ORGANIC COMPOUND IBUPROFEN C 13 H 18 O

ORGANIC COMPOUND IBUPROFEN C 13 H 18 O 2 BRAND NAMES: ADVIL, GENPRIL, MIDOL IB, MOTRIN IB, PROPRINAL, SMART SENSE CHILDREN'S IBUPROFEN Emma Van Der Mark

History of Ibuprofen In the 1960 s Ibuprofen was developed by the British drug manufacturer and retailer Boots Laboratories as a result of research in the 1950 and 1960 s to find a safer, more powerful alternative to aspirin. A team led by Stewart Adams, part of Boots, discovered that carboxylic acid was the agent in aspirin that gave the anti-inflammatory property. Therefore after synthesizing and testing more than 600 compounds created from these acids, the most active was chosen for a clinical trial. After they found it to be ineffective to treat rheumatoid arthritis they tried other compounds and ended up finding that ibuprofen was the most effective and useful compound. Adams initial idea was to use ibuprofen as a cure for hangovers but in 1969 in the United Kingdom it was released as a treatment for rheumatoid arthritis. Then, later in the early 1980 s ibuprofen was available for over the counter uses in the United States and United Kingdom. As a result of the development of ibuprofen, Boots was awarded the Queens Award for Technical Achievement in 1987.
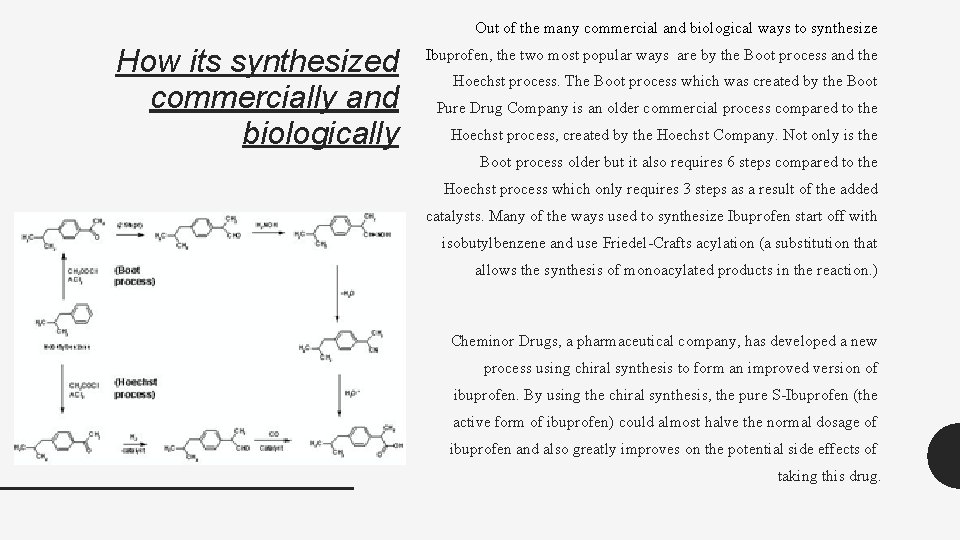
Out of the many commercial and biological ways to synthesize How its synthesized commercially and biologically Ibuprofen, the two most popular ways are by the Boot process and the Hoechst process. The Boot process which was created by the Boot Pure Drug Company is an older commercial process compared to the Hoechst process, created by the Hoechst Company. Not only is the Boot process older but it also requires 6 steps compared to the Hoechst process which only requires 3 steps as a result of the added catalysts. Many of the ways used to synthesize Ibuprofen start off with isobutylbenzene and use Friedel-Crafts acylation (a substitution that allows the synthesis of monoacylated products in the reaction. ) Cheminor Drugs, a pharmaceutical company, has developed a new process using chiral synthesis to form an improved version of ibuprofen. By using the chiral synthesis, the pure S-Ibuprofen (the active form of ibuprofen) could almost halve the normal dosage of ibuprofen and also greatly improves on the potential side effects of taking this drug.
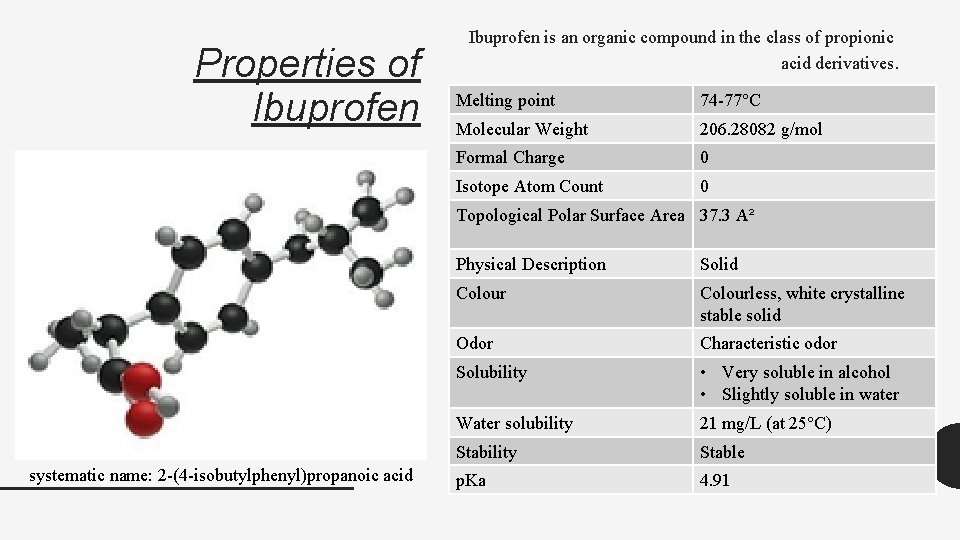
Properties of Ibuprofen is an organic compound in the class of propionic acid derivatives. Melting point 74 -77°C Molecular Weight 206. 28082 g/mol Formal Charge 0 Isotope Atom Count 0 Topological Polar Surface Area 37. 3 A² systematic name: 2 -(4 -isobutylphenyl)propanoic acid Physical Description Solid Colourless, white crystalline stable solid Odor Characteristic odor Solubility • Very soluble in alcohol • Slightly soluble in water Water solubility 21 mg/L (at 25°C) Stability Stable p. Ka 4. 91

