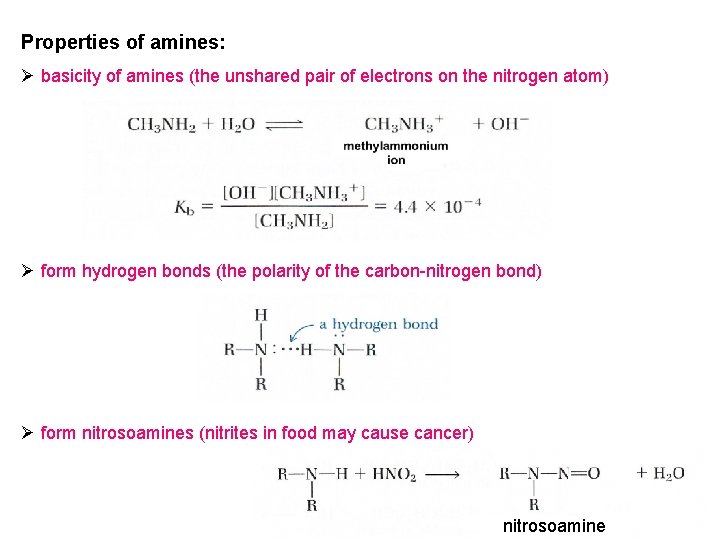
Properties of amines: Ø basicity of amines (the unshared pair of electrons on the nitrogen atom) Ø form hydrogen bonds (the polarity of the carbon-nitrogen bond) Ø form nitrosoamines (nitrites in food may cause cancer) nitrosoamine

Importance of amines: The most important organic bases In biochemistry: Ø Quaternary ammonium salts Ø Biogenic amines Ø Amino acids Ø Proteins Ø Alkaloids

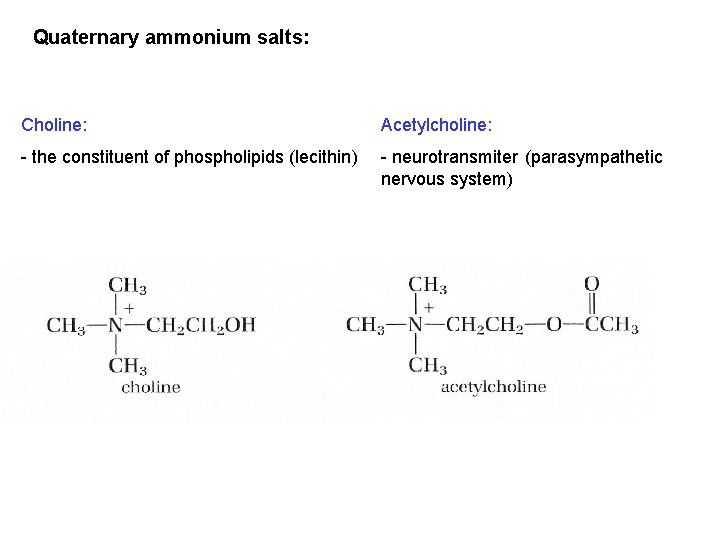
Quaternary ammonium salts: Choline: Acetylcholine: - the constituent of phospholipids (lecithin) - neurotransmiter (parasympathetic nervous system)

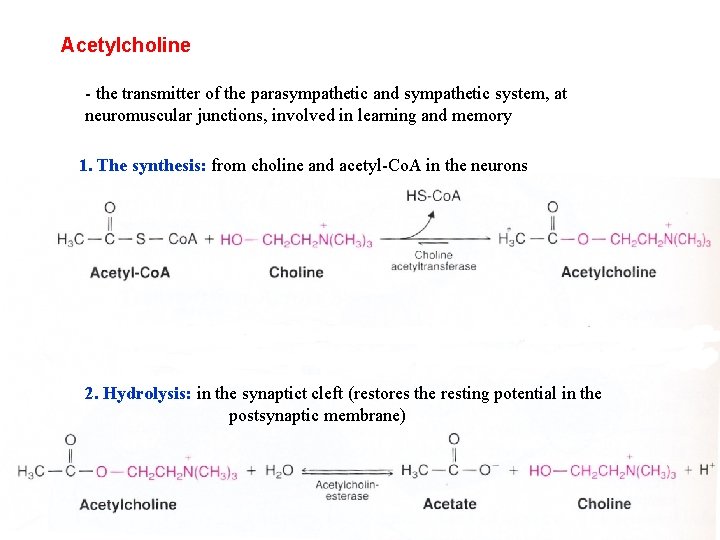
Acetylcholine - the transmitter of the parasympathetic and sympathetic system, at neuromuscular junctions, involved in learning and memory 1. The synthesis: from choline and acetyl-Co. A in the neurons 2. Hydrolysis: in the synaptict cleft (restores the resting potential in the postsynaptic membrane)

