Organic Compounds Always contain carbon and hydrogen generally

Organic Compounds • Always contain carbon and hydrogen & generally Oxygen • Usually long chain of Carbon atoms linked by covalent bonds • The carbon atoms typically form additional bonds with Hydrogen or Oxygen. • Make up 40% of body mass. • 4 Main Classes: Carbohydrates, Lipids, Proteins, and Nucleic Acids. • Known as macromolecules or Polymer; • Lipid is not a polymer.
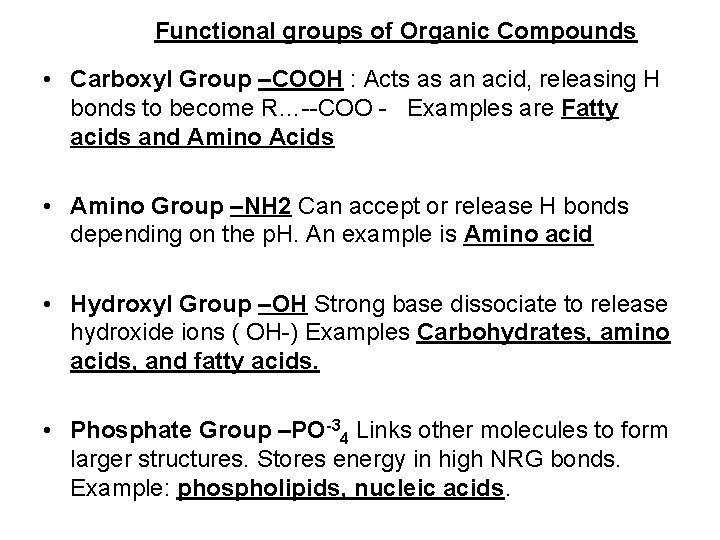
Functional groups of Organic Compounds • Carboxyl Group –COOH : Acts as an acid, releasing H bonds to become R…--COO - Examples are Fatty acids and Amino Acids • Amino Group –NH 2 Can accept or release H bonds depending on the p. H. An example is Amino acid • Hydroxyl Group –OH Strong base dissociate to release hydroxide ions ( OH-) Examples Carbohydrates, amino acids, and fatty acids. • Phosphate Group –PO-34 Links other molecules to form larger structures. Stores energy in high NRG bonds. Example: phospholipids, nucleic acids.
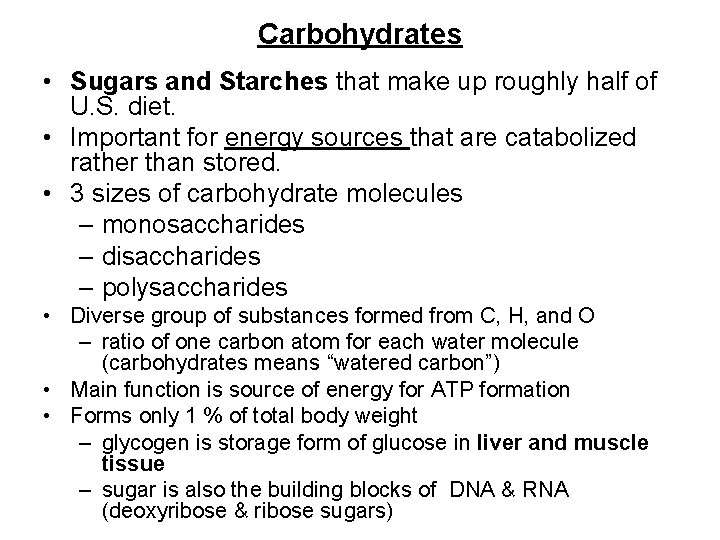
Carbohydrates • Sugars and Starches that make up roughly half of U. S. diet. • Important for energy sources that are catabolized rather than stored. • 3 sizes of carbohydrate molecules – monosaccharides – disaccharides – polysaccharides • Diverse group of substances formed from C, H, and O – ratio of one carbon atom for each water molecule (carbohydrates means “watered carbon”) • Main function is source of energy for ATP formation • Forms only 1 % of total body weight – glycogen is storage form of glucose in liver and muscle tissue – sugar is also the building blocks of DNA & RNA (deoxyribose & ribose sugars)

Monosaccharides • Called simple sugars • Contain 3 to 7 carbon atoms • We can absorb only 3 simple sugars without further digestion in our small intestine – glucose found in syrup or honey (metabolic fuel) – fructose found in fruits – galactose found in dairy products
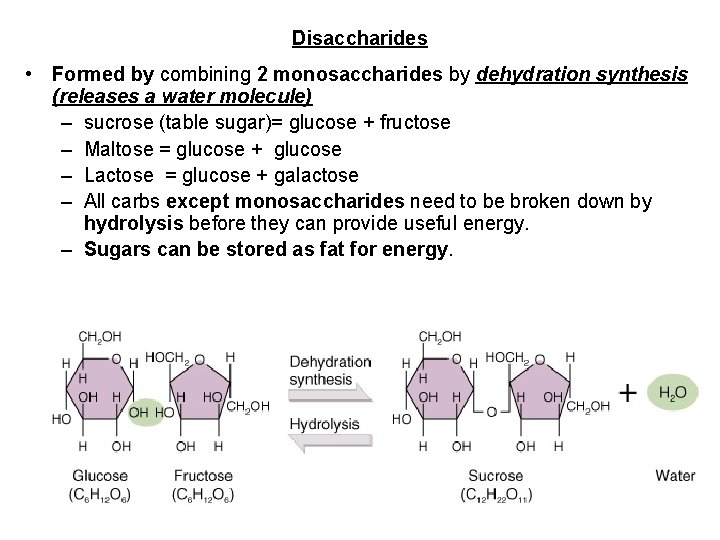
