Organic Compounds All compounds can be classified into

Organic Compounds

All compounds can be classified into 2 main categories: Organic compounds • Any compound that contains carbon. Exception: CO 2 Inorganic Compounds: • Compounds that do not contain carbon.

The Elements of life • The four most common elements in living things are: – Carbon – Hydrogen – Oxygen – Nitrogen • CHON

The Creativity of Carbon • Central to all living things • Also found in non-living things for example –drugs -dyes -plastics • Diamonds are carbon atoms in crystal structure
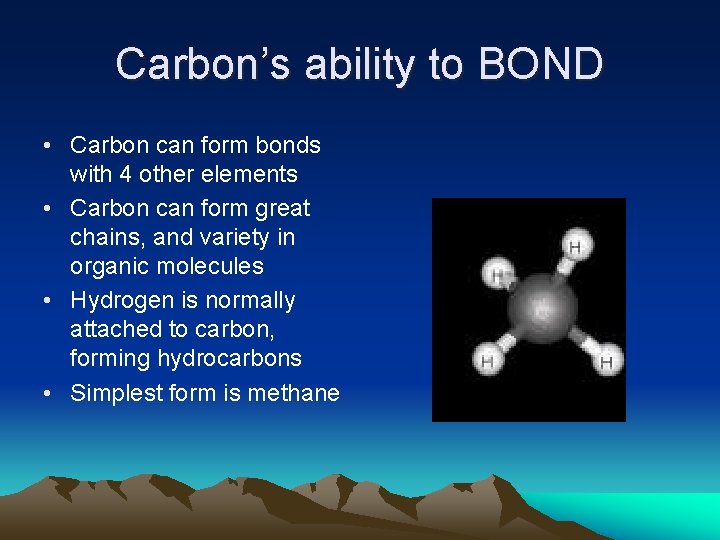
Carbon’s ability to BOND • Carbon can form bonds with 4 other elements • Carbon can form great chains, and variety in organic molecules • Hydrogen is normally attached to carbon, forming hydrocarbons • Simplest form is methane

• Carbon also readily bonds with other carbon atoms • Forming straight chains • Branched chains • Or even rings • This “creativity” of carbon results in a large variety of organic compounds

Organic Compounds Organic compounds can be broken up into four major groups: • Carbohydrates (think sugar and starch) • Lipids (think fat and oils) • Proteins (think of muscles & beef) • Nucleic Acids (think genetic material)

Carbohydrates! • Compounds made of Carbon, Hydrogen, and Oxygen • Ratio of 2: 1 ( for every 2 hydrogen there must be 1 oxygen) • Cells in your body obtain most of their energy from carbohydrates
Common carbohydrates • Starches • Sugars • Cellulose

What is a carbohydrate? • Energy packed compounds • Break down fast, release high energy • However, this energy does not last long
There are 3 types of carbohydrates 1. Monosaccharides (simple sugars)glucose. 2. Disaccharides (Double sugars)- sucrose (table sugar) 3. Polysaccharides ( many sugars)starches, (bread/pasta)-also cellulose

The Monosaccharides • Referred to as the “simple sugars” • Greek-”mono”-single and “sacchar”-sugar • Main fuel molecules for cellular work
Disaccharides and Polysaccharides Disaccharides Called- double sugars Most common is sucrose Polysaccharides Called- complex carbohydrates Common examples include the starches, breads, pasta, and cellulose - the most abundant organic compound on earth. Cellulose is used to build cell walls.
ATP (Quick Intro) • Stands for adenosine triphosphate • Has extra energy-storing phosphate • In cells, once food is broken down, the energy is stored in ATP • All cells need ATP to function
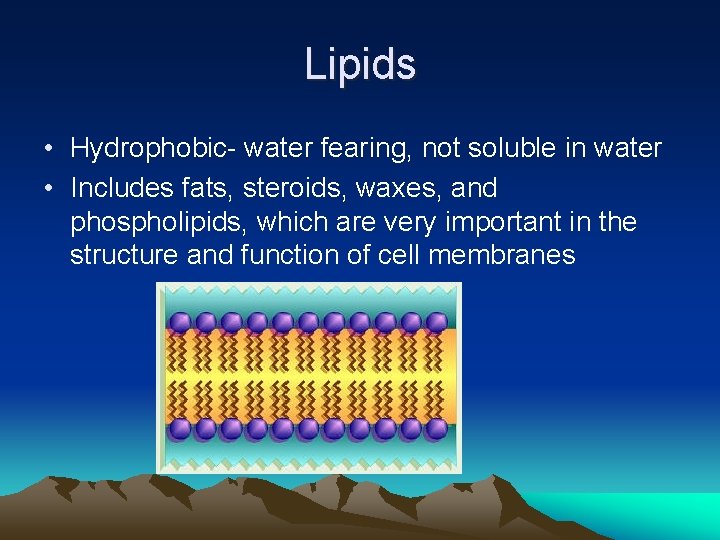
Lipids • Hydrophobic- water fearing, not soluble in water • Includes fats, steroids, waxes, and phospholipids, which are very important in the structure and function of cell membranes

FATS Lipids that store energy Most contain 16 -18 carbon atoms per molecule Saturated fats have only single bonds between their carbons. They are bad for you. They are solid at room temperature. Unsaturated fats have double bonds in the carbon chain and are liquid at room temperature.