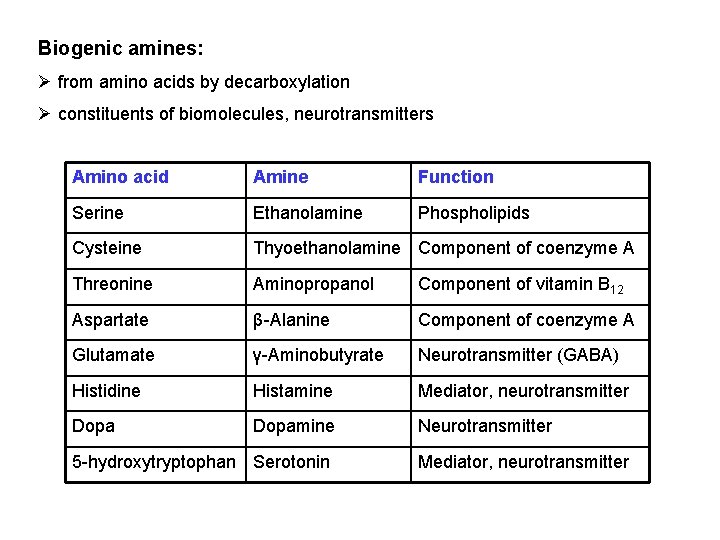
Biogenic amines: Ø from amino acids by decarboxylation Ø constituents of biomolecules, neurotransmitters Amino acid Amine Function Serine Ethanolamine Phospholipids Cysteine Thyoethanolamine Component of coenzyme A Threonine Aminopropanol Component of vitamin B 12 Aspartate β-Alanine Component of coenzyme A Glutamate γ-Aminobutyrate Neurotransmitter (GABA) Histidine Histamine Mediator, neurotransmitter Dopamine Neurotransmitter 5 -hydroxytryptophan Serotonin Mediator, neurotransmitter

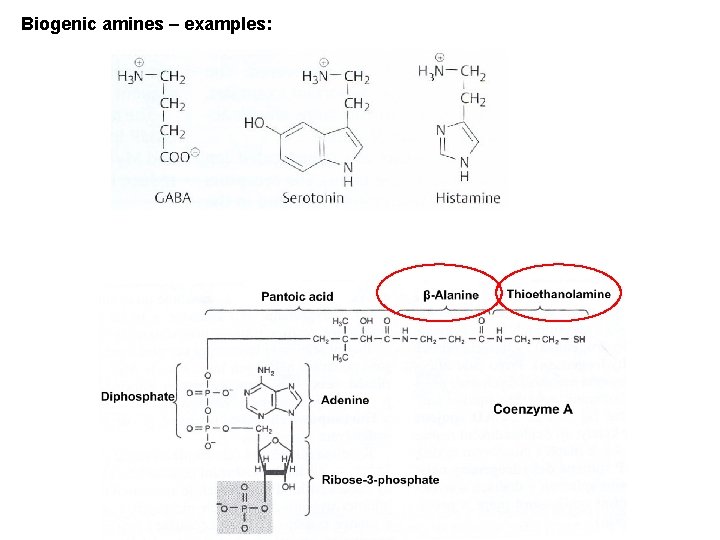
Biogenic amines – examples:

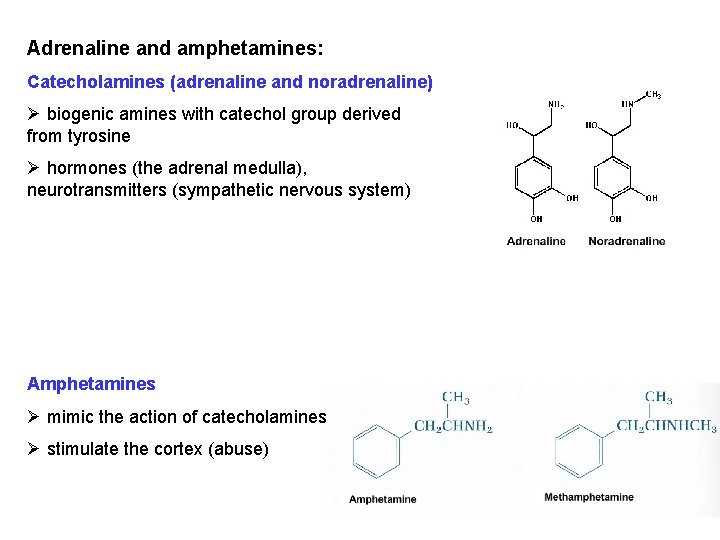
Adrenaline and amphetamines: Catecholamines (adrenaline and noradrenaline) Ø biogenic amines with catechol group derived from tyrosine Ø hormones (the adrenal medulla), neurotransmitters (sympathetic nervous system) Amphetamines Ø mimic the action of catecholamines Ø stimulate the cortex (abuse)

