Organic Chemistry What is pencil lead made of
























- Slides: 114

Organic Chemistry

What is pencil lead made of if it isn't lead? • Pencil lead is a mixture of graphite and clay. • Graphite is one form of the element carbon. • Other forms of carbon are diamond - the hardest naturally occurring substance on the earth, soot, charcoal and coke.

What is pencil lead made of if it isn't lead? • Pencils used to be made with lead, many years ago. Lead is poisonous and so sucking the end of your pencil could be quite dangerous. • We now use graphite and clay because it is safer and because we can make pencils of different hardness

Chemistry of Living Things • Living things are a lot like laboratories… • There’s some serious chemistry going on inside. • Your body is an incredibly complex chemical machine taking in chemicals & food, and causing countless reactions to occur every second. • Biochemistry is the study of substances & processes occurring in all living organisms.

What are living things made of?

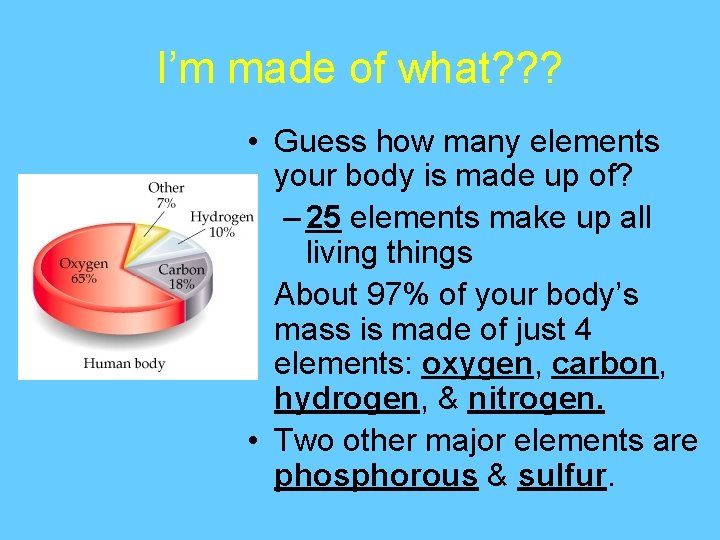
I’m made of what? ? ? • Guess how many elements your body is made up of? – 25 elements make up all living things • About 97% of your body’s mass is made of just 4 elements: oxygen, carbon, hydrogen, & nitrogen. • Two other major elements are phosphorous & sulfur.

Minor Elements • Of course, other elements are also important, but they’re often found in small amounts. • They may seem insignificant, but they’re not. • For example, iron makes up only 0. 004% of your body mass, but you can’t live without it!

Major Compounds • The human body also relies on many compounds, especially water & salt. • The human body typically consists of 60 -65% water. • In other words, 2/3 of your body weight is water. • Water is important because many of our body’s chemical reactions can only occur in solutions containing water.

Major Compounds • Blood, sweat, urine… all mostly water! • Salt is also important because of how it can separate into its two ions: Na+ and Cl-. • Sodium ions regular the amount of water in our cells, while chlorine ions help our body digest food.

The most important element is… Carbon • If you take away the water, the rest of the human body is 53% carbon. • It may not be the most abundant element in living things, but it certainly is the most important. At one time, scientists thought that the chemical reactions that took place inside of living things could not occur outside of them. • The carbon molecules were so complex, scientists thought they must have been made in some unknown way. They called these carbon compounds organic compounds

The most important element is… • The word “organic” has lots of meanings. Eventually, scientists realized that the reactions occurring inside the body could occur outside it as well. • They also learned how important carbon is in all living things, because of its ability to bond with other atoms.

The most important element is… • Not all substances made of carbon are living. Diamonds & graphite are pure forms of carbon. • Non-organic carbon compounds, and compounds without carbon, are called inorganic compounds.

What is organic chemistry? • We used to describe organic chemistry as the chemistry of living things. • Since the chemistry of living things is based on carbon, the chemistry of carbon compounds has come to be known as organic chemistry. • It now includes the study of carbon compounds which are not found in living things and so is an incredibly large branch of modern chemistry.

Organic Chemistry- The study of carbon & carbon compounds • Organic compounds are the primary constituents of all living organisms.

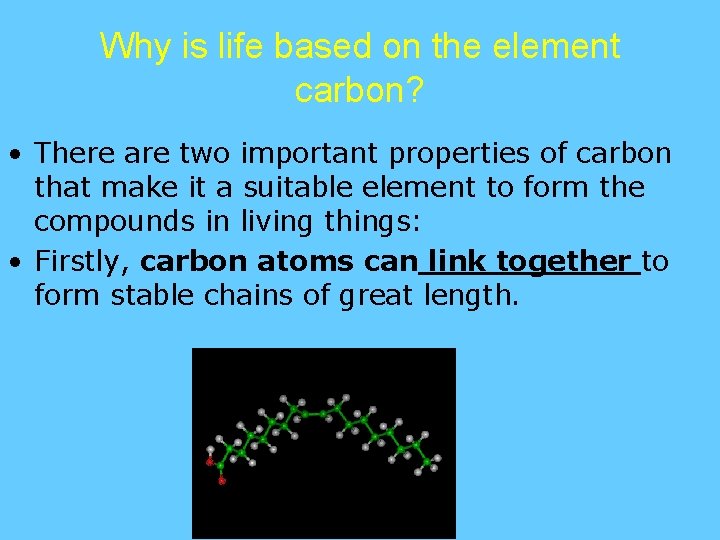
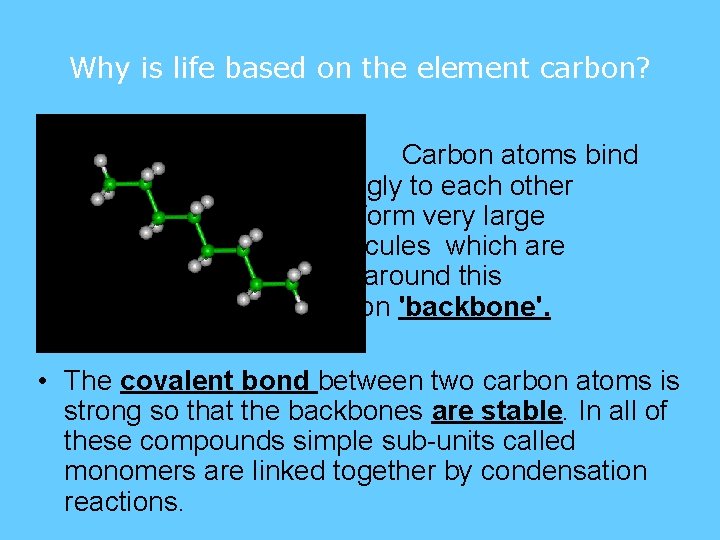
Why is life based on the element carbon? • There are two important properties of carbon that make it a suitable element to form the compounds in living things: • Firstly, carbon atoms can link together to form stable chains of great length.

Why is life based on the element carbon? • Carbon atoms bind strongly to each other and form very large molecules which are built around this carbon 'backbone'. • The covalent bond between two carbon atoms is strong so that the backbones are stable. In all of these compounds simple sub-units called monomers are linked together by condensation reactions.

We are not silicon based life forms!

Silicon is similar to carbon. Why are there no life forms based on silicon? Silicon is unsuitable because, although it is a valence IV element like carbon (4 electrons to share), BUT the silicon-silicon covalent bond is not strong enough for it to form long stable chains. So, it can not form molecules of the complexity needed to make up cells like carbon can!

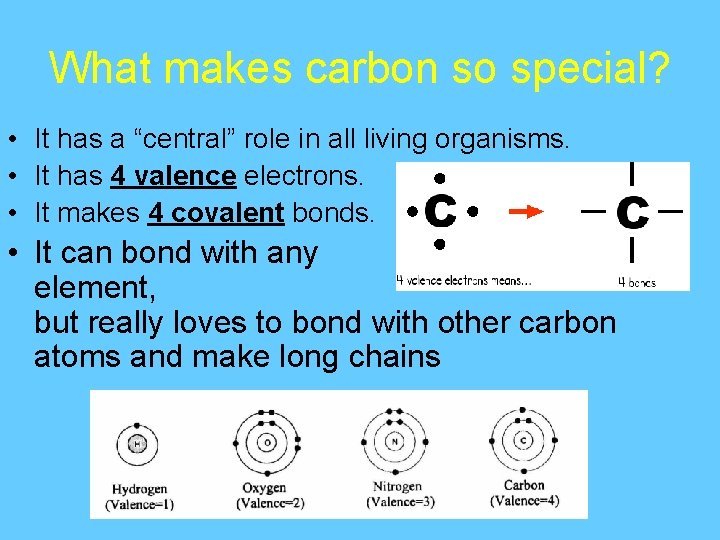
What makes carbon so special? • It has a “central” role in all living organisms. • It has 4 valence electrons. • It makes 4 covalent bonds. • It can bond with any element, but really loves to bond with other carbon atoms and make long chains

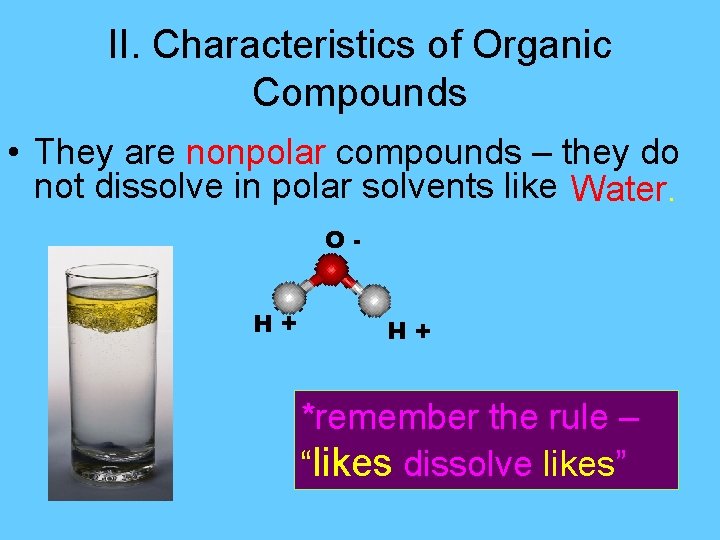
II. Characteristics of Organic Compounds • They are nonpolar compounds – they do not dissolve in polar solvents like Water. O+H H+ *remember the rule – “likes dissolve likes”

4) They have low melting points – due to weak intermolecular forces. C-C ● ● ● C-C STRONG weak STRONG 5) They react slower than ionic compounds – due to strong covalent bonds between atoms.

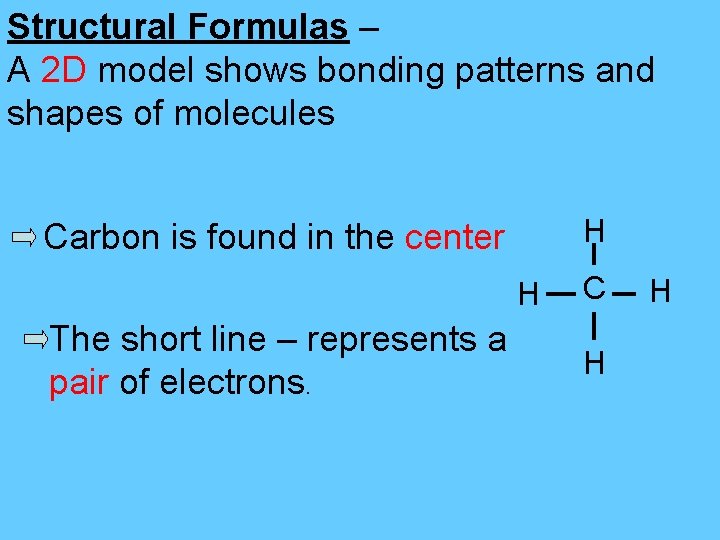
Structural Formulas – A 2 D model shows bonding patterns and shapes of molecules H Carbon is found in the center H The short line – represents a pair of electrons. C H H

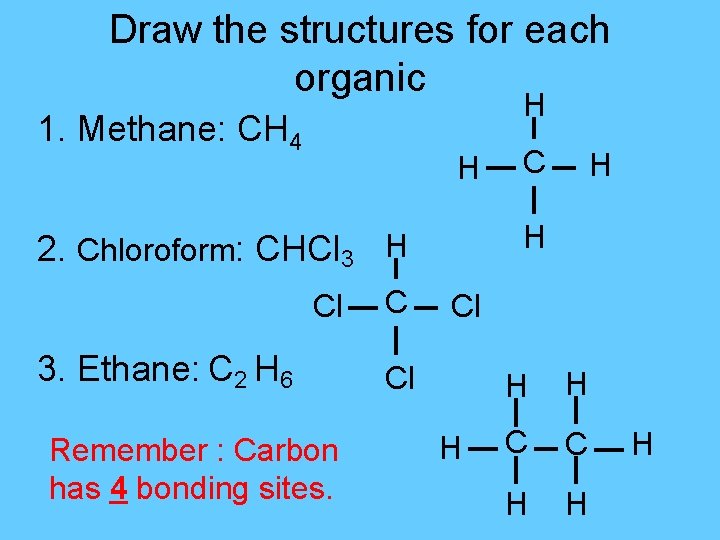
Draw the structures for each organic H 1. Methane: CH 4 H 3. Ethane: C 2 H 6 Remember : Carbon has 4 bonding sites. C H H 2. Chloroform: CHCl 3 H Cl Cl H H H C C H H H

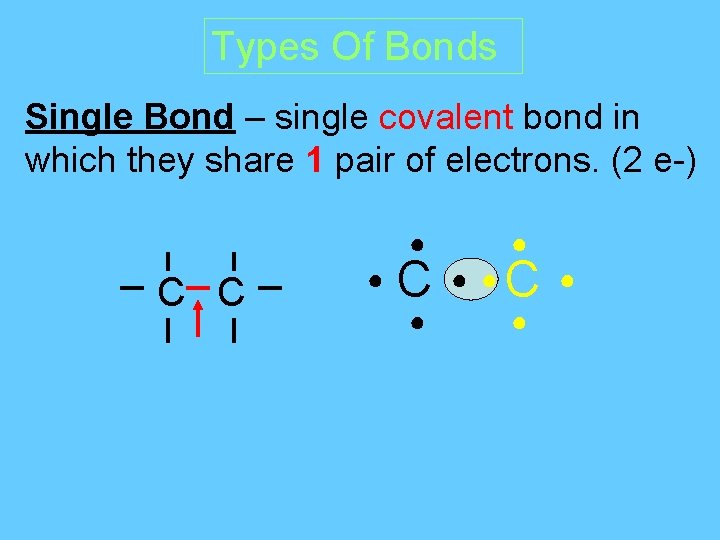
Types Of Bonds Single Bond – single covalent bond in which they share 1 pair of electrons. (2 e-) ● C C ● ● ● ● C ● ●

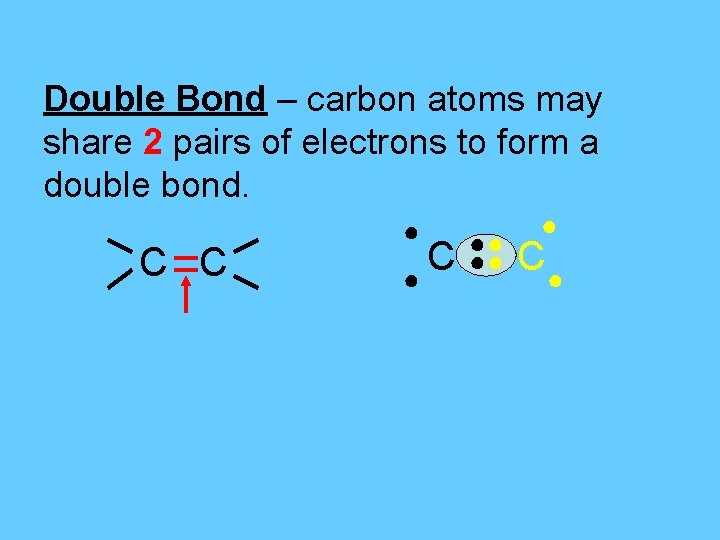
Double Bond – carbon atoms may share 2 pairs of electrons to form a double bond. C C ● ●● ●● ● C●