Disaccharides • Formed by combining 2 monosaccharides by dehydration synthesis (releases a water molecule) – sucrose (table sugar)= glucose + fructose – Maltose = glucose + glucose – Lactose = glucose + galactose – All carbs except monosaccharides need to be broken down by hydrolysis before they can provide useful energy. – Sugars can be stored as fat for energy.

Polysaccharides: Complex Carbohydrates • Contain 10 or 100’s of monosaccharides joined by dehydration synthesis. • STARCH: The storage form of glucose in plants. High concentrations of glucose in plant or animal cells is TOXIC. Surplus stores of glucose is converted to starch for safety. • Glycogen is the storage form of glucose in animals. Glycogen is stored in the liver (hepatocytes) and in muscle cells. If serum glucose levels fall, glycogen is converted into glucose • Cellulose: can only digest small amounts via bacterial action. Cellulose is called dietary fiber. Water soluble found in fruits veggies. Insoluble fiber is in nuts and grains.
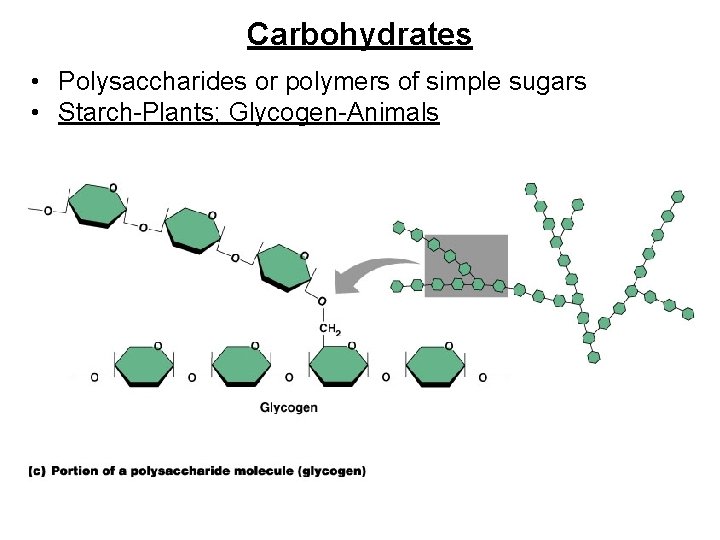
Carbohydrates • Polysaccharides or polymers of simple sugars • Starch-Plants; Glycogen-Animals
Lipids or fats • Formed from C, H and O – includes Fats, oils, waxes, • 18 -25% of body weight • Hydrophobic – insoluble in polar solvents like water • Can combines with proteins for transport in blood (lipoproteins LDL’s, HDL’s ) • They form the essential structural component of all cells • 5 Classes: Fatty acids, eicosanoids, glycerides, steroids, phospholipids.

Triglycerides • Neutral fats composed of a single glycerol molecule and 3 fatty acid molecules – three-carbon glycerol molecule is the backbone • Very concentrated form of energy – 9 calories/gram compared to 4 for proteins & carbohydrates – our bodies store triglycerides in fat cells if we eat extra food. Major source of stored energy in the body. – Fatty Acids help make up Triglycerides in 2 forms. SATURATED & UNSATURATED.