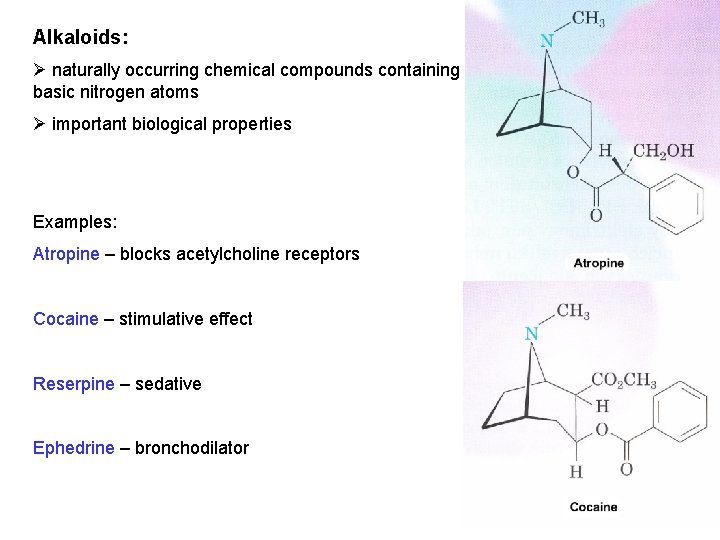
Alkaloids: Ø naturally occurring chemical compounds containing basic nitrogen atoms Ø important biological properties Examples: Atropine – blocks acetylcholine receptors Cocaine – stimulative effect Reserpine – sedative Ephedrine – bronchodilator

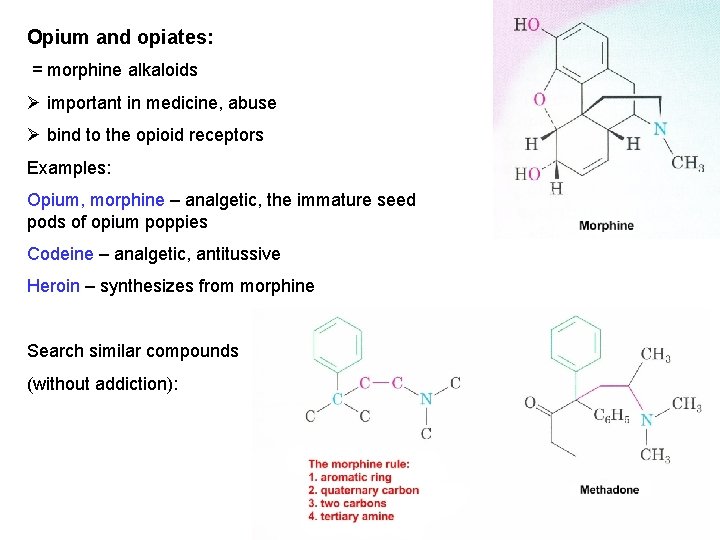
Opium and opiates: = morphine alkaloids Ø important in medicine, abuse Ø bind to the opioid receptors Examples: Opium, morphine – analgetic, the immature seed pods of opium poppies Codeine – analgetic, antitussive Heroin – synthesizes from morphine Search similar compounds (without addiction):