Steroids • Classified as lipids because they are hydrophobic • Most common is cholesterol. Cell membrane contains cholesterol. • Controversial drugs called synthetic anabolic steroidsvariants of testosterone

Proteins! • Involved in every aspect of living things • Extremely important in structure and support of cells and our bodies • Some bind and carry cells throughout the body • All are made from amino acids
The Building Blocks of Protein • There are 20 different amino acids found in proteins. • Amino acids are produced in the cells Each amino acid is linked in certain order, and each protein has unique order and number of amino acids
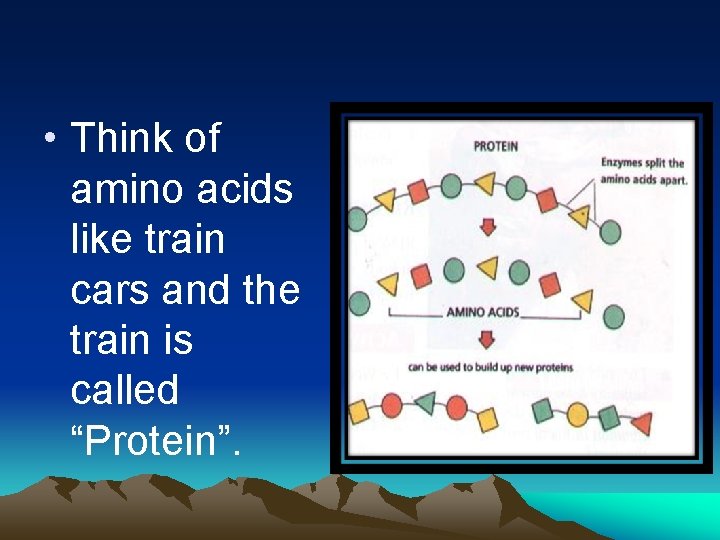
• Think of amino acids like train cars and the train is called “Protein”.
Nucleic Acids • Found in ALL of your cells. • Store important information (genetic and heredity) in the cell. • Made and assembled from nucleotides.
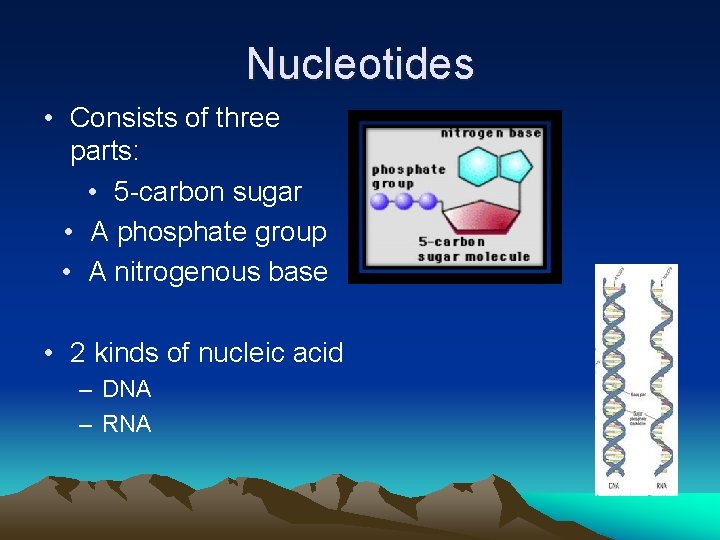
Nucleotides • Consists of three parts: • 5 -carbon sugar • A phosphate group • A nitrogenous base • 2 kinds of nucleic acid – DNA – RNA

why saturated fats are bad for you. • Fats in your body are these molecules called triglycerides. Triglycerides have a polar head and 3 hydrophobic tails. What hydrophic means is that they cannot dissolve in water. So that is why when you place fats in water, the fats clump up together. This is why they can clog arteries and blood vessels, because they don't dissolve. -The differenece between saturated and unsaturated, lies in the structure of the tails. saturated means that the tails all contain the maximum amount of hydrogen and can clump easier together. Unsaturated means that some parts of the tail contains double bonds which means that they don't have the maximum amount of hydrogen attached to them. Hench the term"unsaturated". They are unsaturated in terms of hydrogen. Since they have these kinks caused by double bonds, they don't clump up together as well and it is less likely that they will cause clots in blood vessels. In some cases these fats can also help break up saturated fats because they can make it hard for them to clump together as well. -
- Slides: 23