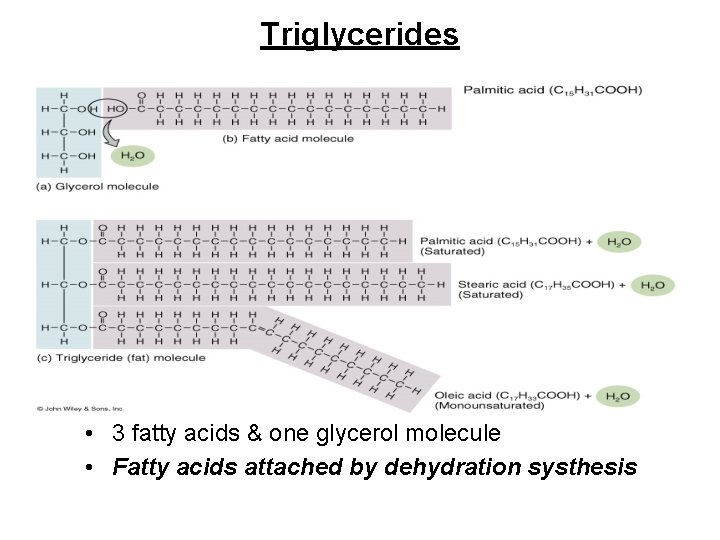
Triglycerides • 3 fatty acids & one glycerol molecule • Fatty acids attached by dehydration systhesis

Triglycerides continued • Saturated fatty acids are found in animal products and are solid at room temperature and linked to cardiovascular disease. • Unsaturated Fatty Acids are liquids at room temperature and found in plants. • Margarine is made of corn oil a polyunsaturated fatty acid. Corn oil is hydrogenated by bubbling hydrogen gas through it. Hydrogenating polyunsaturated fats produces trans fats which are harmful to the body.

Oils & Waxes • Oils are very similar to polyunsaturated fatty acids. Oils are lipids that are liquids at room temperature. Oils we produce act as natural sun block, barrier to bacteria and lubricant for skin. • Waxes- Are lipids that are solid at room temperature. Produced to protect dust particles form entering the external canal.
Phospholipids • Consists of a lipid molecule joined to a phosphate containing organic group. • They act as the structural component of the cell • They have a phosphate group ( hydrophilic end ) • They have a lipid ( Fatty Acids tails) ( hydrophobic end) • Composition of phospholipid molecule – a polar head • a phosphate group (PO 4 -3) & glycerol molecule • can form hydrogen bonds with water – 2 non-polar fatty acid tails • interact only with lipids • Composition of cell membrane – double layer of phospholipids – Participates in transport of lipids in plasma