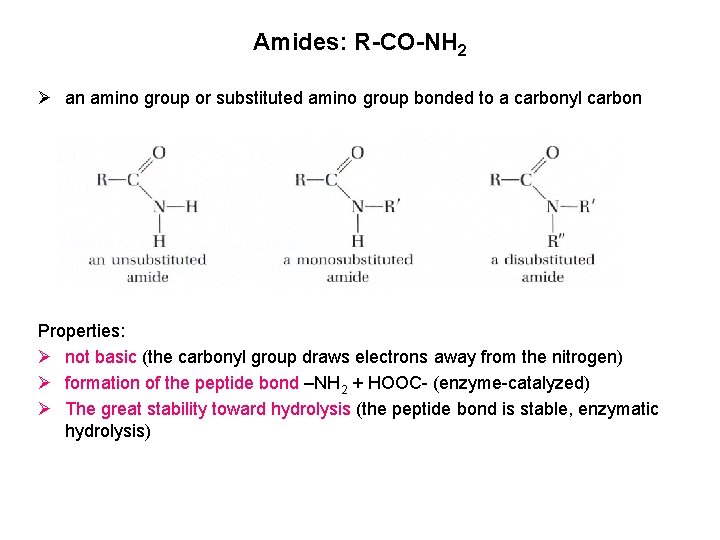
Amides: R-CO-NH 2 Ø an amino group or substituted amino group bonded to a carbonyl carbon Properties: Ø not basic (the carbonyl group draws electrons away from the nitrogen) Ø formation of the peptide bond –NH 2 + HOOC- (enzyme-catalyzed) Ø The great stability toward hydrolysis (the peptide bond is stable, enzymatic hydrolysis)

Important amides in medicine: Øasparagine, glutamine Øurea, carbamate, creatine Ønicotinamide – coenzyme NAD Øpeptides Øbarbiturates

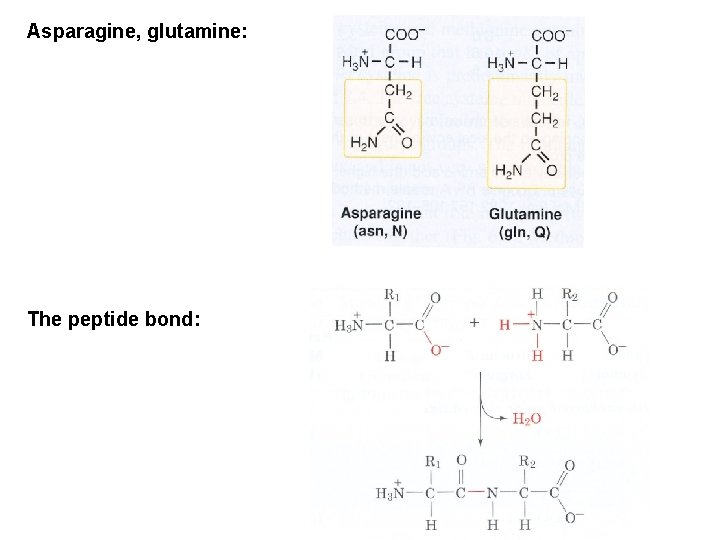
Asparagine, glutamine: The peptide bond:

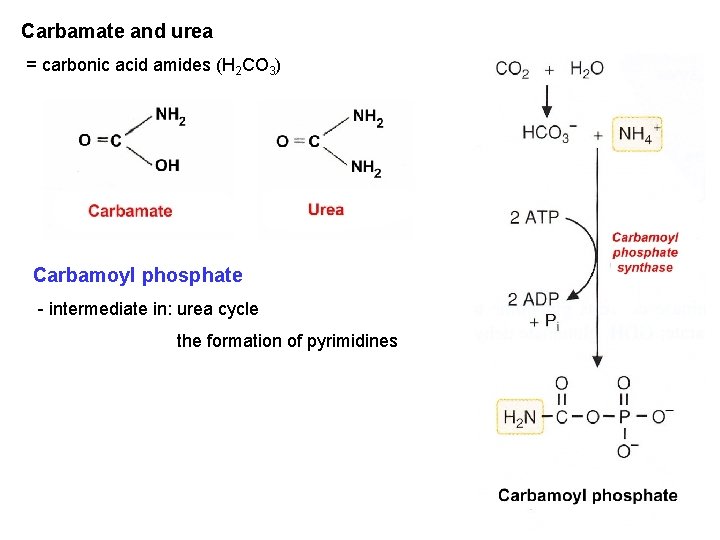
Carbamate and urea = carbonic acid amides (H 2 CO 3) Carbamoyl phosphate - intermediate in: urea cycle the formation of pyrimidines