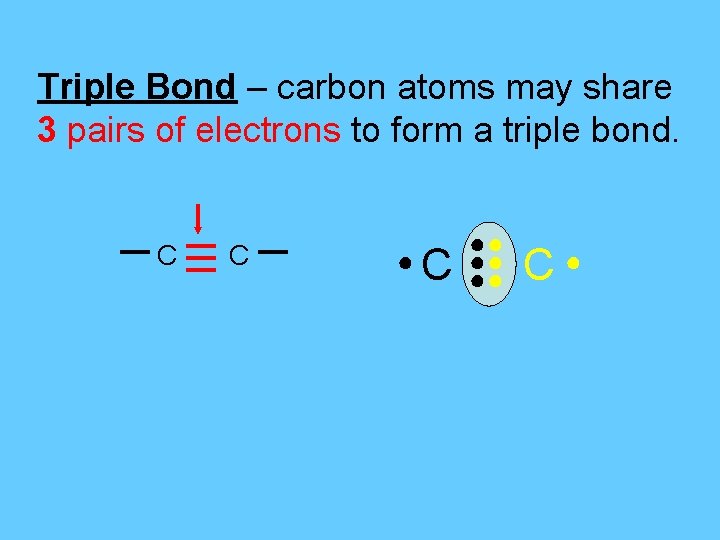
Triple Bond – carbon atoms may share 3 pairs of electrons to form a triple bond. C C ●C ●● ●● ●● C●

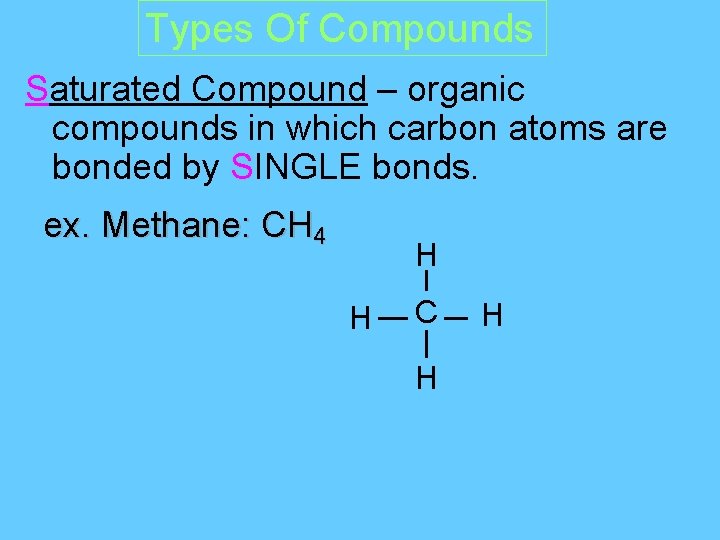
Types Of Compounds Saturated Compound – organic compounds in which carbon atoms are bonded by SINGLE bonds. ex. Methane: CH 4 H H C H H

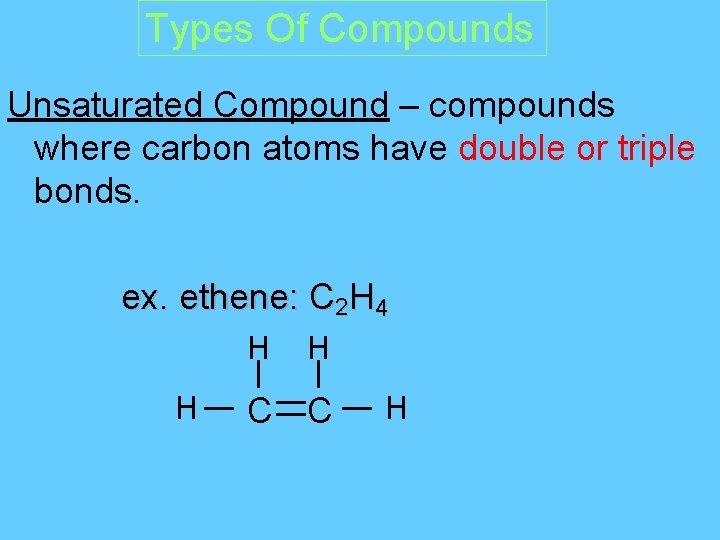
Types Of Compounds Unsaturated Compound – compounds where carbon atoms have double or triple bonds. ex. ethene: C 2 H 4 H H H C C H

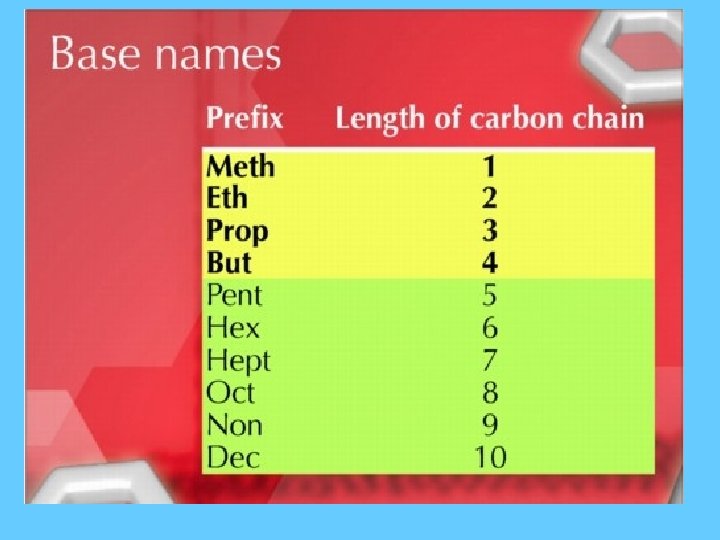
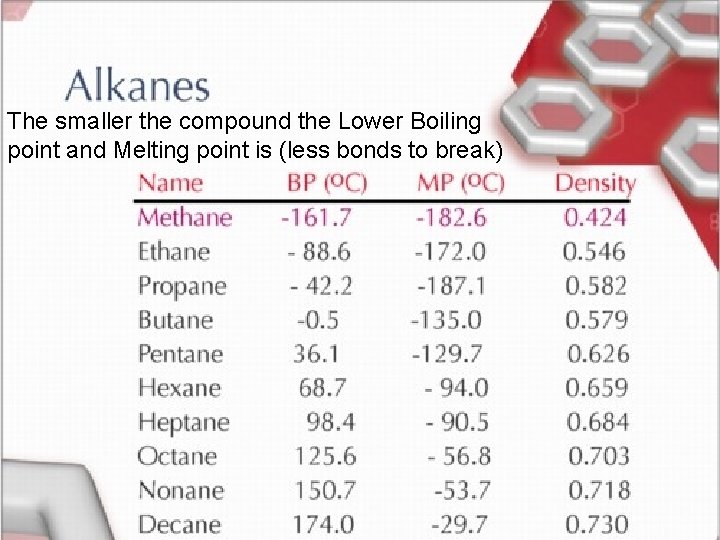
Homologous Series of Hydrocarbons • Organic compounds can be classified into groups with related structures and properties. ***As size of molecule increases the boiling and freezing points increase.

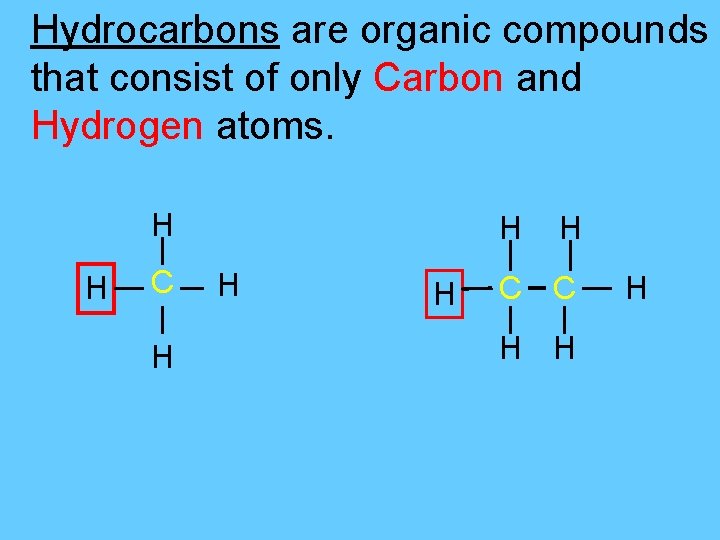
Hydrocarbons are organic compounds that consist of only Carbon and Hydrogen atoms. H H C H H H C C H H H

< TARGET="display">

single ● Saturated hydrocarbons

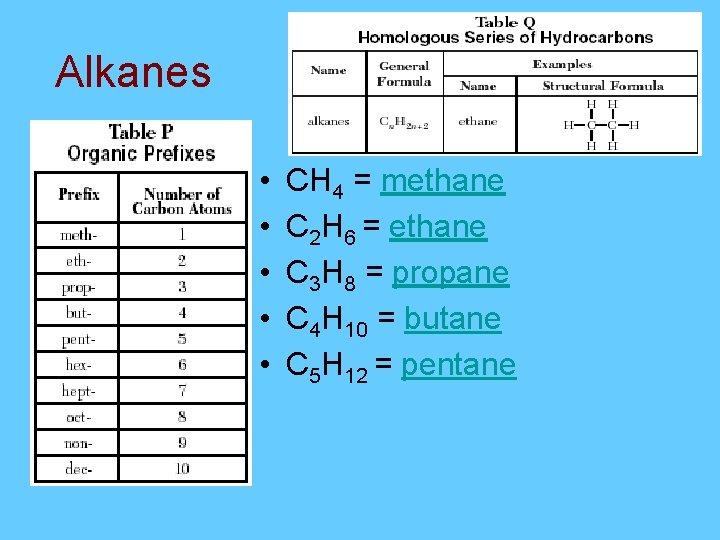
Alkanes = Cn. H 2 n+2 • A saturated hydrocarbon contains 5 carbons. What is the formula? C 5 H 2(5)+2 = C 5 H 12 • A saturated hydrocarbon contains 20 carbons. What is the formula? C 20 H 2(20)+2 = C 20 H 42 Saturated = Single

Alkanes • • • CH 4 = methane C 2 H 6 = ethane C 3 H 8 = propane C 4 H 10 = butane C 5 H 12 = pentane

The smaller the compound the Lower Boiling point and Melting point is (less bonds to break) < TARGET="display">

Naming Organic Compounds • Organic compounds are named according to the IUPAC (international union of pure & applied chemistry) system of nomenclature. Alkanes – end in Alkenes – end in Alkynes – end in ane ene yne

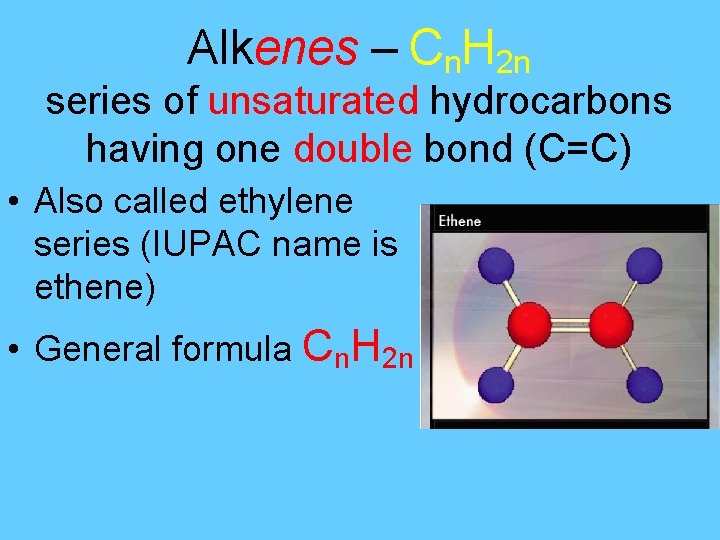
Alkenes – Cn. H 2 n series of unsaturated hydrocarbons having one double bond (C=C) • Also called ethylene series (IUPAC name is ethene) • General formula Cn. H 2 n

Alkenes • • • C 2 H 4 = Ethene C 3 H 6 = Propene C 4 H 8 = Butene C 5 H 10 = Pentene To find the number of hydrogens, double the number of carbons.

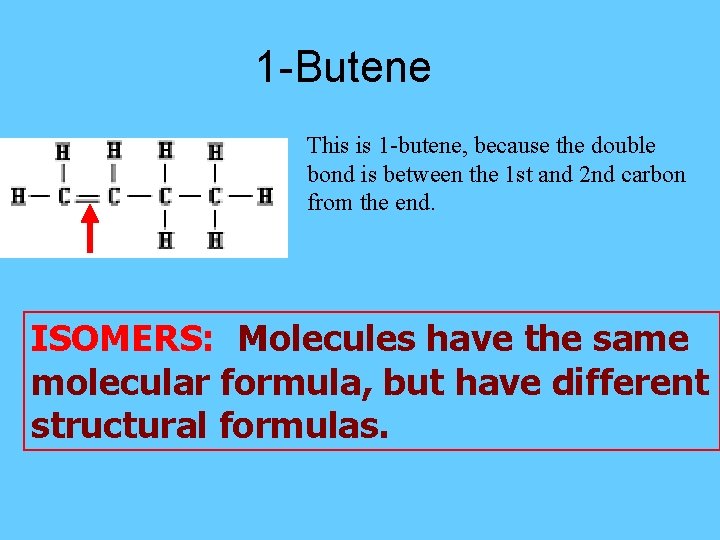
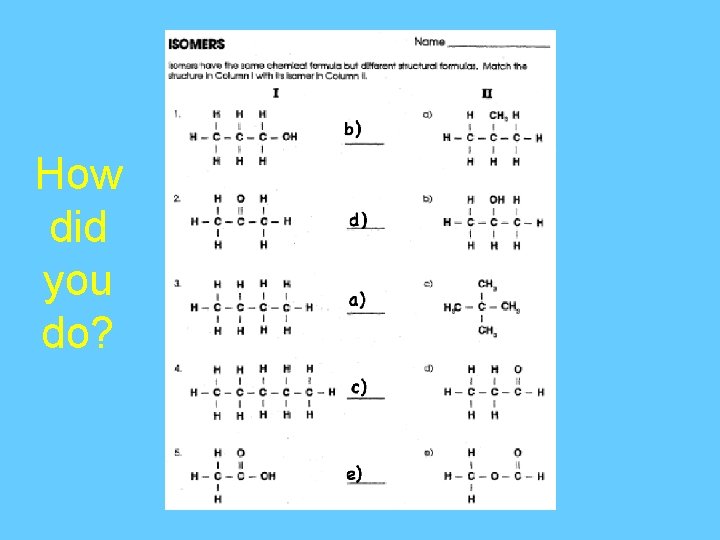
1 -Butene This is 1 -butene, because the double bond is between the 1 st and 2 nd carbon from the end. ISOMERS: Molecules have the same molecular formula, but have different structural formulas.

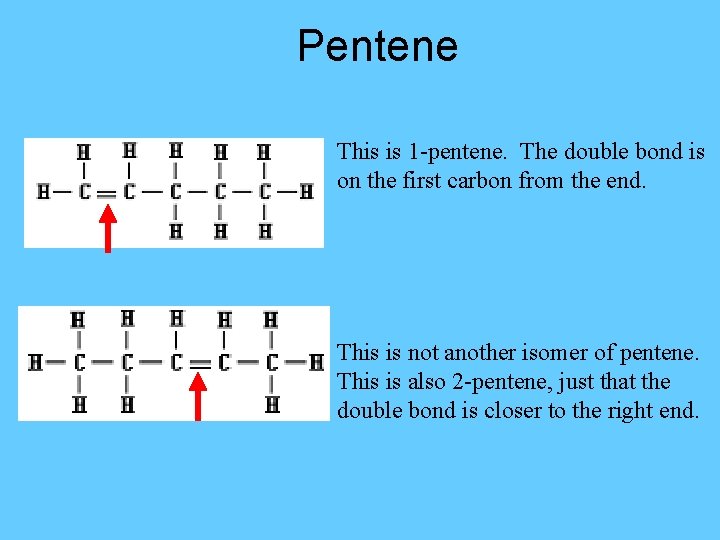
Pentene This is 1 -pentene. The double bond is on the first carbon from the end. This is not another isomer of pentene. This is also 2 -pentene, just that the double bond is closer to the right end.