Phospholipids • Composition of phospholipid molecule – a polar head • a phosphate group (PO 4 -3) & glycerol molecule • can form hydrogen bonds with water – 2 nonpolar fatty acid tails • interact only with lipids • Composition of cell membrane – double layer of phospholipids – Participates in transport of lipids in plasma
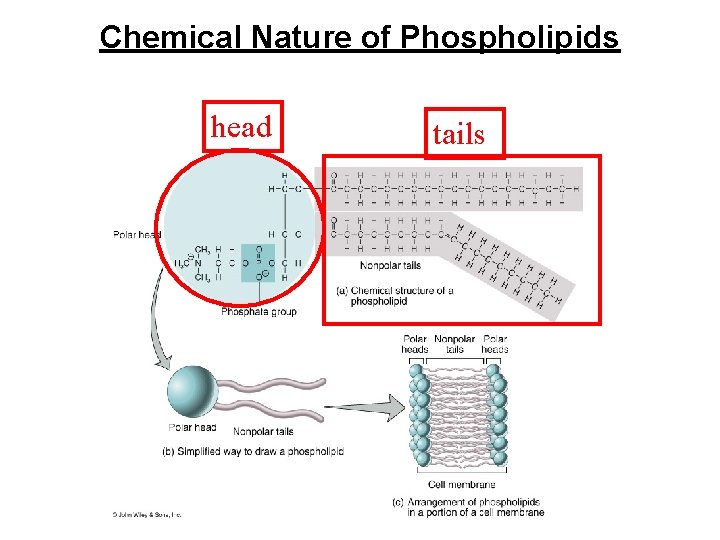
Chemical Nature of Phospholipids head tails
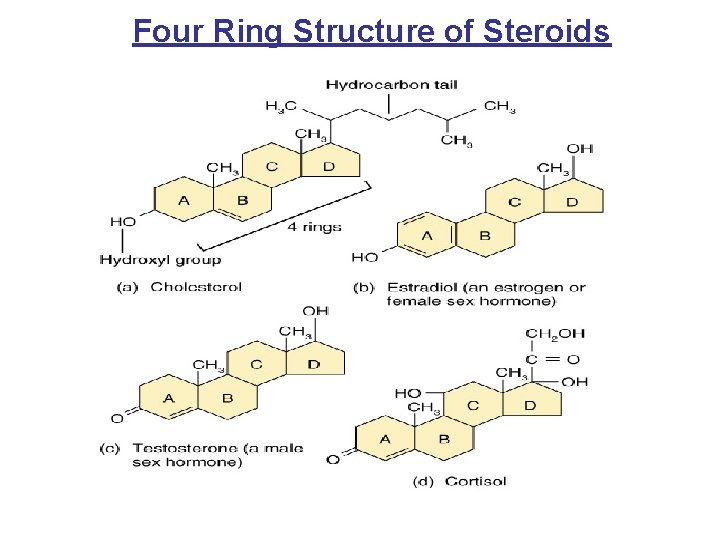
Four Ring Structure of Steroids

Steroids and Eicosanoids • Steroids: – Cholesterol- structural basis of all body steroids. We ingest it form eggs, milk, cheese, and our liver makes 80% of what we need. – Bile salts-these breakdown products of cholesterol are released by the liver into the digestive tract, where they aid in fat digestion and absorption. – Vitamin D- A fat soluble vitamin produced in the skin on exposure to UV radiation. Needed for bone growth and calcium absorption, and immune function. – Sex hormones- Estrogen and progesterone(female) and Testosterone(male). Needed for normal sexual function – Adrenocorticol hormones- Cortisol and Aldosterone • Eicosanoids: Lipid type derived from a fatty acid called arachidonic acid (e. g. Prostaglandins)
Proteins • The most abundant organic component of the body. • Functions consist of: • Support- Structural proteins: framework of the body providing strength. Collagen, keratin, elastin • Movement- Contractile proteins needed for muscle contraction. Actin any Myosin. • Transport proteins- Hemoglobin transports oxygen in blood. Lipoproteins transport lipids and cholesterol. • Buffering- Assist in p. H changes. Albumin acts as acid and base to prevent wide swing in Ph. • Catalysis- ENZYMES accelerate chemical reactions