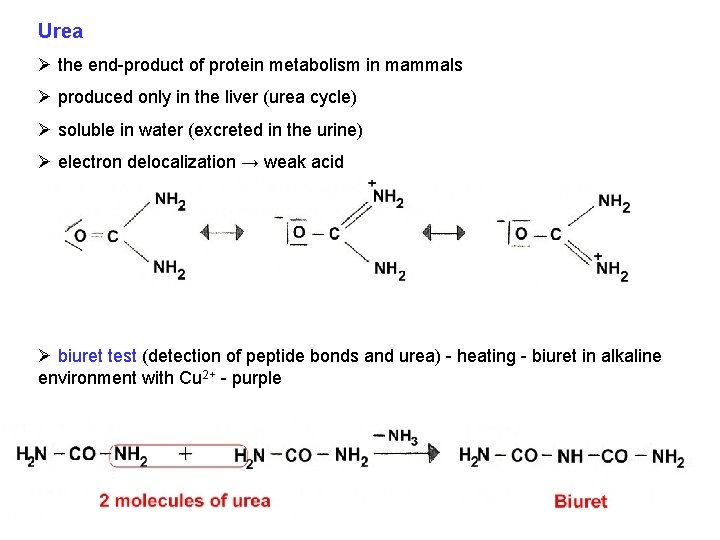
Urea Ø the end-product of protein metabolism in mammals Ø produced only in the liver (urea cycle) Ø soluble in water (excreted in the urine) Ø electron delocalization → weak acid Ø biuret test (detection of peptide bonds and urea) - heating - biuret in alkaline environment with Cu 2+ - purple

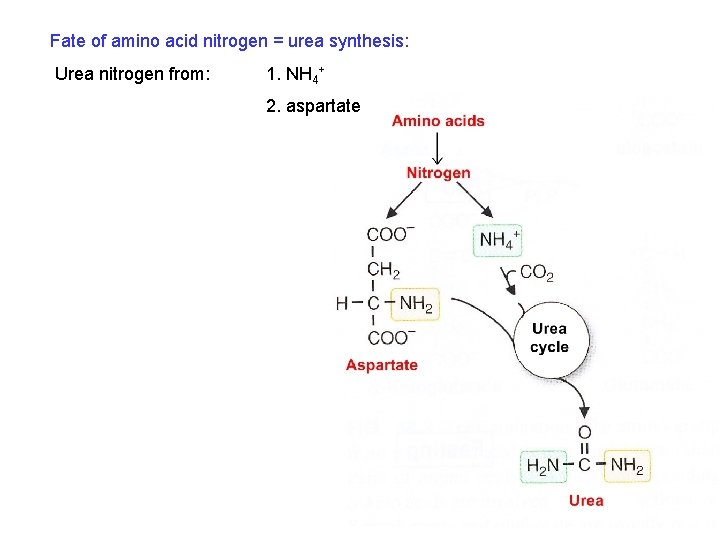
Fate of amino acid nitrogen = urea synthesis: Urea nitrogen from: 1. NH 4+ 2. aspartate

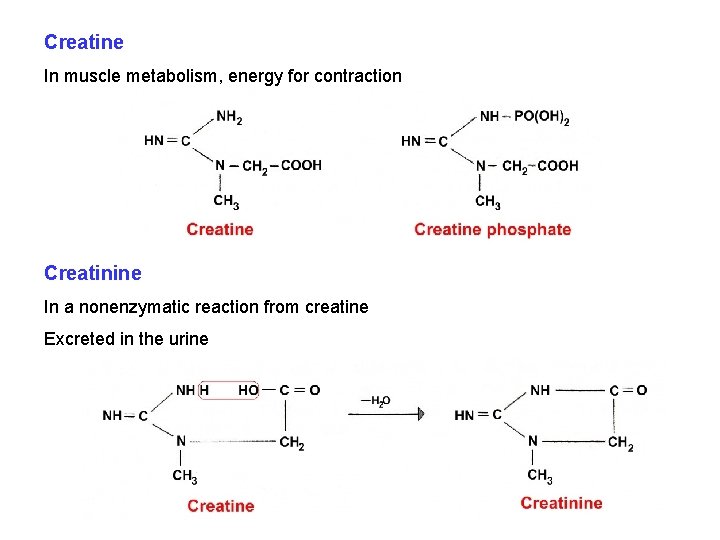
Creatine In muscle metabolism, energy for contraction Creatinine In a nonenzymatic reaction from creatine Excreted in the urine