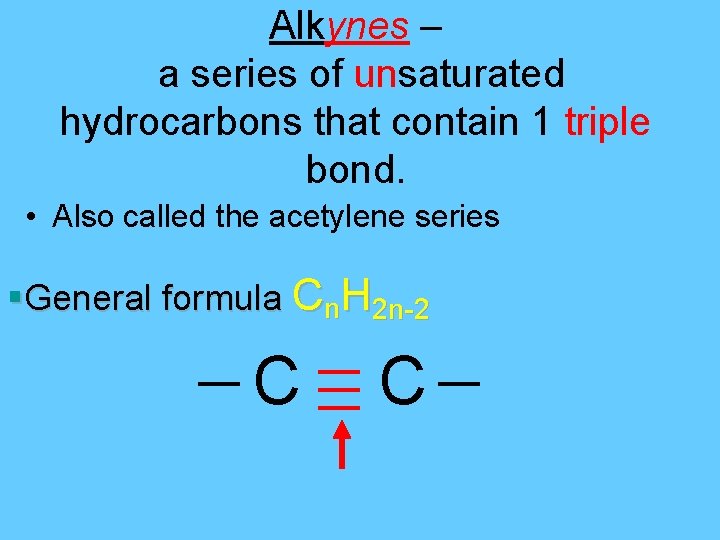
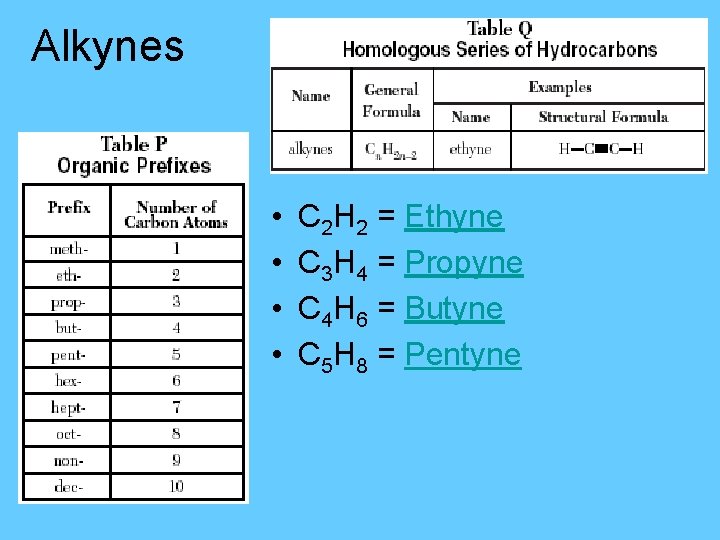
Alkynes – a series of unsaturated hydrocarbons that contain 1 triple bond. • Also called the acetylene series §General formula Cn. H 2 n-2 C C

Alkynes • • C 2 H 2 = Ethyne C 3 H 4 = Propyne C 4 H 6 = Butyne C 5 H 8 = Pentyne

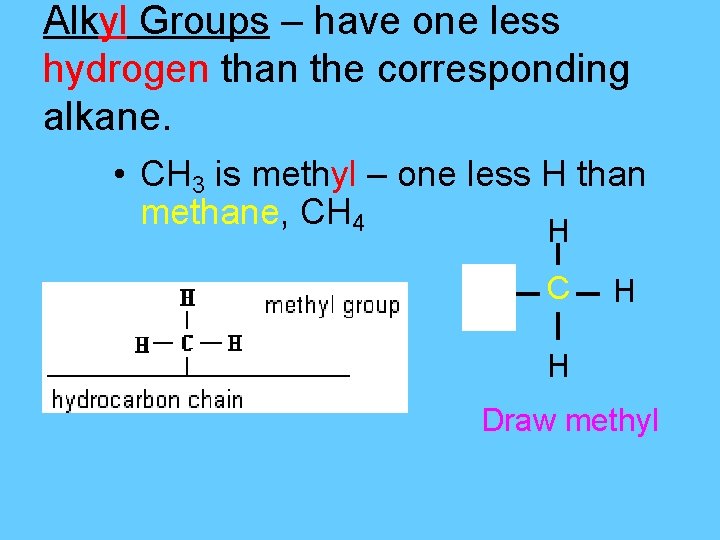
Alkyl Groups – have one less hydrogen than the corresponding alkane. • CH 3 is methyl – one less H than methane, CH 4 H H C H H Draw methyl

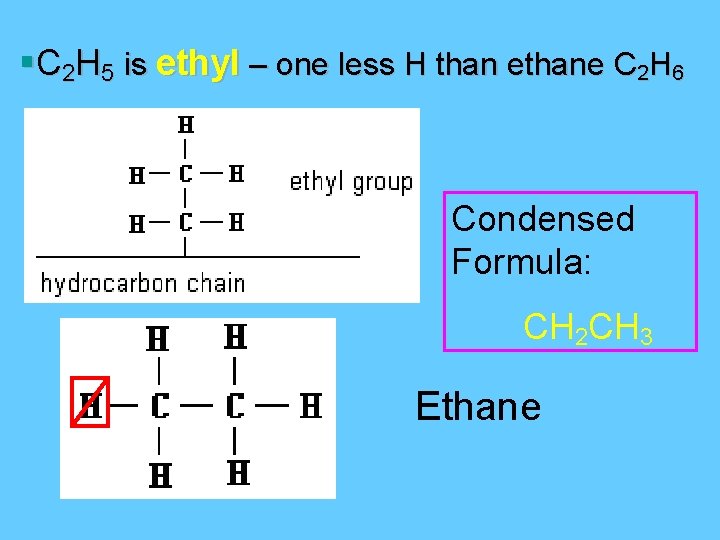
§C 2 H 5 is ethyl – one less H than ethane C 2 H 6 Condensed Formula: CH 2 CH 3 Ethane

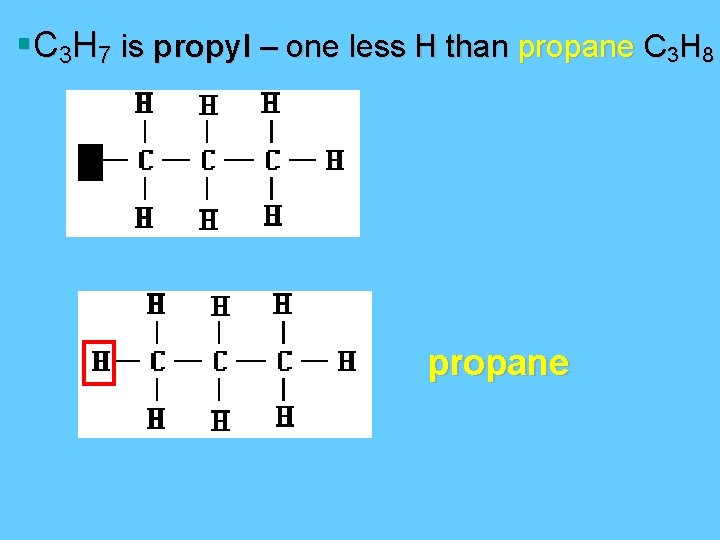
§C 3 H 7 is propyl – one less H than propane C 3 H 8 propane

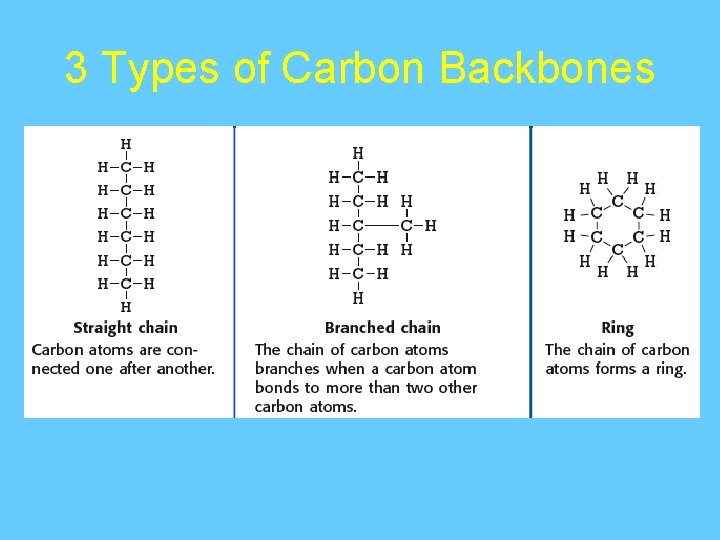
3 Types of Carbon Backbones

Carbon forms long chains • One carbon chain may contain hundreds of carbon atoms. • Unlike other elements, carbon atoms can bond to each other to form very long chains. • One carbon chain may contain hundreds of carbon atoms. Notice how the CH 2 units repeat. • A very large carbon-based molecule made of repeating units is called a polymer. Each unit of a polymer is called a monomer. • Polymers can be thousands of atoms long

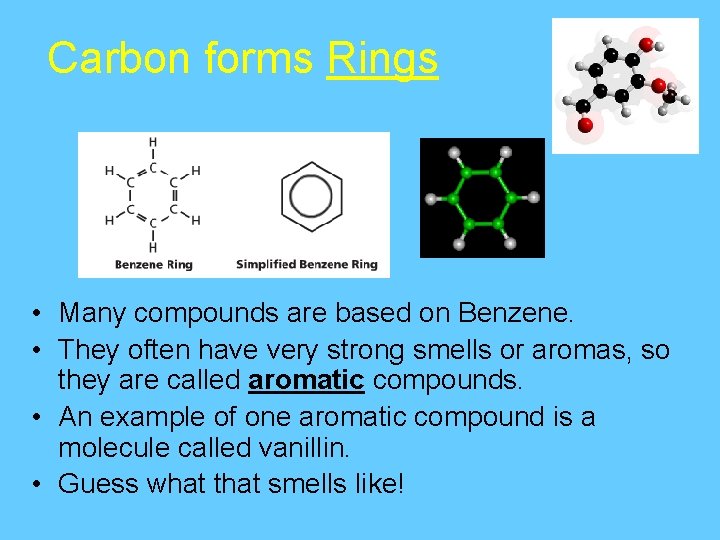
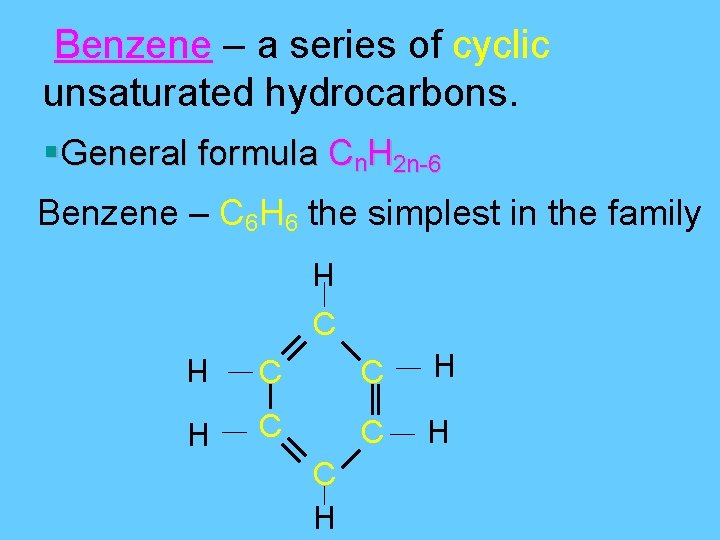
Carbon forms Rings • Carbon-based molecules also can be shaped like rings. Most carbon rings contain 5 or 6 carbon atoms. • One of the most important carbon rings is benzene. • It has 6 carbons & 6 hydrogens , with alternating double bonds.

Carbon forms Rings • Many compounds are based on Benzene. • They often have very strong smells or aromas, so they are called aromatic compounds. • An example of one aromatic compound is a molecule called vanillin. • Guess what that smells like!

Benzene – a series of cyclic unsaturated hydrocarbons. §General formula Cn. H 2 n-6 Benzene – C 6 H 6 the simplest in the family H C C H

IUPAC Naming Branched Hydrocarbon Chains

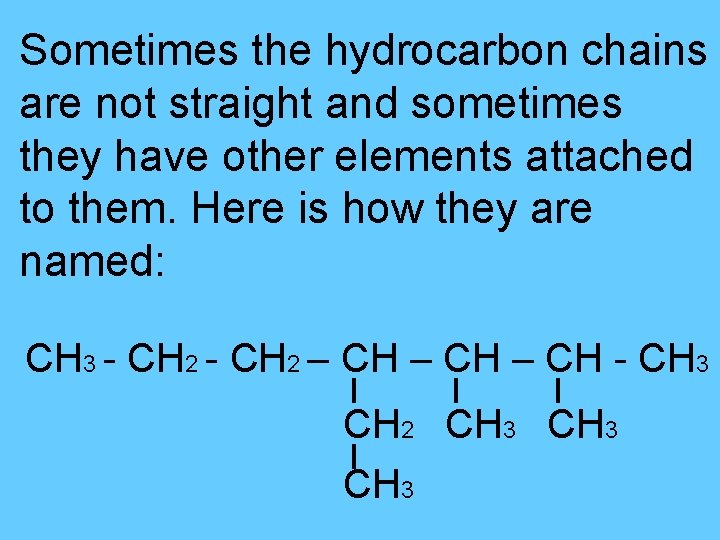
Sometimes the hydrocarbon chains are not straight and sometimes they have other elements attached to them. Here is how they are named: CH 3 - CH 2 – CH - CH 3 CH 2 CH 3

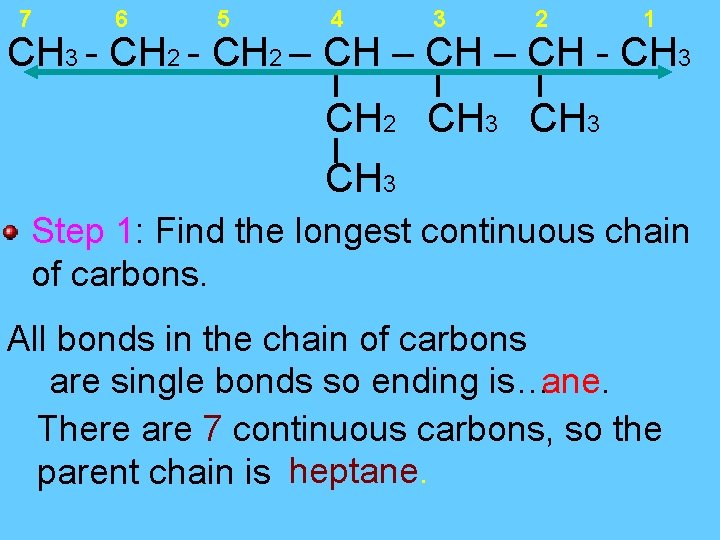
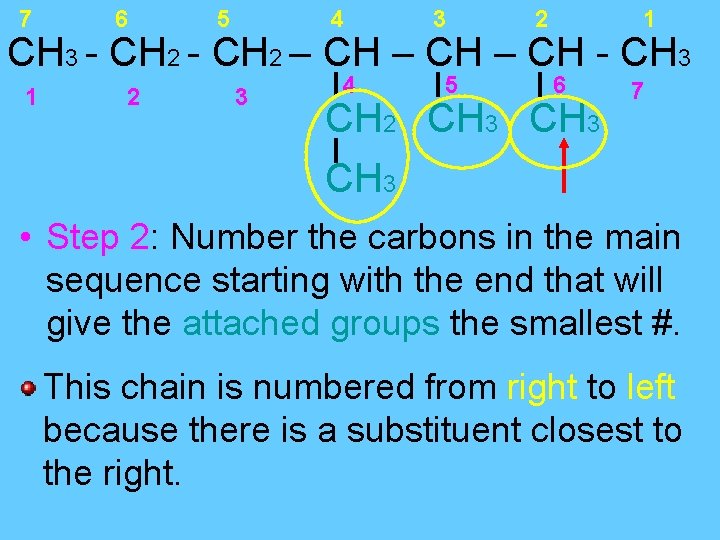
7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 CH 2 CH 3 Step 1: Find the longest continuous chain of carbons. All bonds in the chain of carbons are single bonds so ending is…ane. There are 7 continuous carbons, so the parent chain is heptane.