in cells. • Regulation of metabolism- growth hormone, insulin. • Defense- Tough waterproof proteins of the skin, hair, nails protect the body from hazards. Proteins called antibodies are components of the immune system, helps protect us from foreign matter. Special clotting proteins restrict bleeding after an injury to the cardiovascular system.
Proteins • 12 -20% of body weight • Contain carbon, hydrogen, oxygen, and nitrogen • Constructed from combinations of 20 amino acids. – dipeptides formed from when 2 amino acids joined by a covalent bond called a peptide bond – polypeptides chains formed from 10 to 2000 amino acids. Levels of structural organization – primary, secondary, tertiary, and Quaternary – shape of the protein influences its ability to form bonds
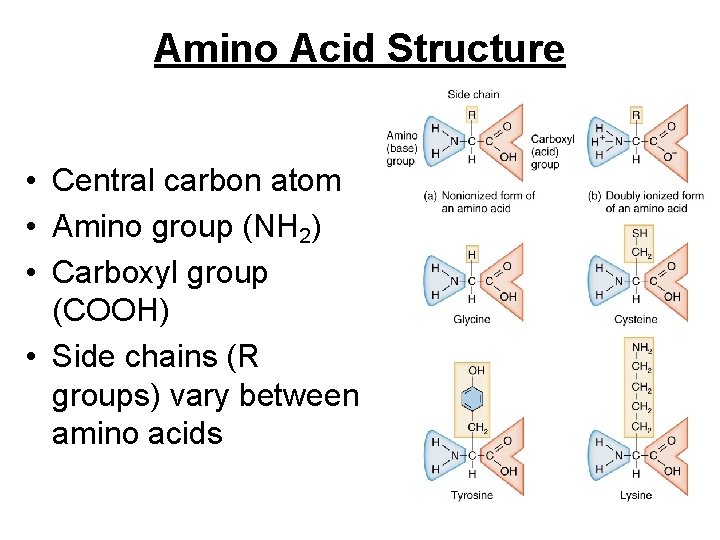
Amino Acid Structure • Central carbon atom • Amino group (NH 2) • Carboxyl group (COOH) • Side chains (R groups) vary between amino acids
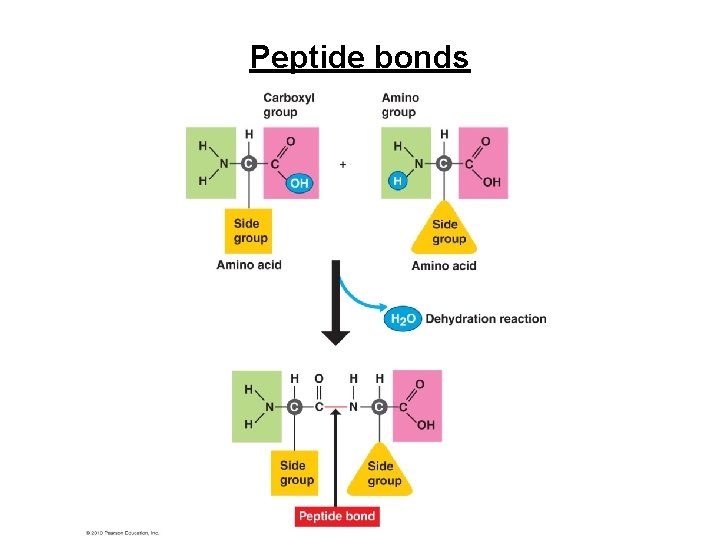
Peptide bonds
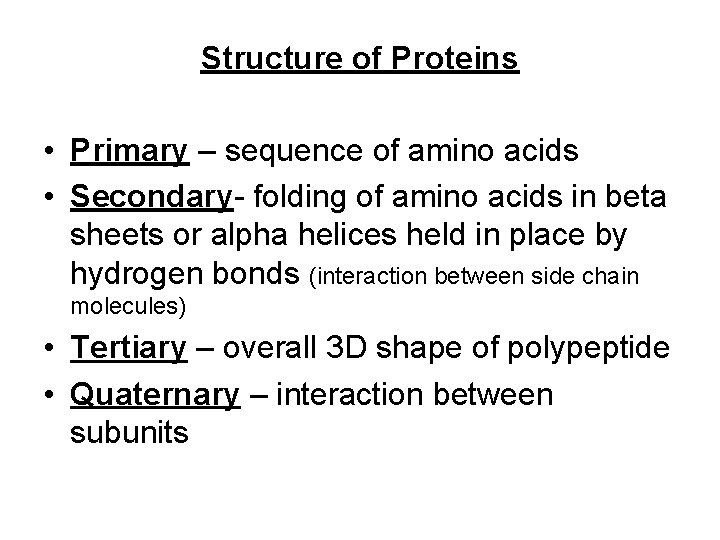
Structure of Proteins • Primary – sequence of amino acids • Secondary- folding of amino acids in beta sheets or alpha helices held in place by hydrogen bonds (interaction between side chain molecules) • Tertiary – overall 3 D shape of polypeptide • Quaternary – interaction between subunits
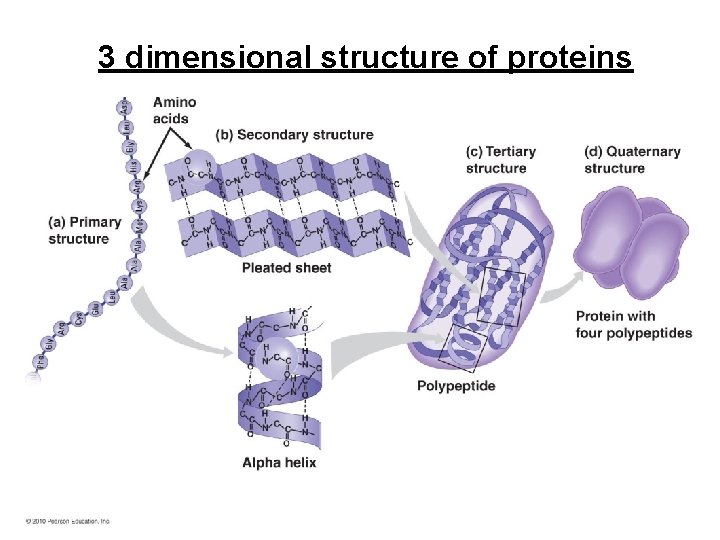
3 dimensional structure of proteins
Enzymes are Protein* Catalysts • Catalysts are not used up in the reaction • Each enzyme performs a specific reaction. • Enzymes end in “ase” (ex: catalase) * All the enzymes are NOT proteins.