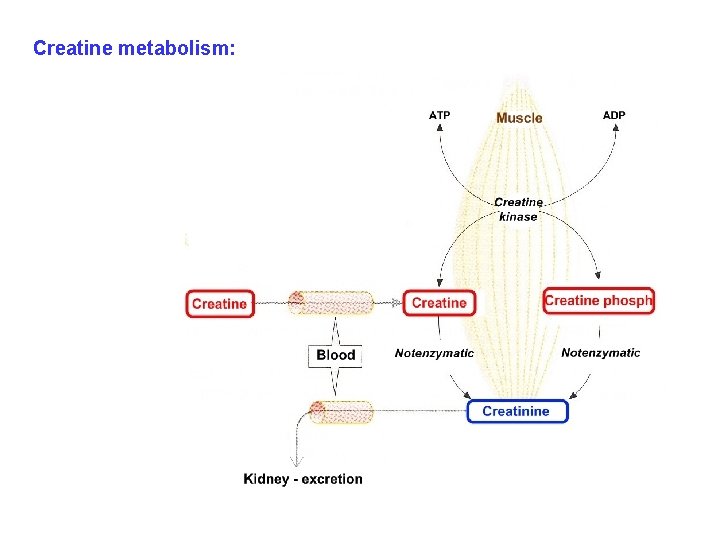
Creatine metabolism:

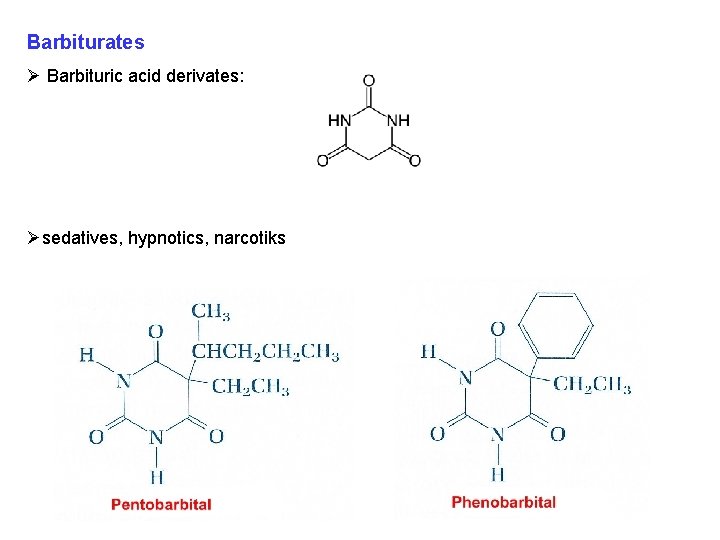
Barbiturates Ø Barbituric acid derivates: Øsedatives, hypnotics, narcotiks

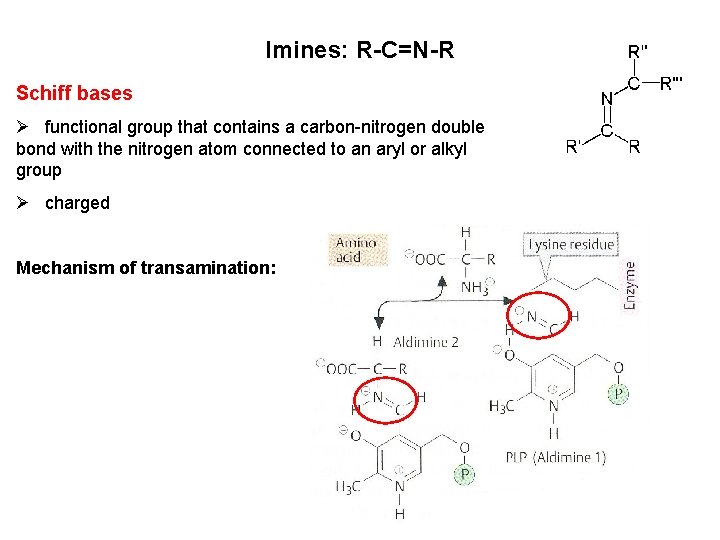
Imines: R-C=N-R Schiff bases Ø functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group Ø charged Mechanism of transamination:

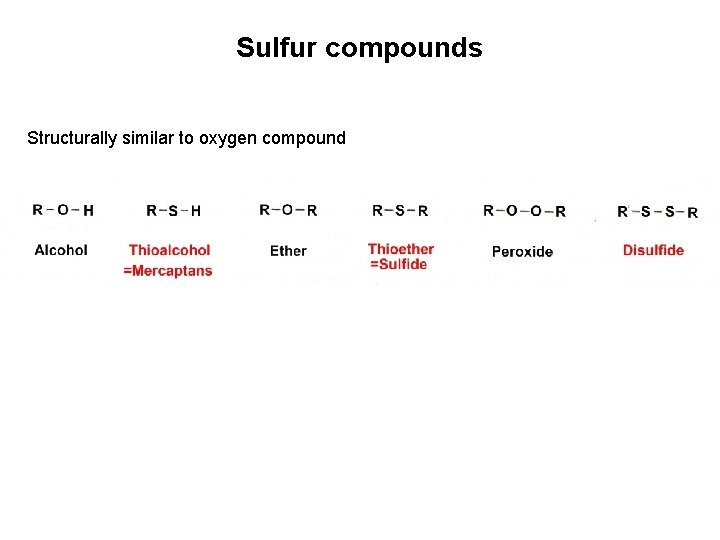
Sulfur compounds Structurally similar to oxygen compound

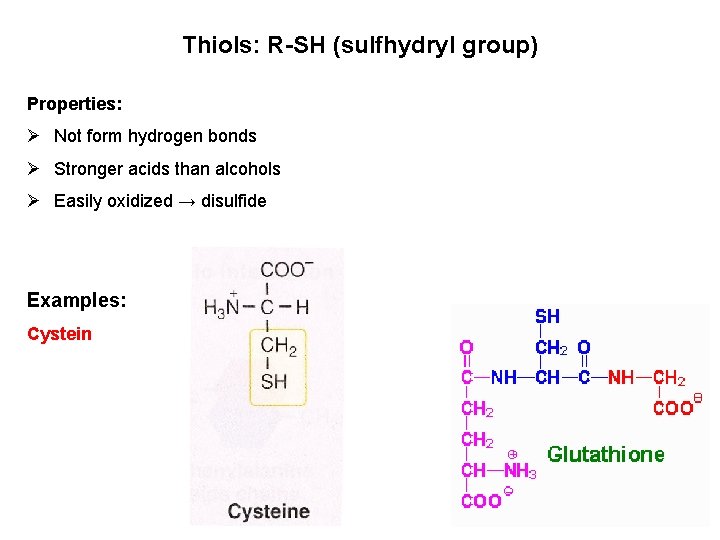
Thiols: R-SH (sulfhydryl group) Properties: Ø Not form hydrogen bonds Ø Stronger acids than alcohols Ø Easily oxidized → disulfide Examples: Cystein

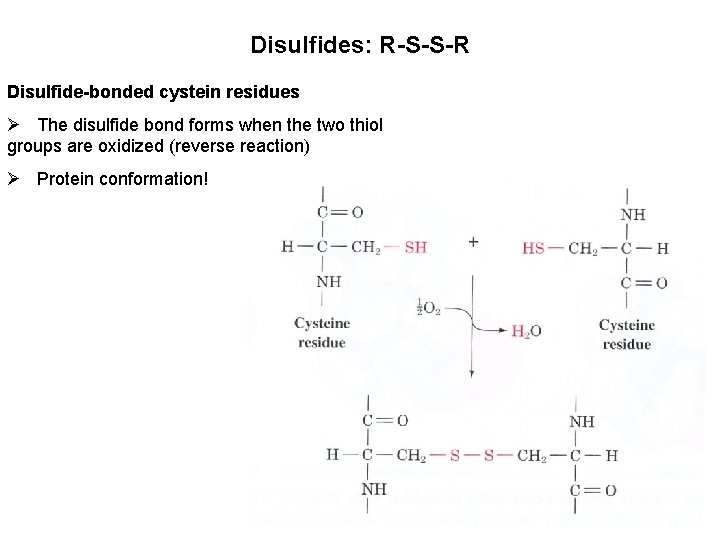
Disulfides: R-S-S-R Disulfide-bonded cystein residues Ø The disulfide bond forms when the two thiol groups are oxidized (reverse reaction) Ø Protein conformation!