7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 1 2 3 4 5 6 CH 2 CH 3 7 CH 3 • Step 2: Number the carbons in the main sequence starting with the end that will give the attached groups the smallest #. This chain is numbered from right to left because there is a substituent closest to the right.

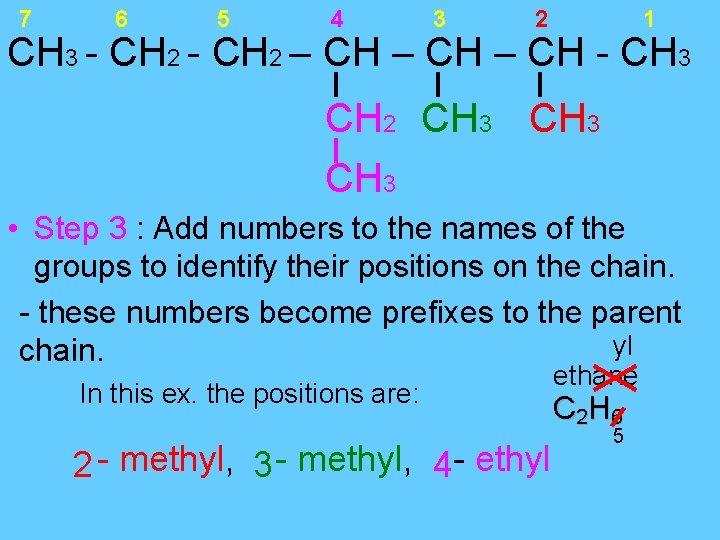
7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 CH 2 CH 3 • Step 3 : Add numbers to the names of the groups to identify their positions on the chain. - these numbers become prefixes to the parent yl chain. In this ex. the positions are: 2 - methyl, 3 - methyl, 4 - ethyl ethane C 2 H 6 5

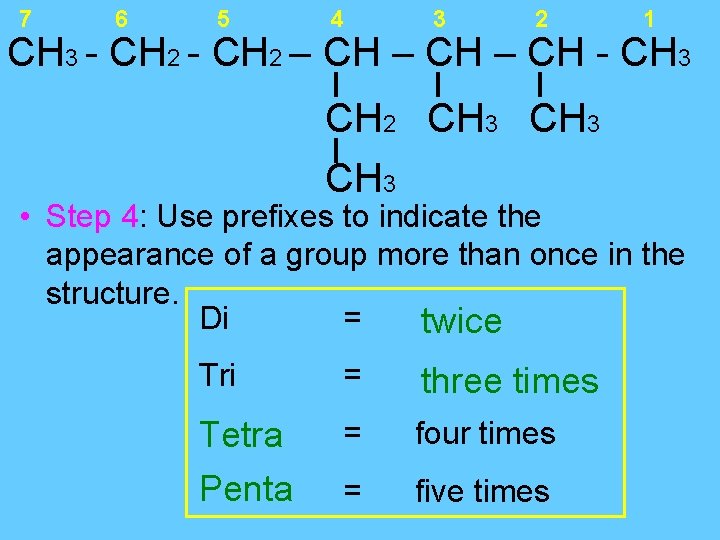
7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 CH 2 CH 3 • Step 4: Use prefixes to indicate the appearance of a group more than once in the structure. Di = twice Tri = three times Tetra Penta = four times = five times

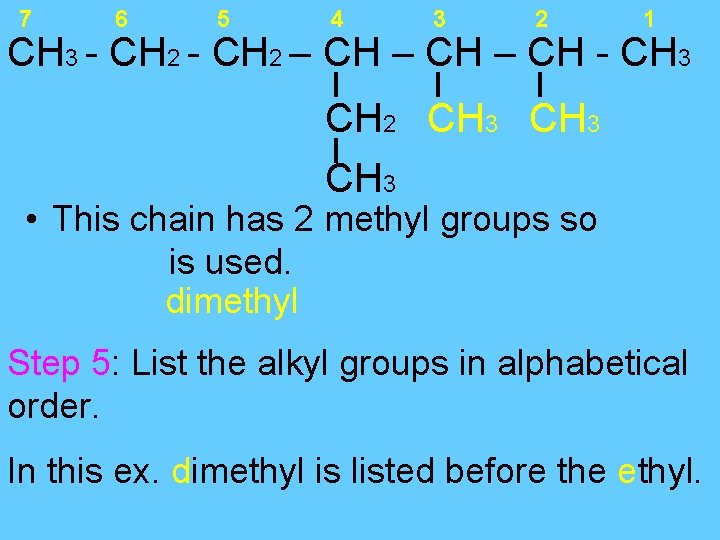
7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 CH 2 CH 3 • This chain has 2 methyl groups so is used. dimethyl Step 5: List the alkyl groups in alphabetical order. In this ex. dimethyl is listed before the ethyl.

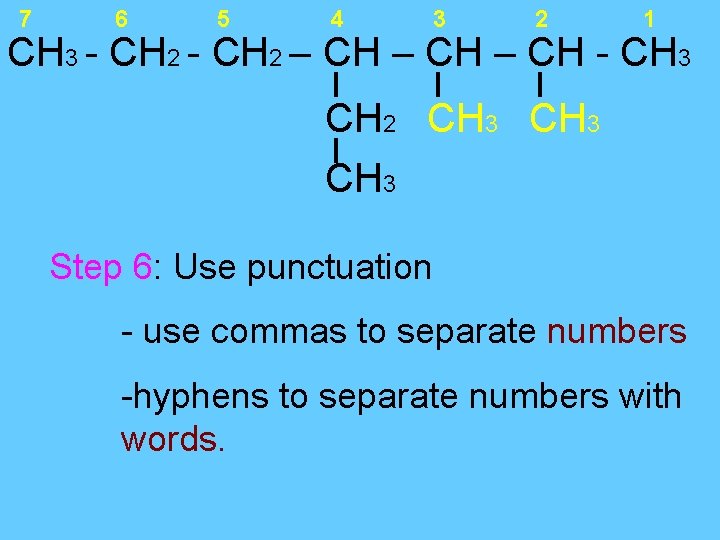
7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 CH 2 CH 3 Step 6: Use punctuation - use commas to separate numbers -hyphens to separate numbers with words.

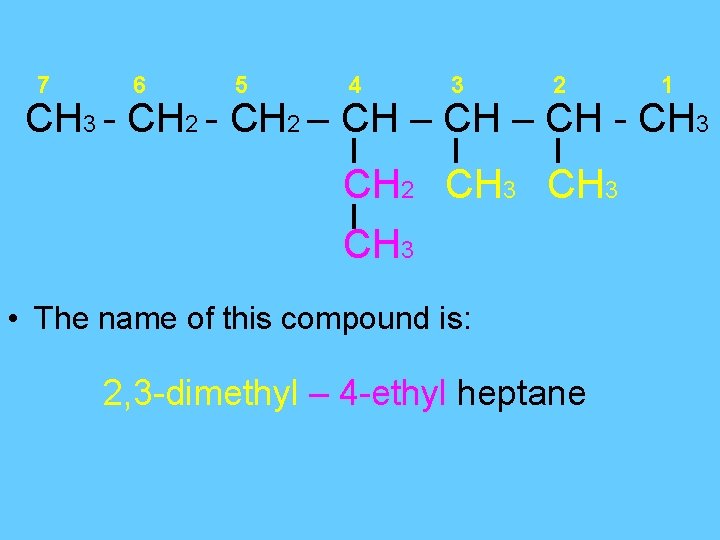
7 6 5 4 3 2 1 CH 3 - CH 2 – CH - CH 3 CH 2 CH 3 • The name of this compound is: 2, 3 -dimethyl – 4 -ethyl heptane

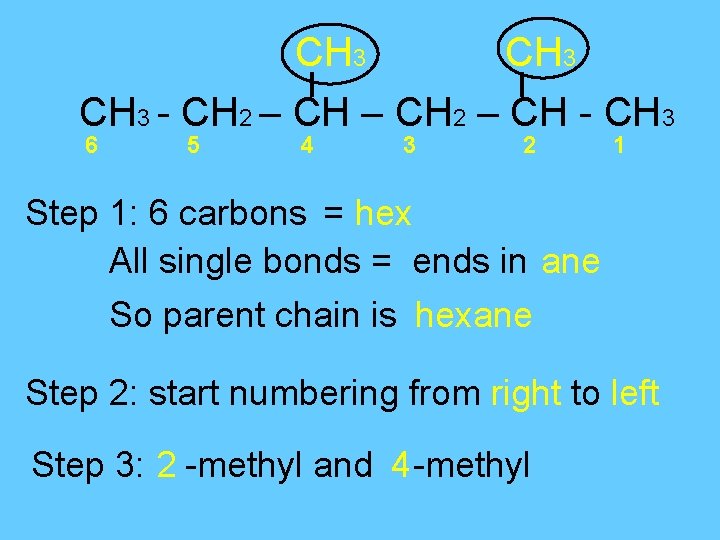
CH 3 - CH 2 – CH - CH 3 6 5 4 3 2 1 Step 1: 6 carbons = hex All single bonds = ends in ane So parent chain is hexane Step 2: start numbering from right to left Step 3: 2 -methyl and 4 -methyl

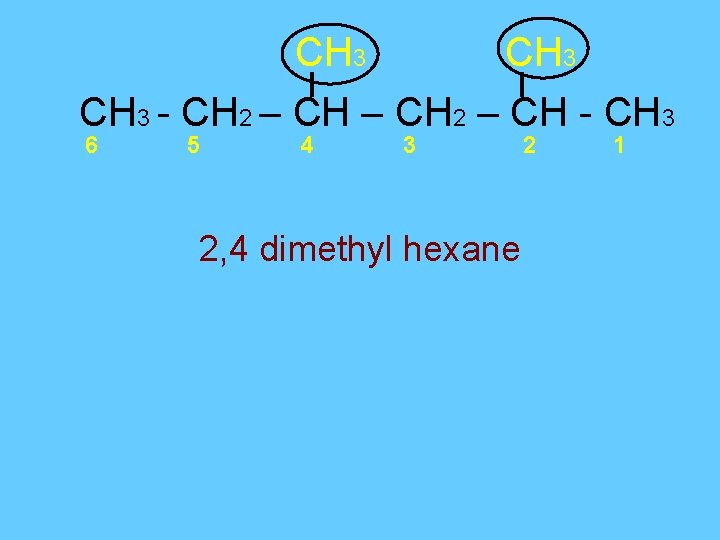
CH 3 - CH 2 – CH - CH 3 6 5 4 3 2, 4 dimethyl hexane 2 1

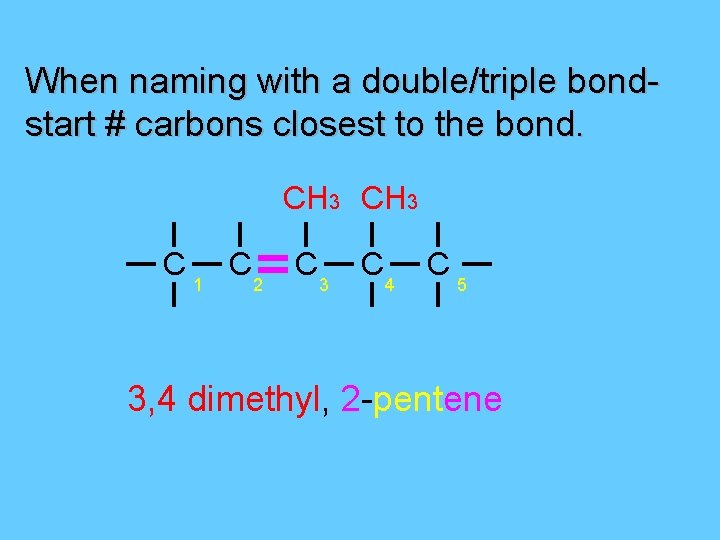
When naming with a double/triple bondstart # carbons closest to the bond. CH 3 C 1 C 2 C 3 C 4 C 5 3, 4 dimethyl, 2 -pentene

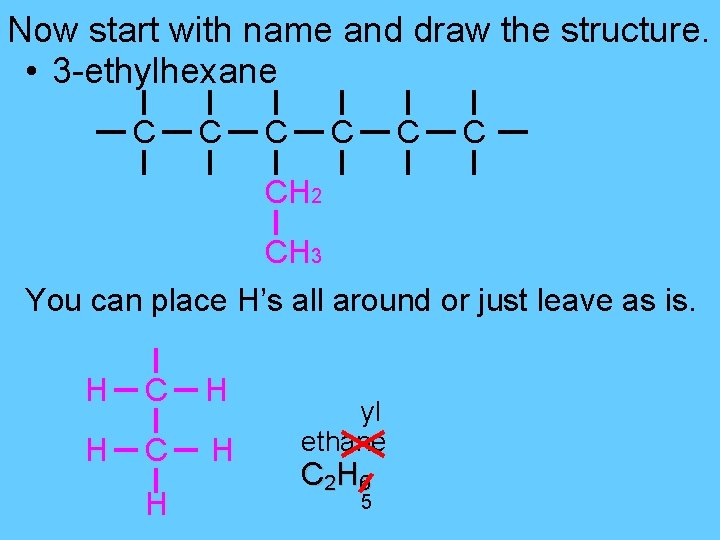
Now start with name and draw the structure. • 3 -ethylhexane C C C CH 2 CH 3 You can place H’s all around or just leave as is. H C H H yl ethane C 2 H 6 5

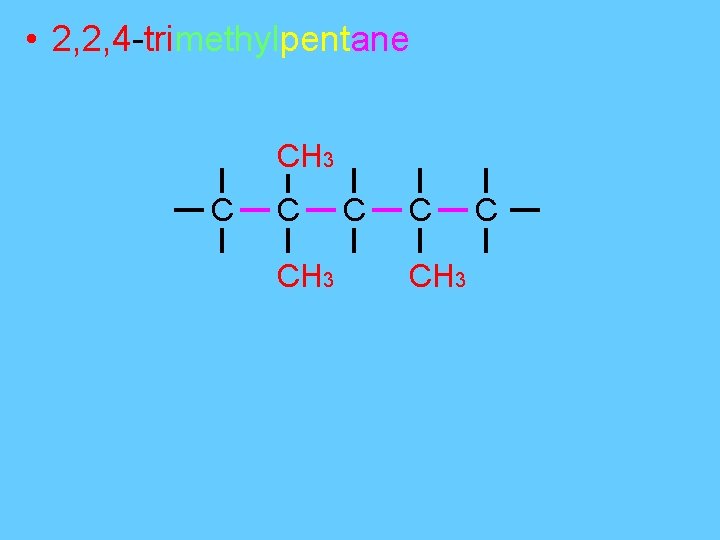
• 2, 2, 4 -trimethylpentane CH 3 C C CH 3 C

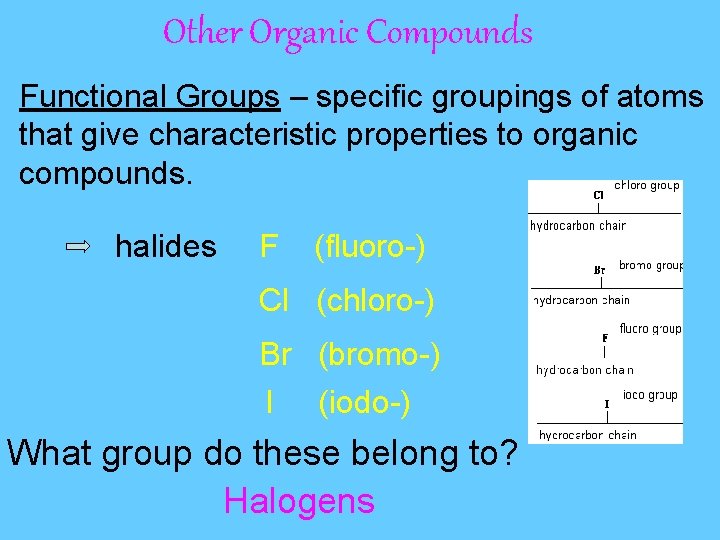
Other Organic Compounds Functional Groups – specific groupings of atoms that give characteristic properties to organic compounds. halides F (fluoro-) Cl (chloro-) Br (bromo-) I (iodo-) What group do these belong to? Halogens