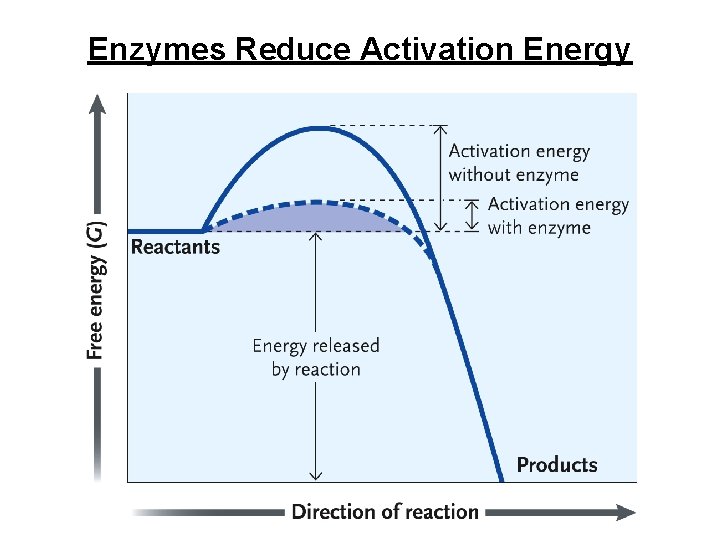
Enzymes Reduce Activation Energy
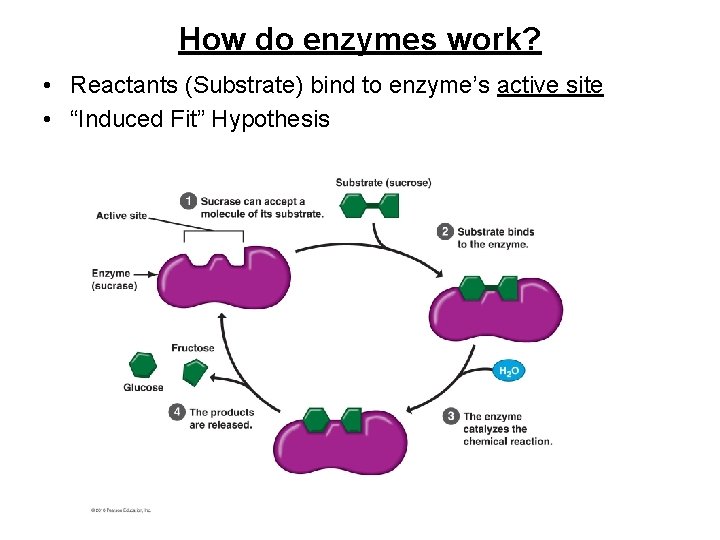
How do enzymes work? • Reactants (Substrate) bind to enzyme’s active site • “Induced Fit” Hypothesis
Factors that affect enzyme activity • • Substrate concentration Enzyme concentration Temperature p. H of the reaction
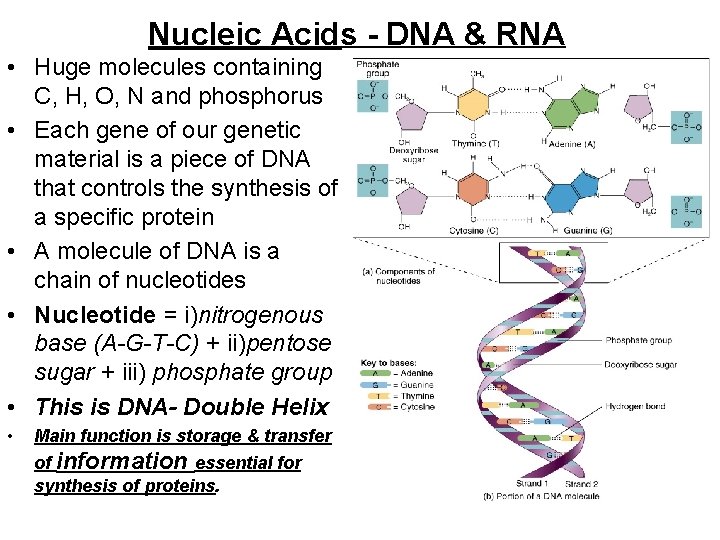
Nucleic Acids - DNA & RNA • Huge molecules containing C, H, O, N and phosphorus • Each gene of our genetic material is a piece of DNA that controls the synthesis of a specific protein • A molecule of DNA is a chain of nucleotides • Nucleotide = i)nitrogenous base (A-G-T-C) + ii)pentose sugar + iii) phosphate group • This is DNA- Double Helix • Main function is storage & transfer of information essential for synthesis of proteins.