Uses and side effects of Ibuprofen is a non-steroidal antiinflammatory drug (NSAID) that reduces hormones that cause pain in the body. Ibuprofen is used to reduce fever, relieve pain or inflammation caused by conditions such as headache, muscle aches, menstrual cramps, minor injuries or arthritis. Although ibuprofen doesn't have very severe side effects, there are still minor ones that are commonly associated with ibuprofen. Common ibuprofen side effects may include: upset stomach, mild heartburn, nausea, vomiting; bloating, gas, diarrhea, constipation; dizziness, headache, nervousness; mild itching or rash; or ringing in your ears. The gastrointestinal related side effects are caused by the ibuprofen stopping the enzyme that produces prostaglandins. This results in stomach irritation because there would be no decrease in stomach acid and no increase in stomach mucus secretion. Uncommon, more severe side effects include: Fatal heart attack or stroke (after long-term use or high doses); Stomach or intestinal bleeding; Taking ibuprofen within the last 3 months of pregnancy could harm the baby

Bibliographie · "Ibuprofen. " Medical Discoveries. 1997, Ian S. Haworth, "ibuprofen. " The Columbia Encyclopedia, 6 th Ed. . 2016, "ibuprofen. " The Oxford Pocket Dictionary of Current English. 2009, "ibuprofen. " A Dictionary of Nursing. 2008, and "ibuprofen. " Oxford Dictionary of Rhymes. 2007. "Ibuprofen. " Encyclopedia. com. High. Beam Research, 1997. Web. 30 May 2016. · "Ibuprofen. " - Wikipedia, the Free Encyclopedia. Web. 30 May 2016. · "Ibuprofen. " 3 D Model: . $9. 95 [buy, Download]. 3 D Rivers S. A. , 2009 -2010. Web. 30 May 2016. · "San Francisco Crossfit. " : Get Off The Ibuprofen Peoples! Web. 30 May 2016. · Broyles, Robyn, and Leigh A. Zaykoski. "Learn About the Chemical Properties of Ibuprofen: Is This Drug Safe? " Bright Hub. 29 Apr. 2009. Web. 30 May 2016. · "Is Ibuprofen Right for a Sinus Headache? - CT Sinus Center. " Connecticut Sinus Center Blog. 2016. Web. 30 May 2016. · Coulter, Andrea. "Wholistic Look at Headaches. " Wholistic Health Care. 17 Sept. 2013. Web. 30 May 2016. · File: (S)-ibuprofen-3 D-vd. W. png. Wikimedia Commons, 11 June 2008. Web. 30 May 2016. · "Friedel-Crafts Acylation. " Friedel-Crafts Acylation. Web. 30 May 2016. · "Ibuprofen Uses, Dosage & Side Effects - Drugs. com. " Ibuprofen Uses, Dosage & Side Effects - Drugs. com. 2 May 2016. Web. 30 May 2016. · "Die Geschichte Von Boots. " Laboratories. 2014. Web. 30 May 2016. · "Synthesis. " Synthesis. Web. 30 May 2016. · "Ibuprofen. " The Pub. Chem Project. Web. 30 May 2016. · "The Discovery of Ibuprofen. " The Royal Society of Chemistry in Your Cupboard. Web. 30 May 2016. · "Ibuprofen. " Water Treatability Database. Environmental Protection Agency, July 2009. Web. 30 May 2016.
- Slides: 6