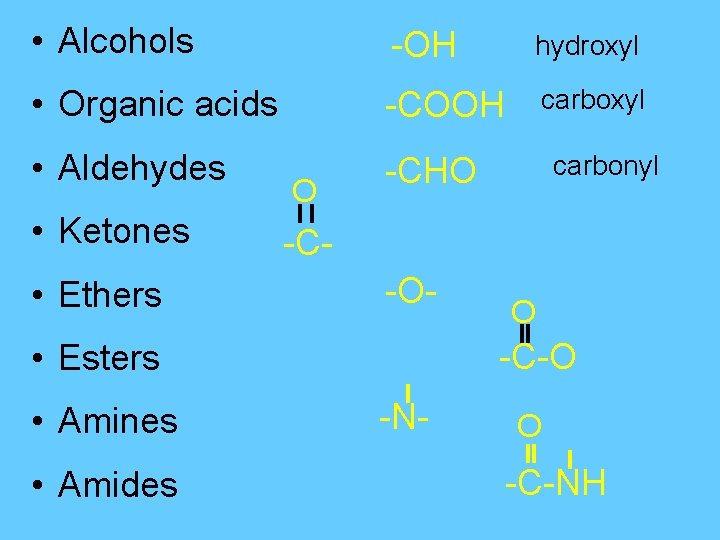
• Alcohols -OH hydroxyl • Organic acids -COOH carboxyl • Aldehydes -CHO • Ethers O • Ketones -C-O- • Amides O -C-O • Esters • Amines carbonyl -N- O -C-NH

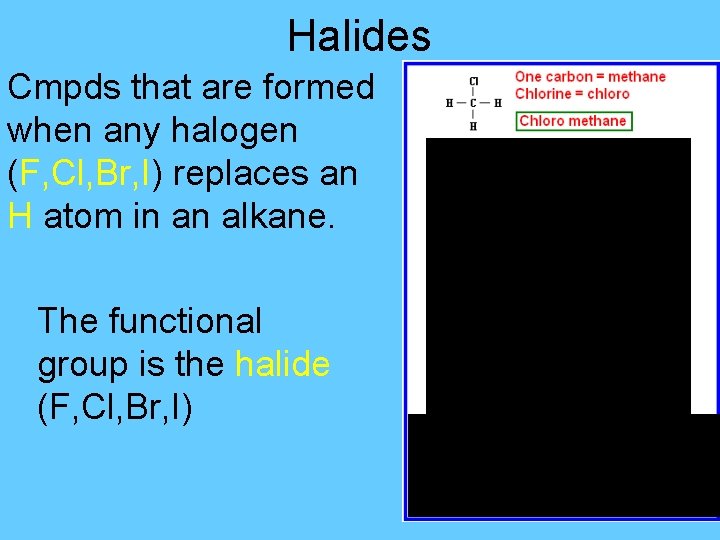
Halides Cmpds that are formed when any halogen (F, Cl, Br, I) replaces an H atom in an alkane. The functional group is the halide (F, Cl, Br, I)

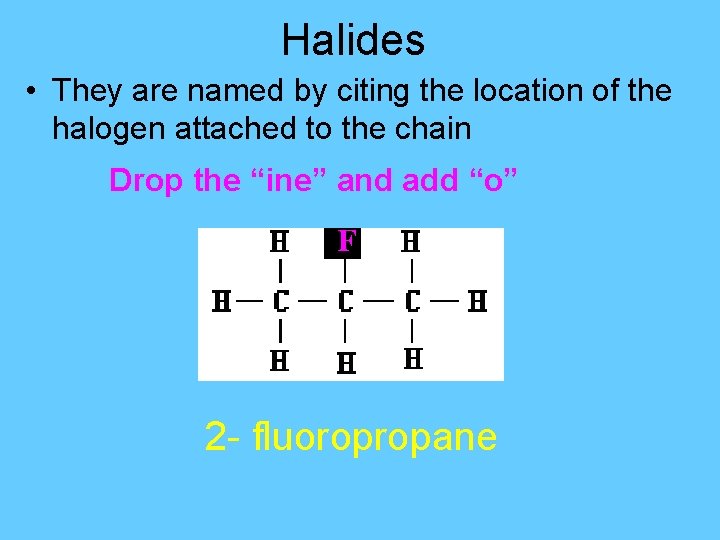
Halides • They are named by citing the location of the halogen attached to the chain Drop the “ine” and add “o” F 2 - fluoropropane

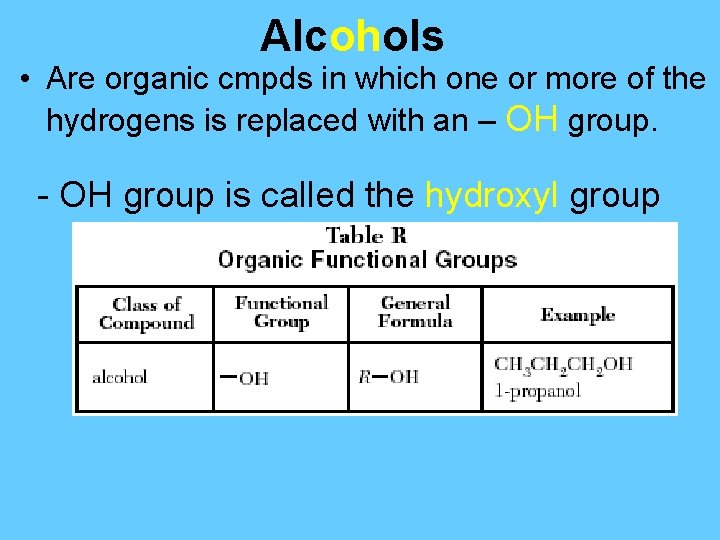
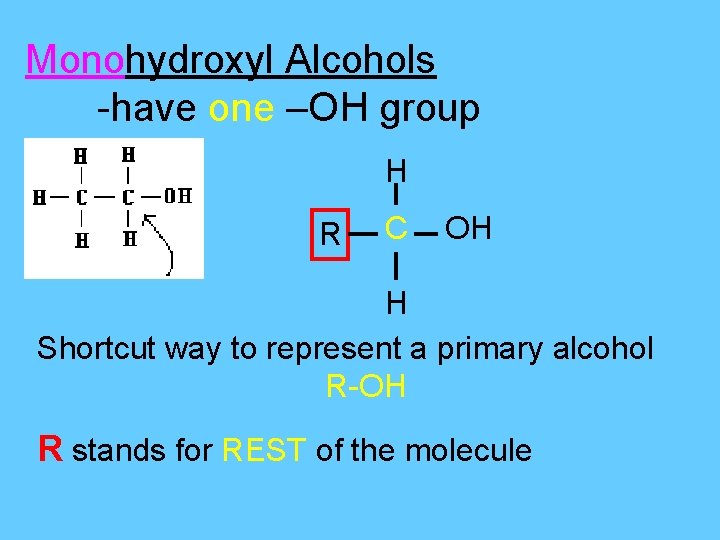
Alcohols • Are organic cmpds in which one or more of the hydrogens is replaced with an – OH group. - OH group is called the hydroxyl group

Monohydroxyl Alcohols -have one –OH group H R C OH H Shortcut way to represent a primary alcohol R-OH R stands for REST of the molecule

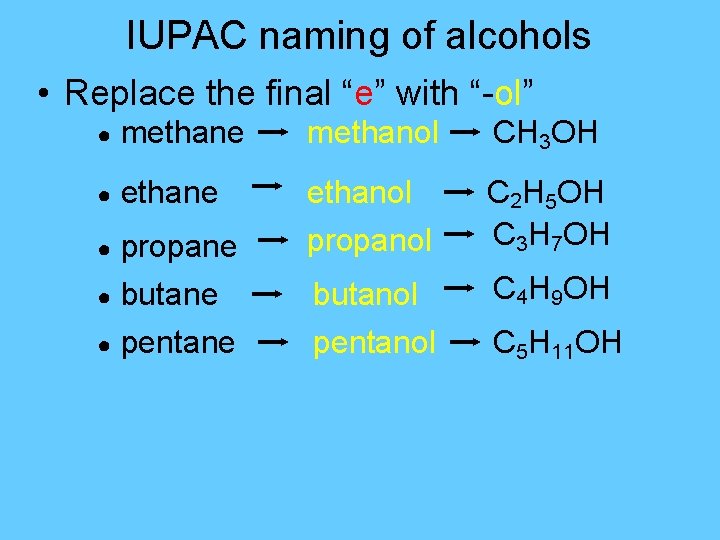
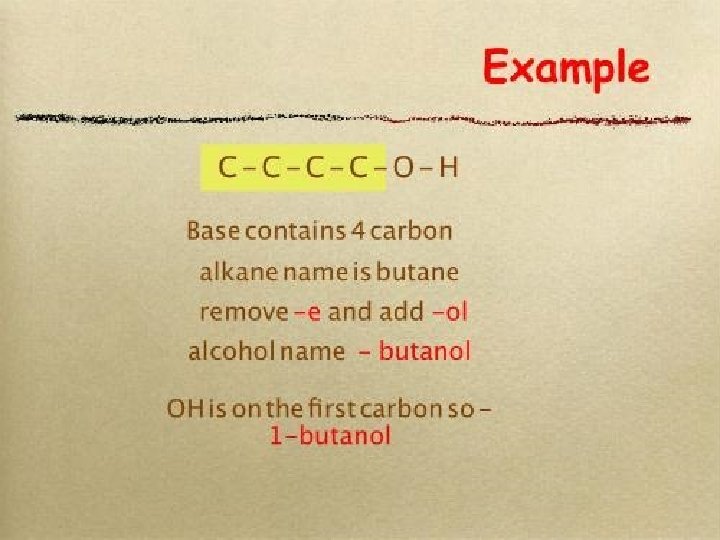
IUPAC naming of alcohols • Replace the final “e” with “-ol” ● methane methanol CH 3 OH ● ethane ethanol propanol C 2 H 5 OH C 3 H 7 OH butanol pentanol C 4 H 9 OH propane ● butane ● pentane ● C 5 H 11 OH


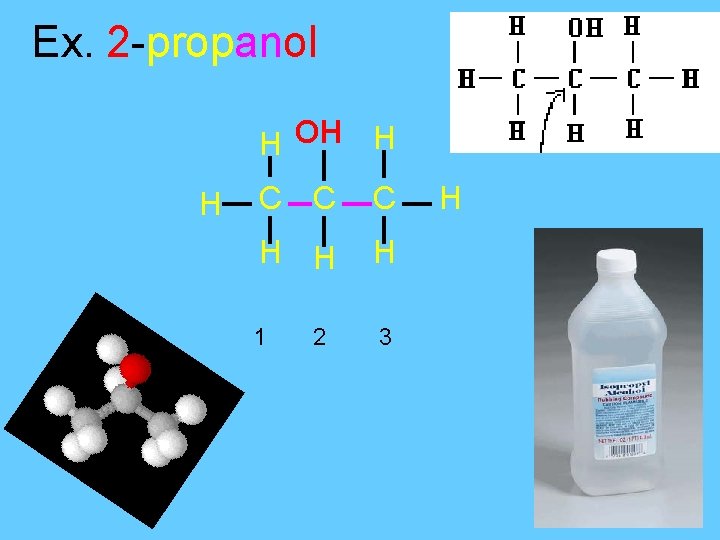
Ex. 2 -propanol H OH H H C C C H H H 1 3 2 H

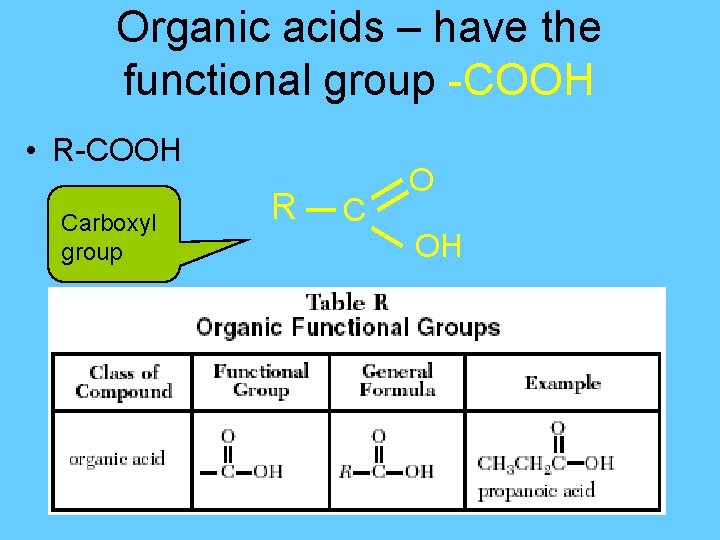
Organic acids – have the functional group -COOH • R-COOH Carboxyl group R C O OH

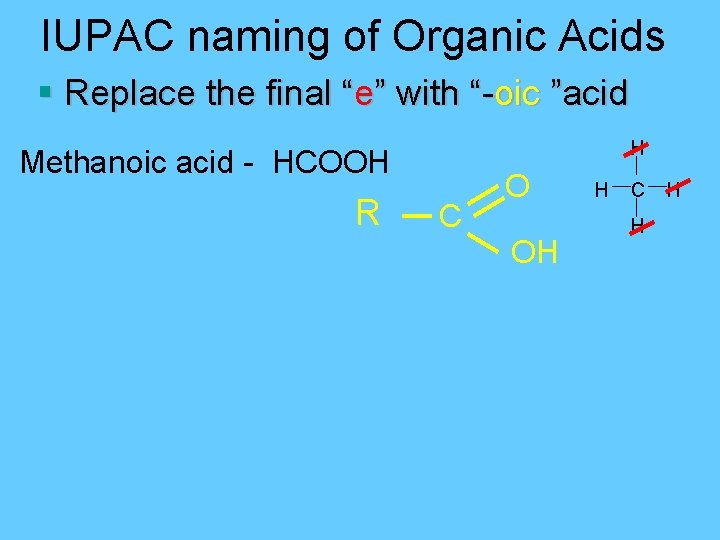
IUPAC naming of Organic Acids § Replace the final “e” with “-oic ”acid H Methanoic acid - HCOOH R C O OH H C H H

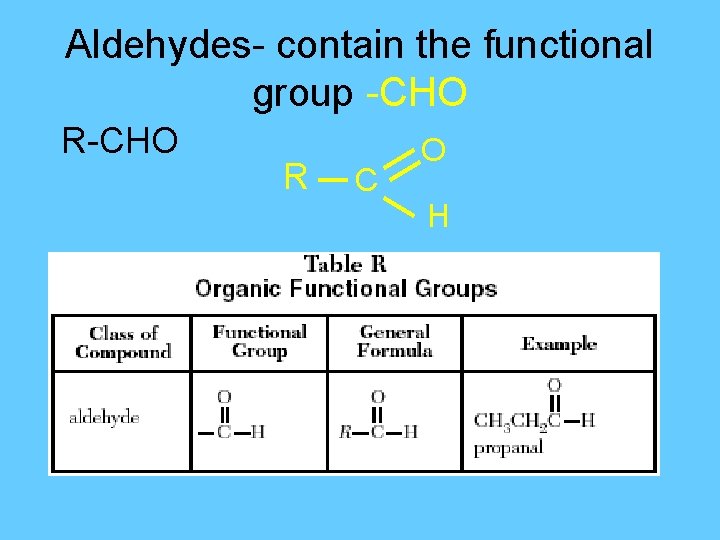
Aldehydes- contain the functional group -CHO R C O H

