Organic Compounds carbonbased compounds used by organisms 1

Organic Compounds - carbon-based compounds used by organisms 1) Carbohydrates (-ose) Function: source of energy Subunits: monosaccharides (simple sugar) polysaccharides ( complex sugar) Examples: glucose, cellulose (plants), glycogen (starch), insulin
2) Lipids Function: Subunits: Examples: stored energy, cell membranes glycerol & fatty acids(chains of C & H) fats and oils 3) Protein Function: aid chemical reactions, transport materials Subunits: amino acids Examples: hemoglobin (transports O 2 in blood) enzymes (speed up reactions)
4) Nucleic Acids Function: store & transmit genetic information Subunits: nucleotides - sugar, phosphate, nitrogen base Examples: DNA, RNA Tests for Organic Compounds Iodine: starch (glycogen) Brown paper: lipids Biuret’s solution: protein Benedict’s solution: simple sugars
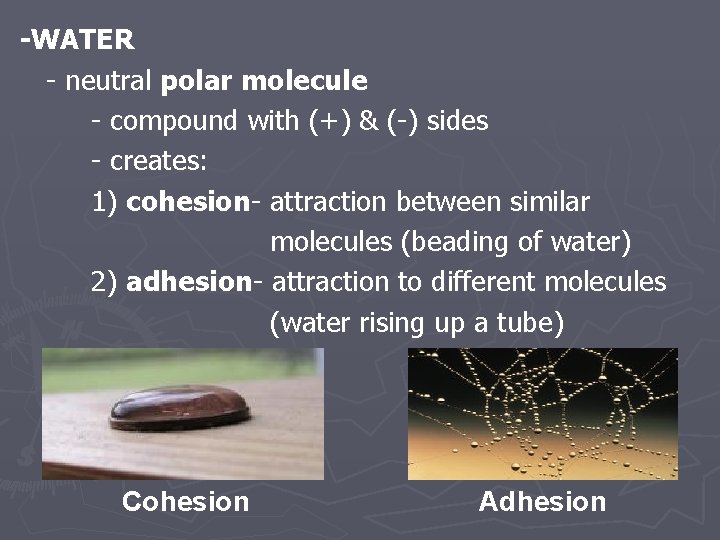
-WATER - neutral polar molecule - compound with (+) & (-) sides - creates: 1) cohesion- attraction between similar molecules (beading of water) 2) adhesion- attraction to different molecules (water rising up a tube) Cohesion Adhesion
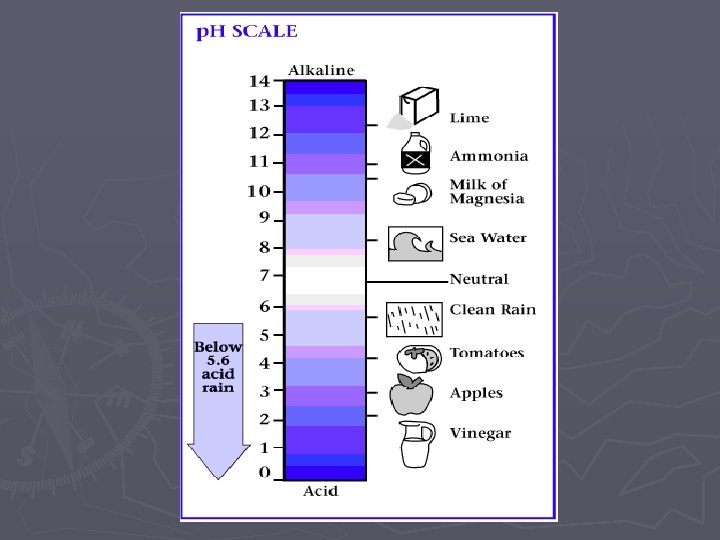
p. H Scale - measure of H+ ions in a solution Example (1 -6) = Acid – high concentration of H+ ions (7) = Neutral- equal concentration of H+ OH- ions (8 -14)= Base – high concentration of OH- ions *Buffer- compounds used to neutralize acids & bases to stabilize p. H
Enzymes (-ase) - proteins that act as a catalyst(speed up reactions) - have specific functions ex. - breaking down carbohydrates into energy - re-usable - affected by changes in p. H & temperature ex. - human enzymes need 39 C 0 (98. 6 F 0) Reaction Examples: starch + amylase maltose + maltase fats/oils + lipase maltose glucose fatty acids + glycerol
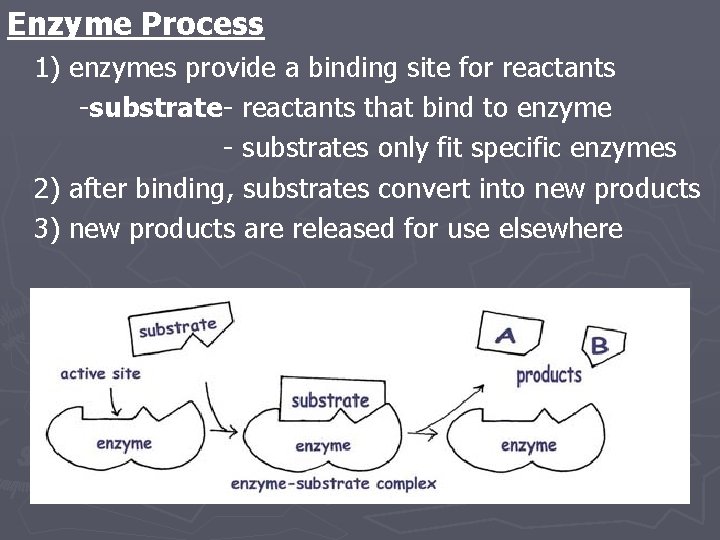
Enzyme Process 1) enzymes provide a binding site for reactants -substrate- reactants that bind to enzyme - substrates only fit specific enzymes 2) after binding, substrates convert into new products 3) new products are released for use elsewhere
- Slides: 9