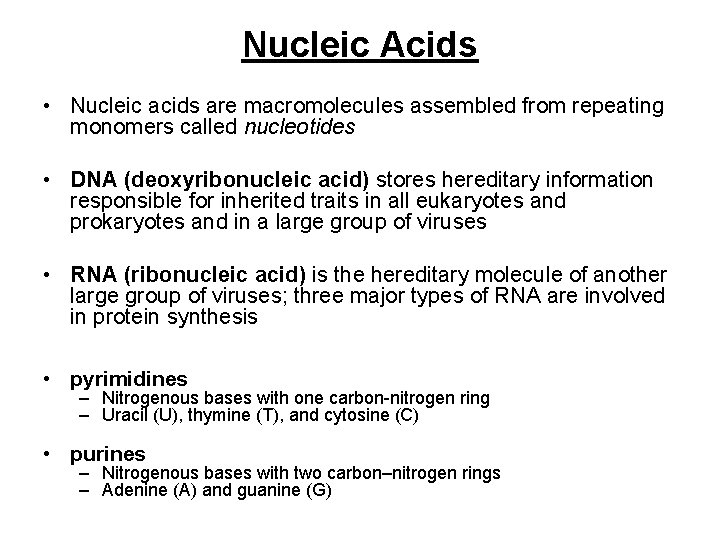
Nucleic Acids • Nucleic acids are macromolecules assembled from repeating monomers called nucleotides • DNA (deoxyribonucleic acid) stores hereditary information responsible for inherited traits in all eukaryotes and prokaryotes and in a large group of viruses • RNA (ribonucleic acid) is the hereditary molecule of another large group of viruses; three major types of RNA are involved in protein synthesis • pyrimidines – Nitrogenous bases with one carbon-nitrogen ring – Uracil (U), thymine (T), and cytosine (C) • purines – Nitrogenous bases with two carbon–nitrogen rings – Adenine (A) and guanine (G)
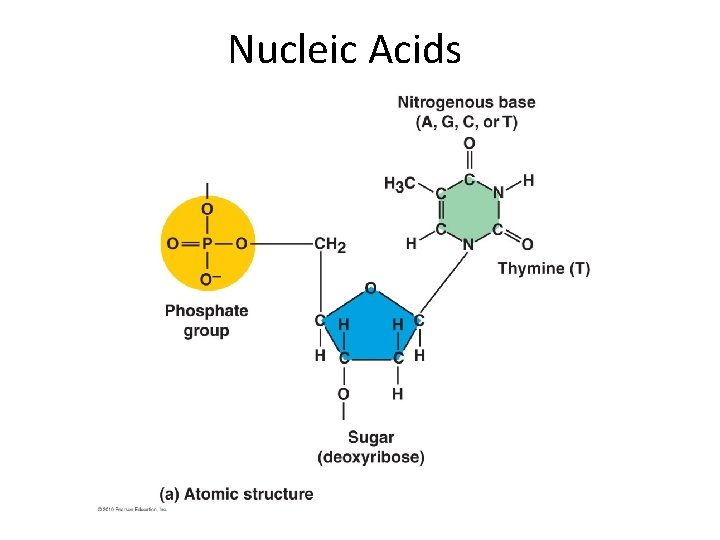
Nucleic Acids
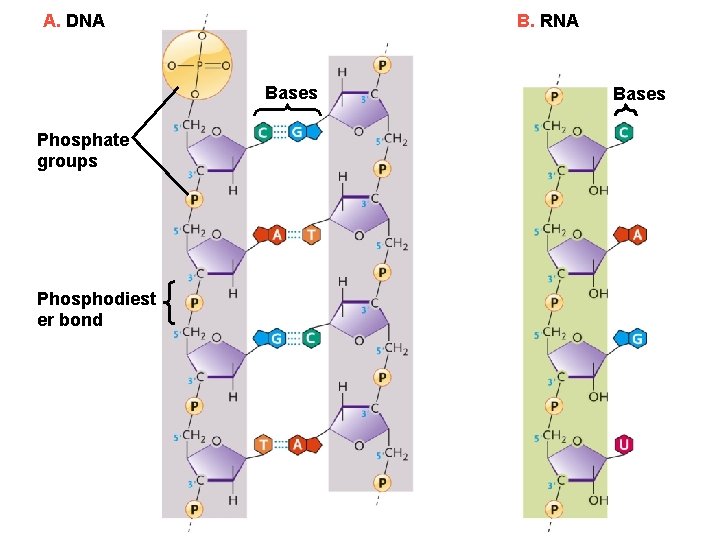
A. DNA B. RNA Bases Phosphate groups Phosphodiest er bond Bases
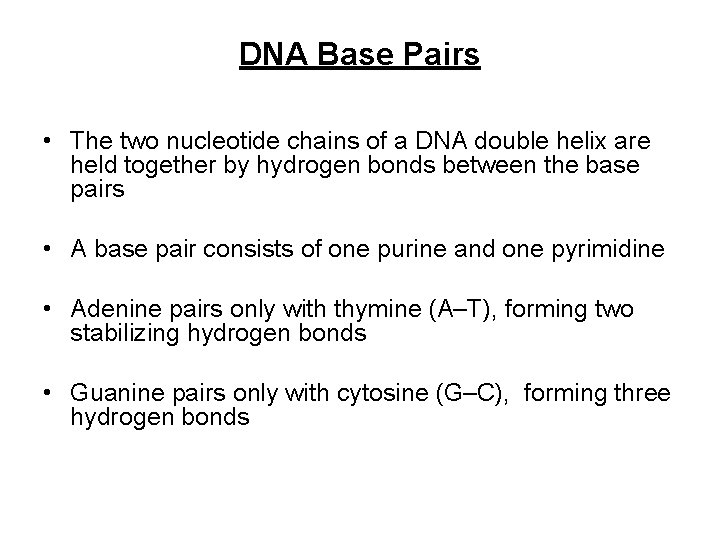
DNA Base Pairs • The two nucleotide chains of a DNA double helix are held together by hydrogen bonds between the base pairs • A base pair consists of one purine and one pyrimidine • Adenine pairs only with thymine (A–T), forming two stabilizing hydrogen bonds • Guanine pairs only with cytosine (G–C), forming three hydrogen bonds