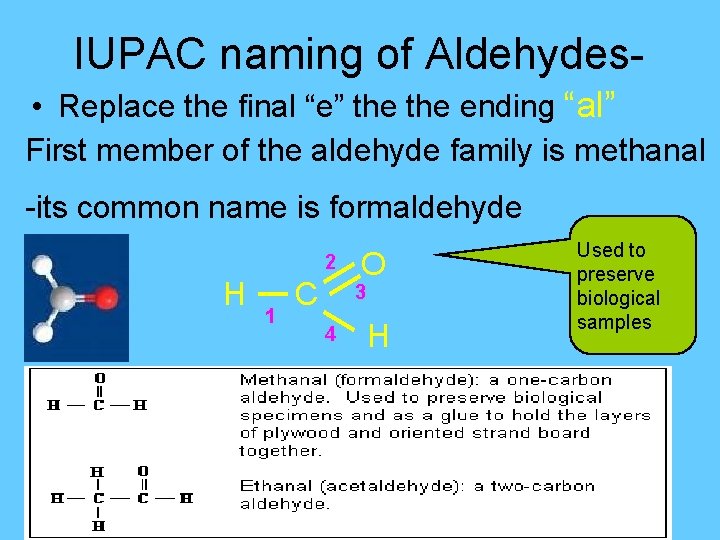
IUPAC naming of Aldehydes • Replace the final “e” the ending “al” First member of the aldehyde family is methanal -its common name is formaldehyde 2 H 1 C O 3 4 H Used to preserve biological samples

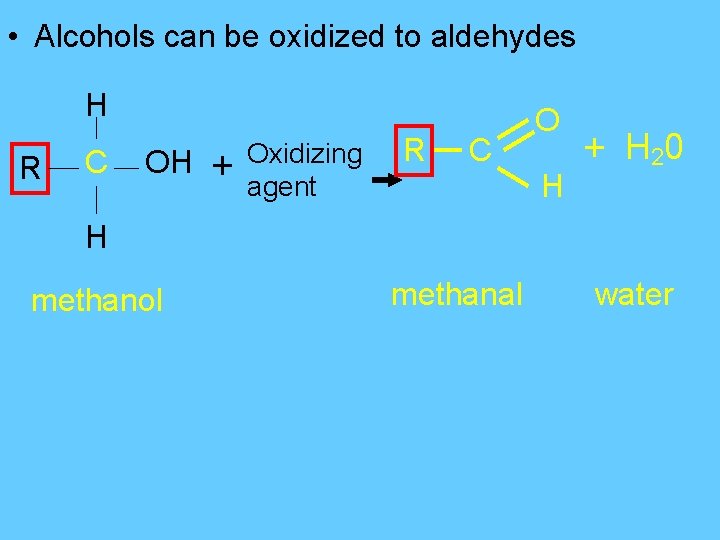
• Alcohols can be oxidized to aldehydes H R C OH + Oxidizing agent R C O H + H 20 H methanol methanal water

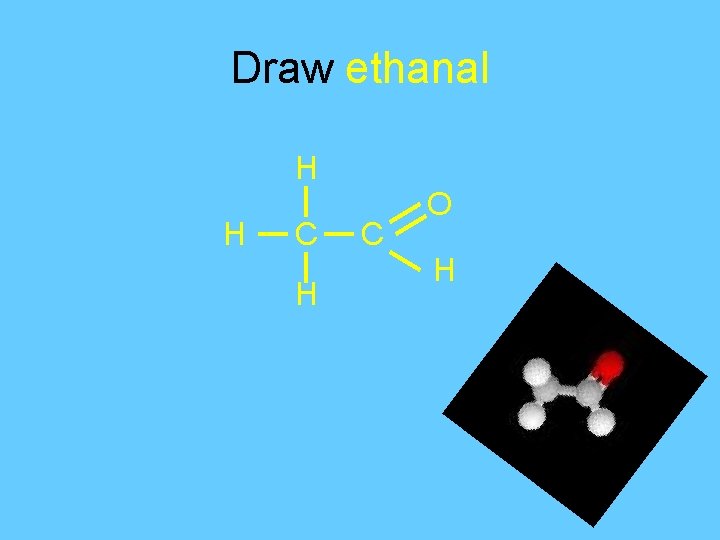
Draw ethanal H H C O H

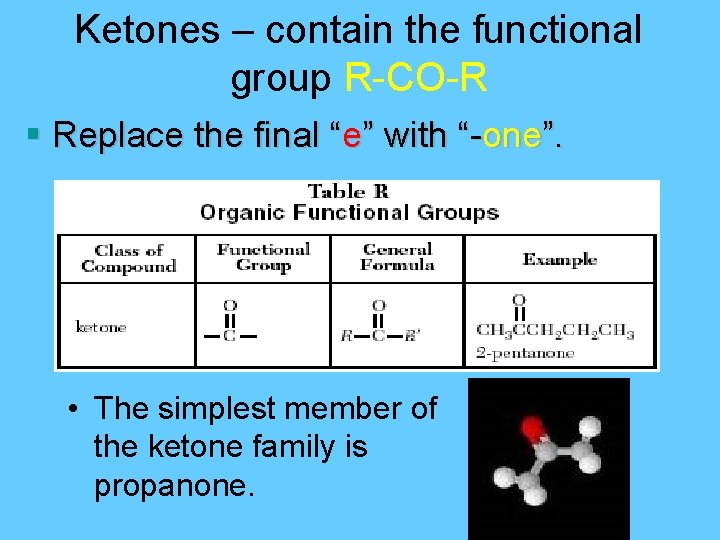
Ketones – contain the functional group R-CO-R § Replace the final “e” with “-one”. • The simplest member of the ketone family is propanone.

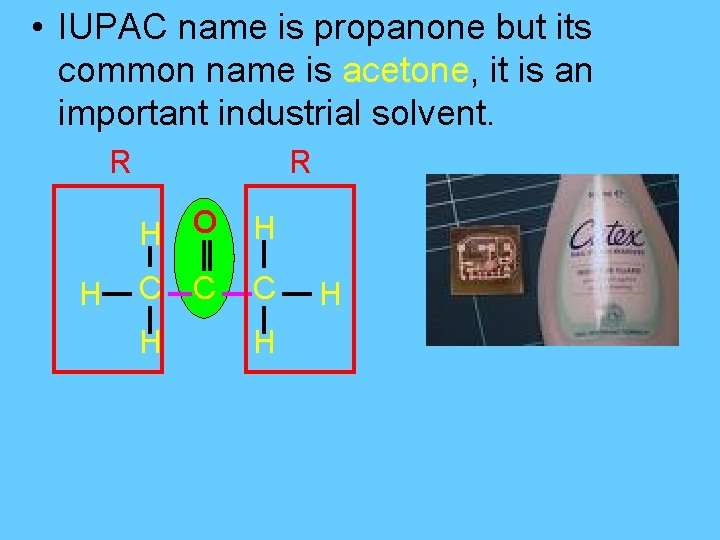
• IUPAC name is propanone but its common name is acetone, it is an important industrial solvent. R H O H C C C H H H

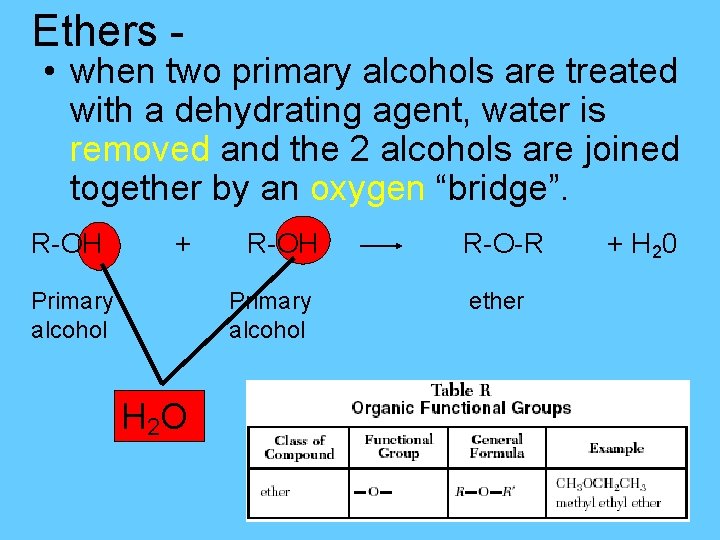
Ethers - • when two primary alcohols are treated with a dehydrating agent, water is removed and the 2 alcohols are joined together by an oxygen “bridge”. R-OH + Primary alcohol R-OH Primary alcohol H 2 O R-O-R ether + H 20

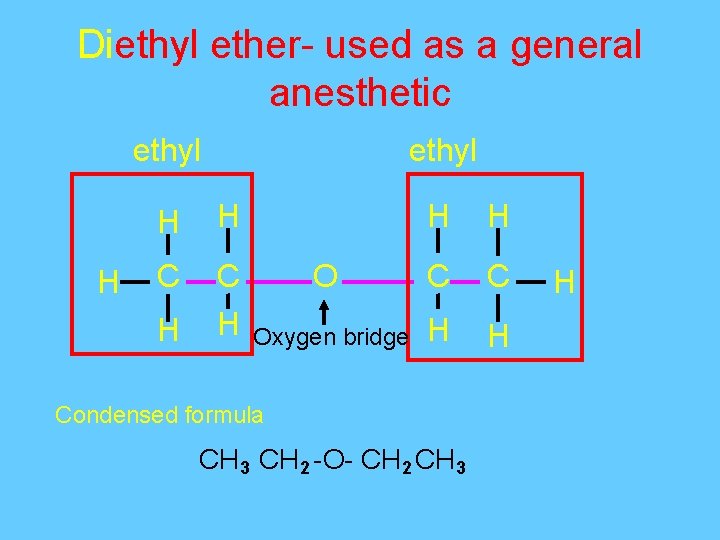
Diethyl ether- used as a general anesthetic ethyl H H C C H H O C C Oxygen bridge H H Condensed formula CH 3 CH 2 -O- CH 2 CH 3 H

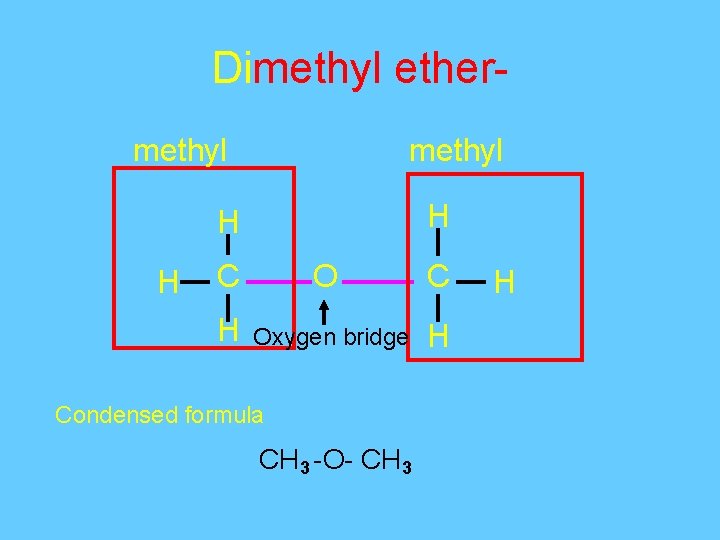
Dimethyl ethermethyl H H H C O C H Oxygen bridge H Condensed formula CH 3 -O- CH 3 H

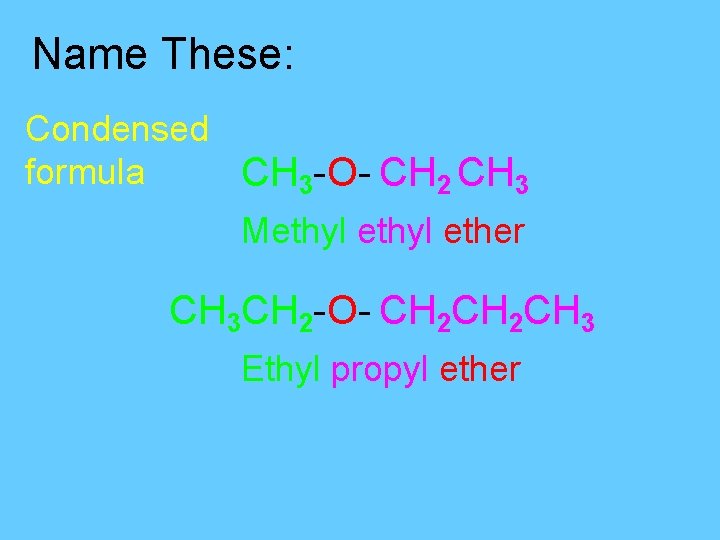
Name These: Condensed formula CH 3 -O- CH 2 CH 3 Methyl ether CH 3 CH 2 -O- CH 2 CH 3 Ethyl propyl ether

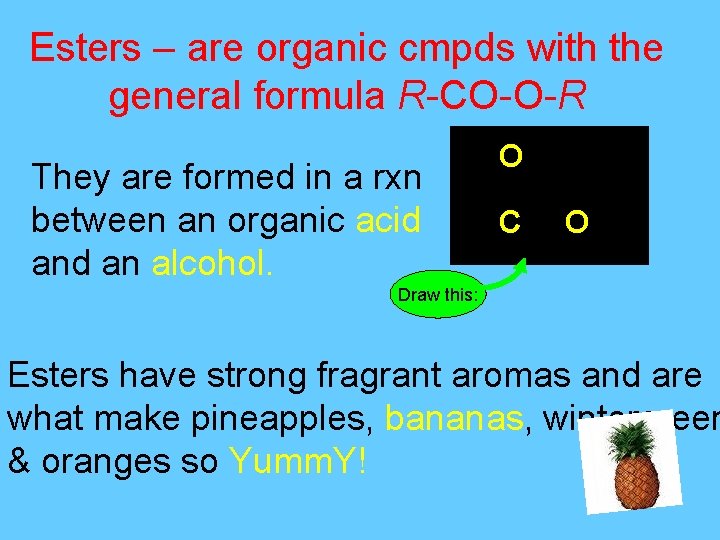
Esters – are organic cmpds with the general formula R-CO-O-R They are formed in a rxn between an organic acid an alcohol. O C O Draw this: Esters have strong fragrant aromas and are what make pineapples, bananas, wintergreen & oranges so Yumm. Y!