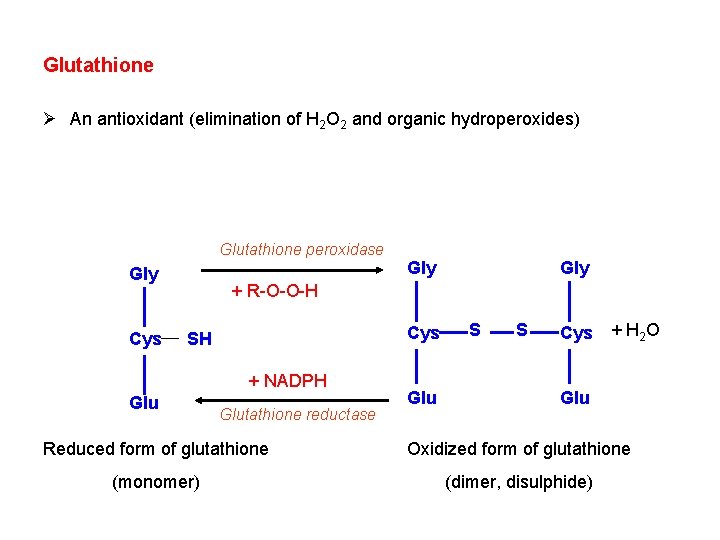
Glutathione Ø An antioxidant (elimination of H 2 O 2 and organic hydroperoxides) Glutathione peroxidase Gly Cys Gly + R-O-O-H Cys SH + NADPH Glutathione reductase Glu S S Cys + H 2 O Glu Reduced form of glutathione Oxidized form of glutathione (monomer) (dimer, disulphide)

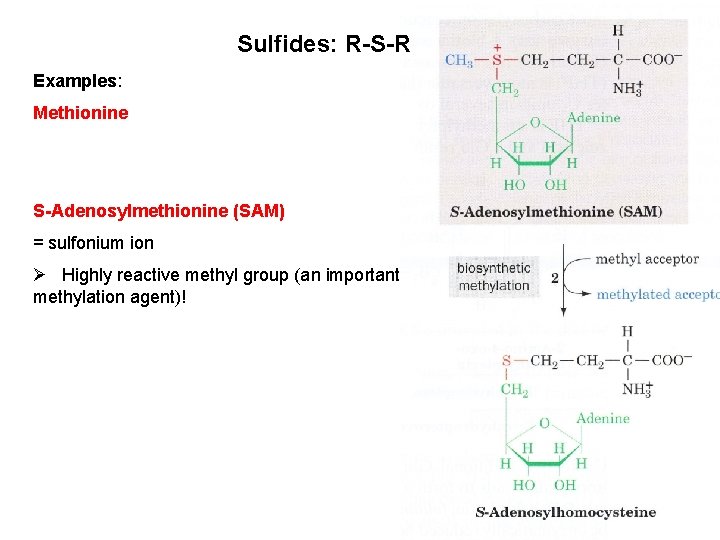
Sulfides: R-S-R Examples: Methionine S-Adenosylmethionine (SAM) = sulfonium ion Ø Highly reactive methyl group (an important methylation agent)!

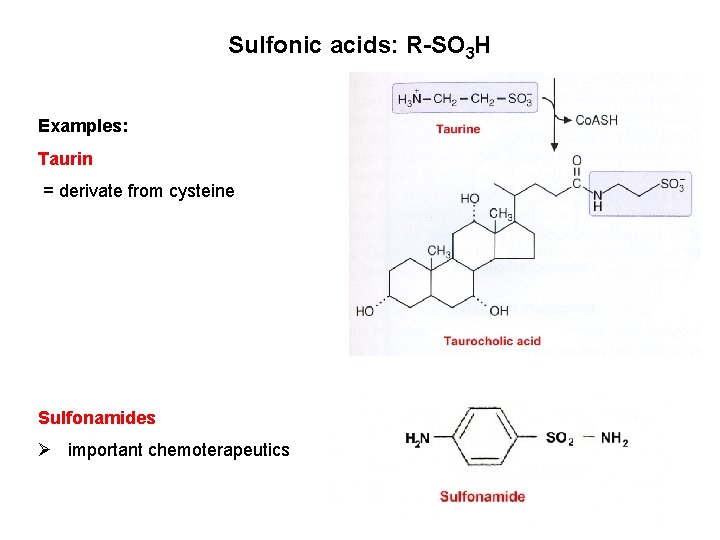
Sulfonic acids: R-SO 3 H Examples: Taurin = derivate from cysteine Sulfonamides Ø important chemoterapeutics

Summary Nitrogen compounds: 1. Amines Ø Ø Ø neurotransmitters (GABA, dopamine, serotonine, catecholamines, acetylcholine) amino acids alkaloids (cocaine, opiates) 2. Amides Ø Ø Ø peptide bond urea creatine 3. Imines Ø Schiff bases Sulfur compounds 1. Thiols - disulfide Ø glutathione, proteins 2. Sulfides Ø S-adenosylmethionine 3. Sulfonic acid Ø Taurin