RNA Molecules • RNA molecules exist mainly as single polynucleotide chains (single-stranded) – however, RNA molecules can fold back on themselves to form double-helical regions • RNA contains ‘ribose’ not ‘deoxyribose’ • In RNA, the uracil (U) base takes the place of thymine (T), forming A–U base pairs • Types of RNA within the cell, each with a specific function. – messenger RNA – ribosomal RNA – transfer RNA
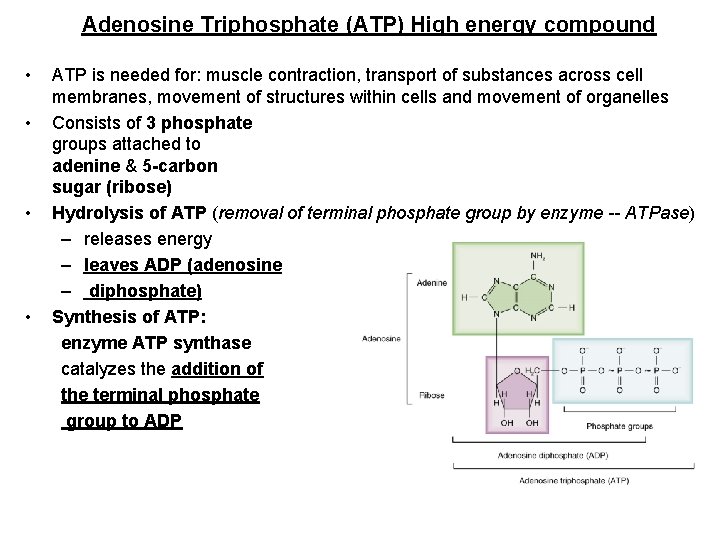
Adenosine Triphosphate (ATP) High energy compound • • ATP is needed for: muscle contraction, transport of substances across cell membranes, movement of structures within cells and movement of organelles Consists of 3 phosphate groups attached to adenine & 5 -carbon sugar (ribose) Hydrolysis of ATP (removal of terminal phosphate group by enzyme -- ATPase) – releases energy – leaves ADP (adenosine – diphosphate) Synthesis of ATP: enzyme ATP synthase catalyzes the addition of the terminal phosphate group to ADP
- Slides: 35