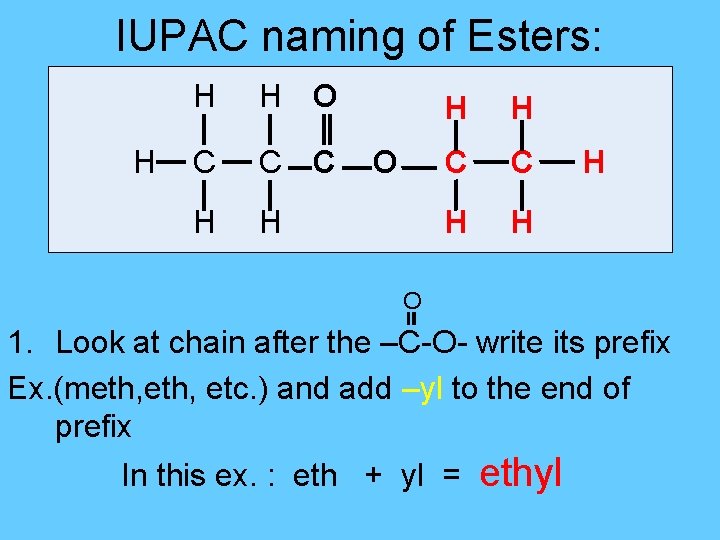
IUPAC naming of Esters: H H H O C C C H H O H H C C H H H O 1. Look at chain after the –C-O- write its prefix Ex. (meth, etc. ) and add –yl to the end of prefix In this ex. : eth + yl = ethyl

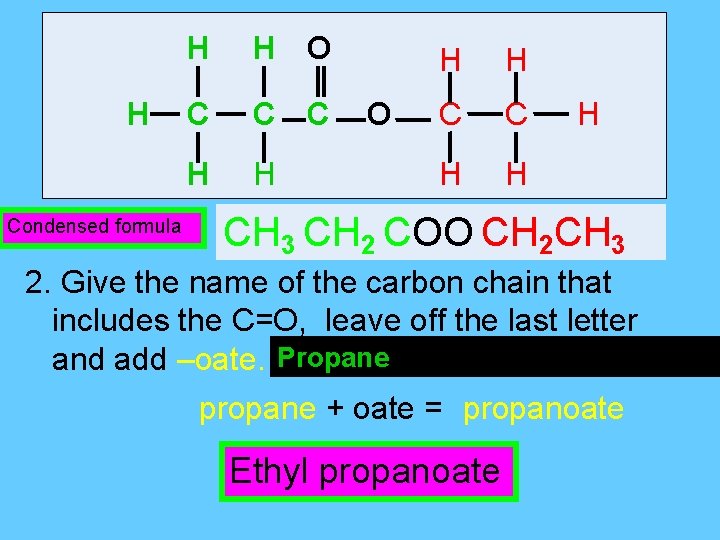
H Condensed formula H H O C C C H H O H H C C H H H CH 3 CH 2 COO CH 2 CH 3 2. Give the name of the carbon chain that includes the C=O, leave off the last letter and add –oate. Propane - 3 C’s and single bonds propane + oate = propanoate Ethyl propanoate

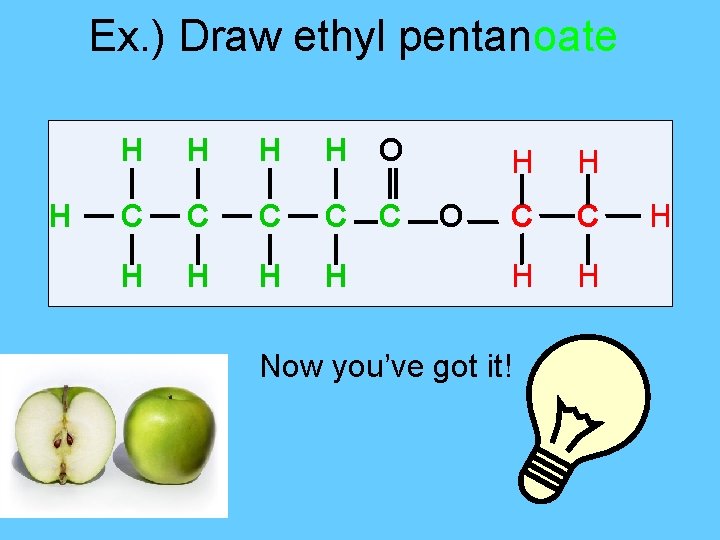
Ex. ) Draw ethyl pentanoate H H H O C C C H H O H H C C H H Now you’ve got it! H

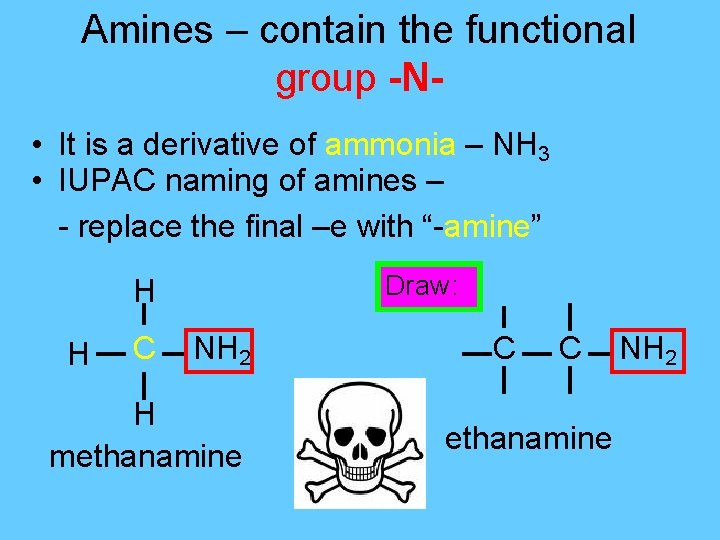
Amines – contain the functional group -N • It is a derivative of ammonia – NH 3 • IUPAC naming of amines – - replace the final –e with “-amine” Draw: H H C NH 2 H methanamine C C ethanamine NH 2

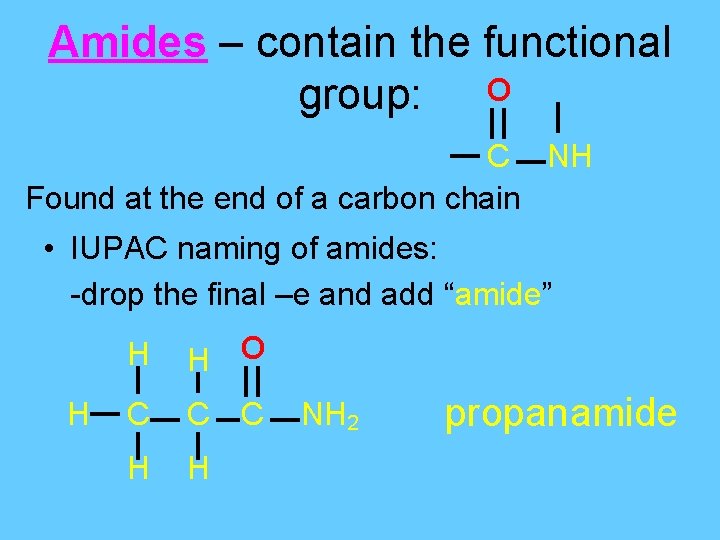
Amides – contain the functional group: O C NH Found at the end of a carbon chain • IUPAC naming of amides: -drop the final –e and add “amide” H H H O C C C H H NH 2 propanamide

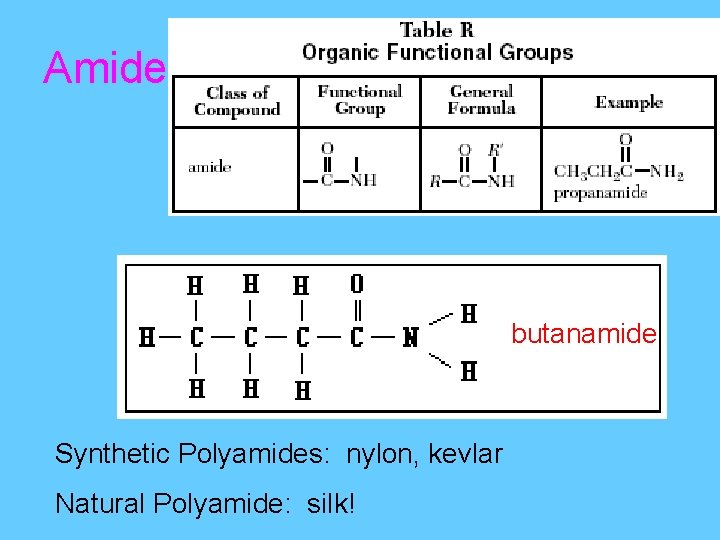
Amide butanamide Synthetic Polyamides: nylon, kevlar Natural Polyamide: silk!

There are two types of isomerism common in organic chemistry: 1. structural isomerism • Which have the atoms of their molecules linked in a different order. • This can come about in one of three ways:

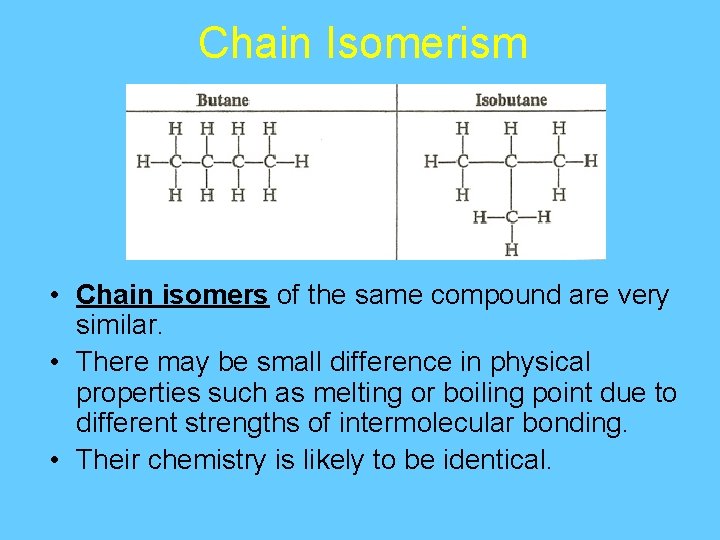
Chain Isomerism • Chain isomers of the same compound are very similar. • There may be small difference in physical properties such as melting or boiling point due to different strengths of intermolecular bonding. • Their chemistry is likely to be identical.

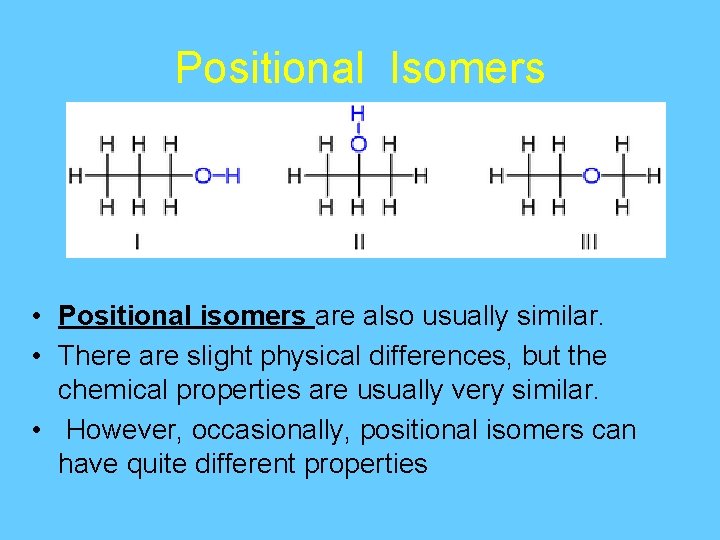
Positional Isomers • Positional isomers are also usually similar. • There are slight physical differences, but the chemical properties are usually very similar. • However, occasionally, positional isomers can have quite different properties

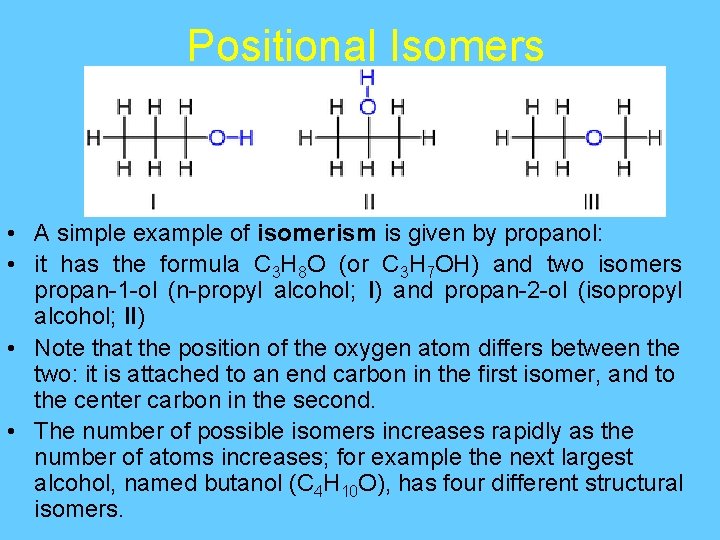
Positional Isomers • A simple example of isomerism is given by propanol: • it has the formula C 3 H 8 O (or C 3 H 7 OH) and two isomers propan-1 -ol (n-propyl alcohol; I) and propan-2 -ol (isopropyl alcohol; II) • Note that the position of the oxygen atom differs between the two: it is attached to an end carbon in the first isomer, and to the center carbon in the second. • The number of possible isomers increases rapidly as the number of atoms increases; for example the next largest alcohol, named butanol (C 4 H 10 O), has four different structural isomers.

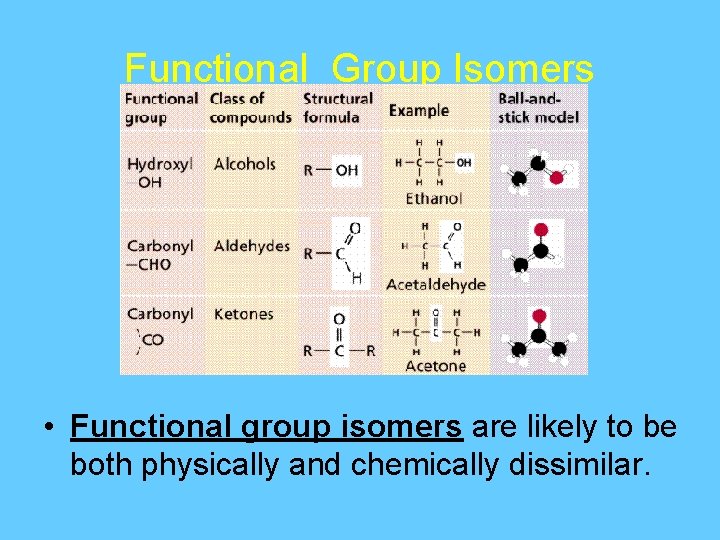
Functional Group Isomers • Functional group isomers are likely to be both physically and chemically dissimilar.

You Try It!

How did you do?

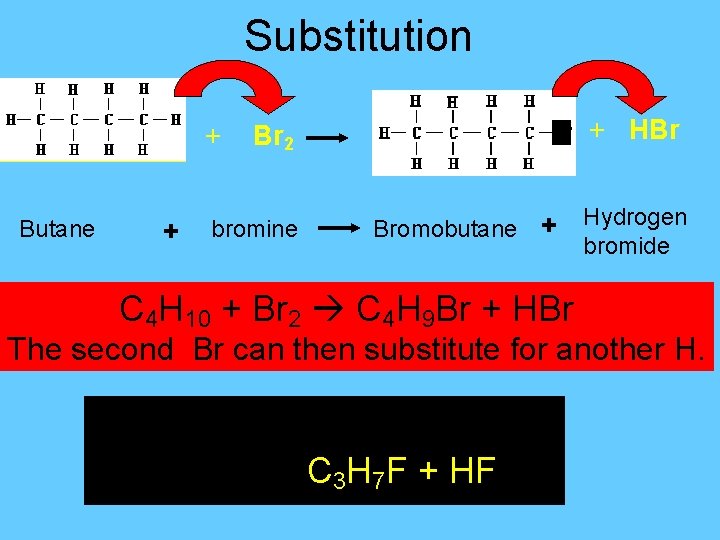
Organic Reactions • Substitution – replacement of one kind of atom or group with another atom or group • If this rxn occurs between an alkane and a halogen, it is called halogenation. *only happens with alkanes – single bonds!!!!

Substitution + Butane + Br Br 2 bromine Bromobutane + C 4 H 10 + Br 2 C 4 H 9 Br + HBr Hydrogen bromide The second Br can then substitute for another H. For Ex: Find the products of C 3 H 8 + F 2 C 3 H 7 F + HF

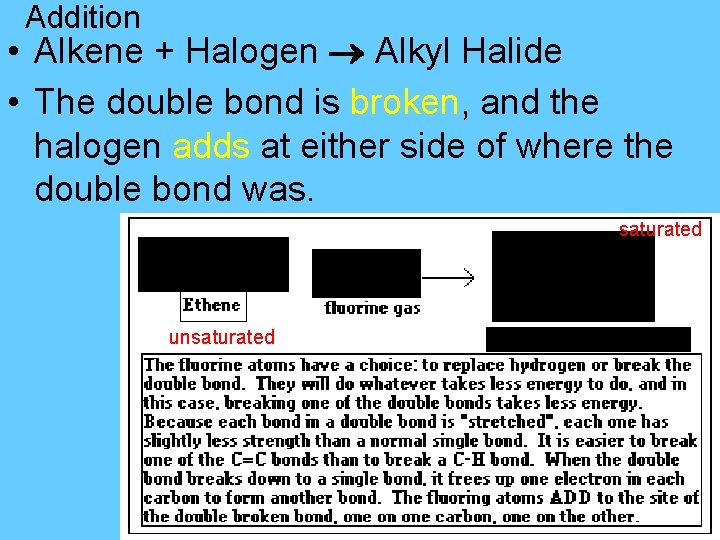
Addition –adding one or more groups at a double or triple bond. • Double bond is broken…becomes a single bond. *only happens with alkenes & alkynes – double/triple bonds!!!!

Addition • Alkene + Halogen Alkyl Halide • The double bond is broken, and the halogen adds at either side of where the double bond was. saturated unsaturated

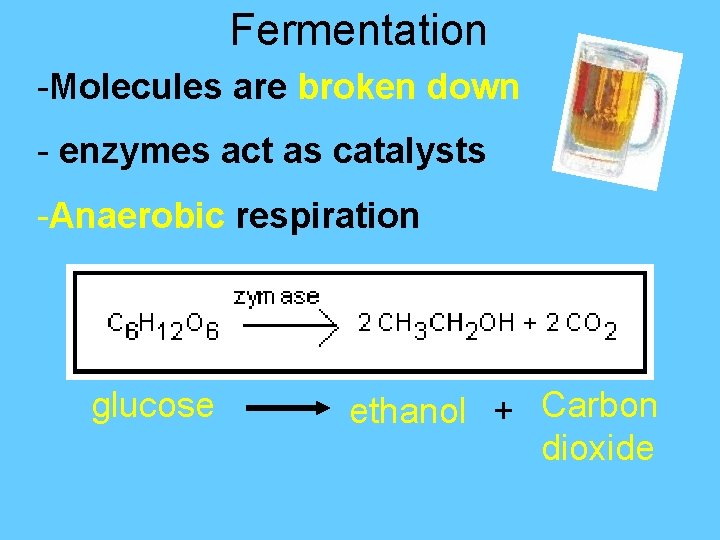
Fermentation -Molecules are broken down - enzymes act as catalysts -Anaerobic respiration glucose ethanol + Carbon dioxide

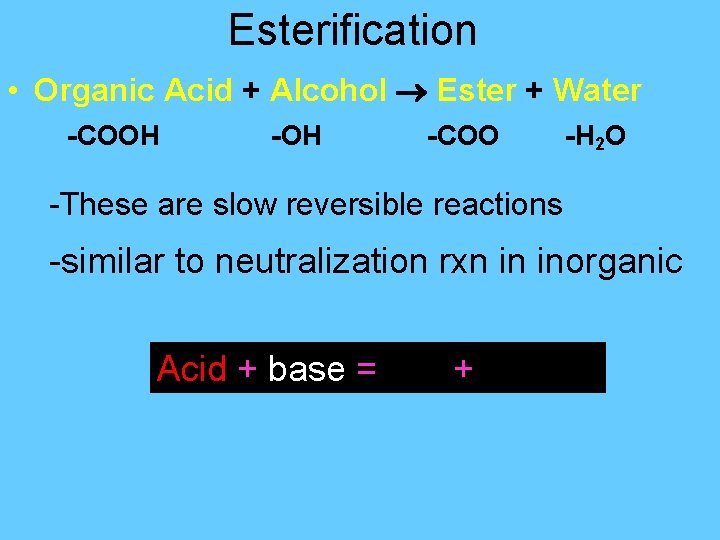
Esterification • Organic Acid + Alcohol Ester + Water -COOH -COO -H 2 O -These are slow reversible reactions -similar to neutralization rxn in inorganic Acid + base = salt + water

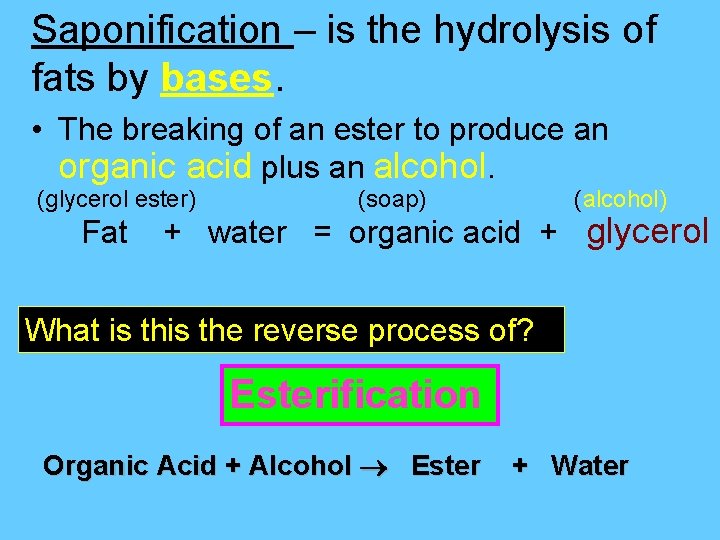
Saponification – is the hydrolysis of fats by bases. • The breaking of an ester to produce an organic acid plus an alcohol. (glycerol ester) Fat (soap) (alcohol) + water = organic acid + glycerol What is the reverse process of? Esterification Organic Acid + Alcohol Ester + Water

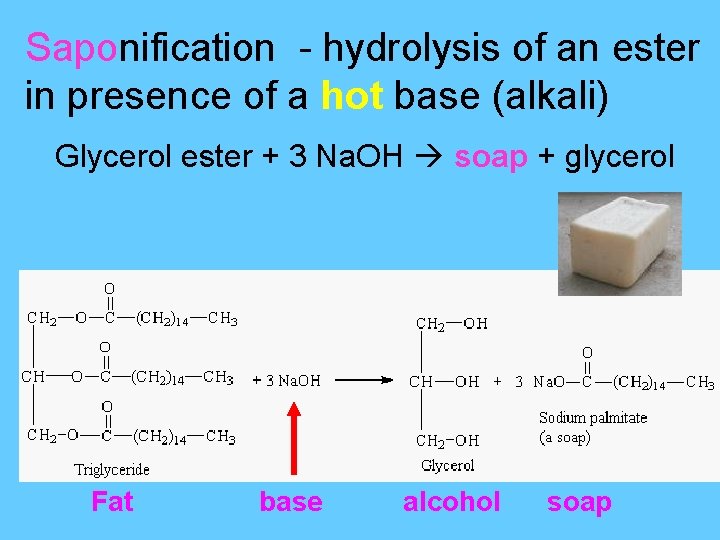
Saponification - hydrolysis of an ester in presence of a hot base (alkali) Glycerol ester + 3 Na. OH soap + glycerol Fat base alcohol soap

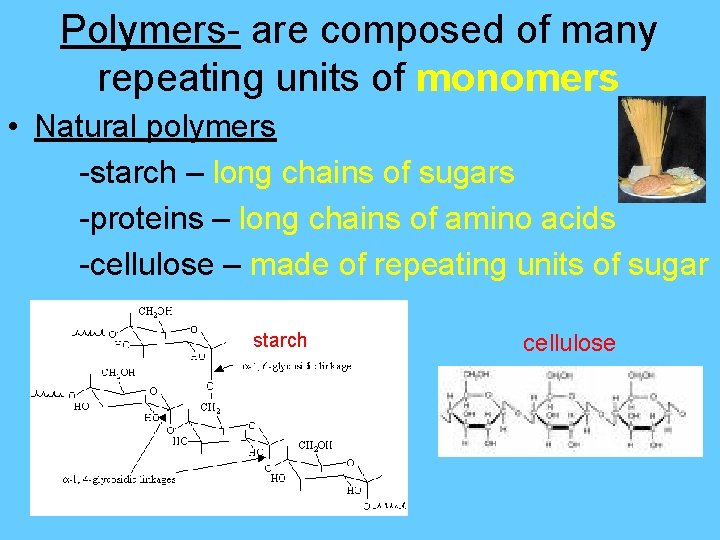
Polymers- are composed of many repeating units of monomers • Natural polymers -starch – long chains of sugars -proteins – long chains of amino acids -cellulose – made of repeating units of sugar starch cellulose

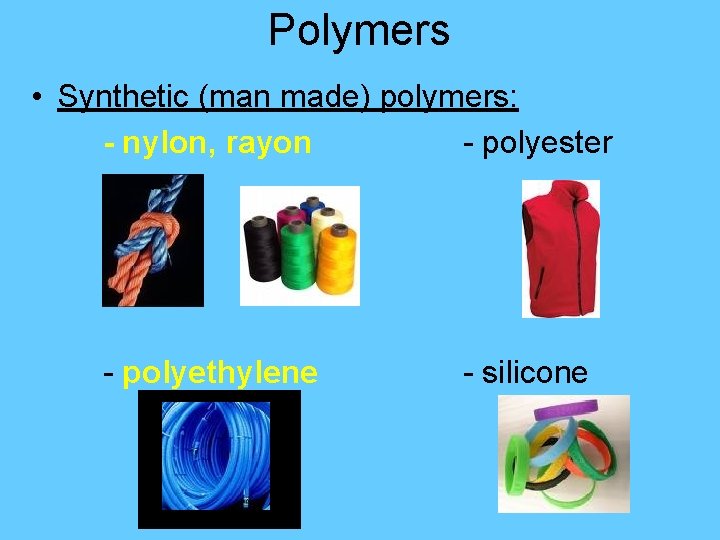
Polymers • Synthetic (man made) polymers: - nylon, rayon - polyester - polyethylene - silicone

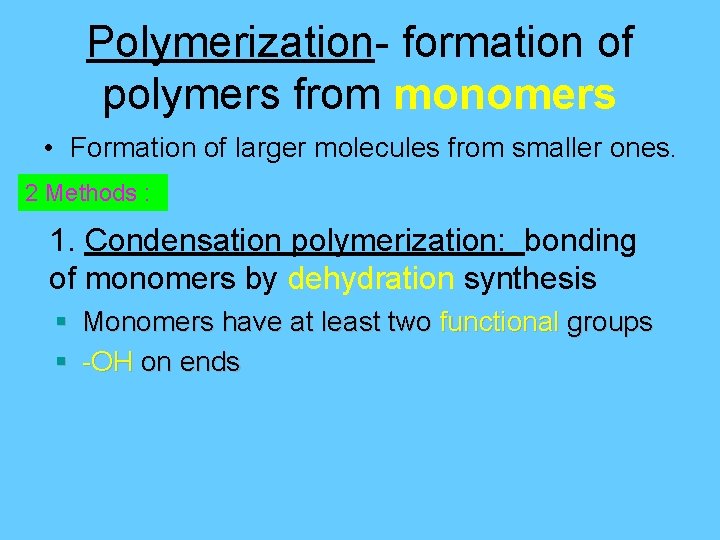
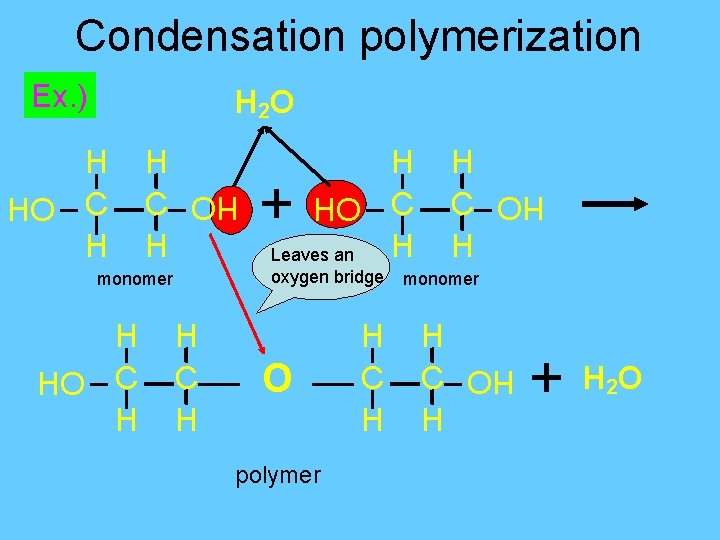
Polymerization- formation of polymers from monomers • Formation of larger molecules from smaller ones. 2 Methods : 1. Condensation polymerization: bonding of monomers by dehydration synthesis § Monomers have at least two functional groups § -OH on ends

Condensation polymerization Ex. ) H 2 O O H C H + oxygen bridge monomer H HO C H Leaves an polymer H C H monomer H C H HO HO H C H HO C H + H 2 O

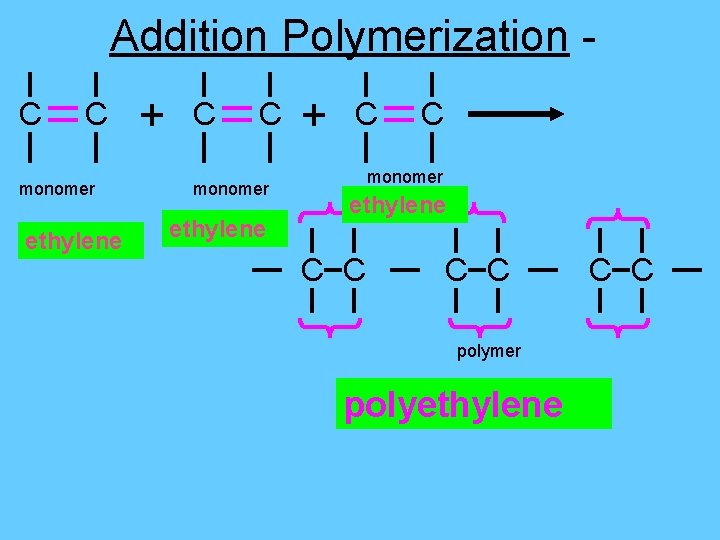
Addition Polymerization C C monomer ethylene + C C monomer ethylene C C polymer polyethylene C C

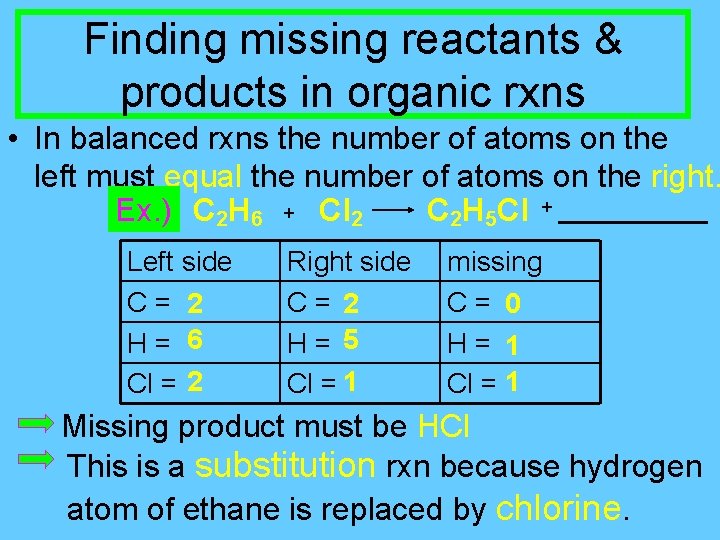
Finding missing reactants & products in organic rxns • In balanced rxns the number of atoms on the left must equal the number of atoms on the right. Ex. ) C 2 H 6 + Cl 2 C 2 H 5 Cl + Left side C= 2 H= 6 Cl = 2 Right side C= 2 H= 5 Cl = 1 missing C= 0 H= 1 Cl = 1 Missing product must be HCl This is a substitution rxn because hydrogen atom of ethane is replaced by chlorine.

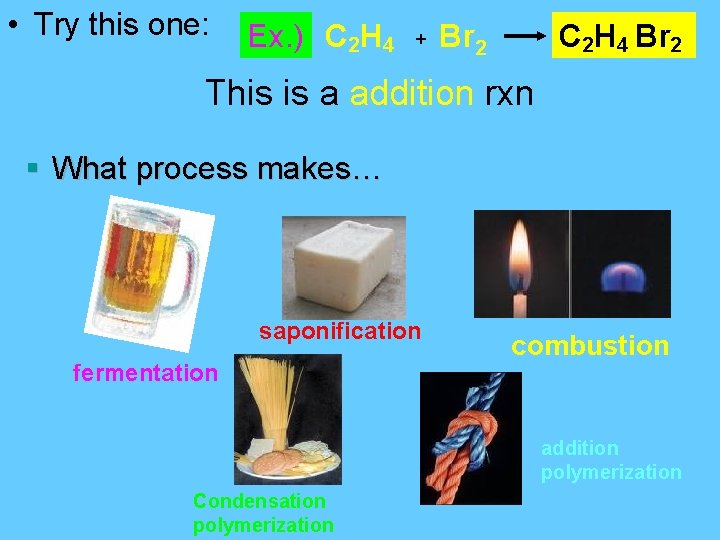
• Try this one: Ex. ) C 2 H 4 + Br 2 C 2 H 4 Br 2 This is a addition rxn § What process makes… saponification fermentation combustion addition polymerization Condensation polymerization