Organic Chemistry What is Organic Chemistry Study of








- Slides: 13

Organic Chemistry

What is Organic Chemistry? § Study of carbon-based compounds

What are some examples of carbon-based compounds? § Fuel § Plastics § Drugs § Food § Explosives § Life

Fuel § Example: petroleum § Used as an energy source § Hydrocarbon: contains carbon and hydrogen § Reacts with oxygen to produce heat for energy and releases carbon dioxide

Plastics § Halocarbons § Contain a halogen, such as flourine covalently bonded to carbon § Other halocarbons: CFCs, DDT § Hazards: plastics do not biodegrade, they photodegrade, CFCs damage the ozone layer, DDT is harmful to animal populations, is a water pollutant, and is a carcinogen

Drugs § Most medicines are organic compounds § Most illegal drugs are organic compounds

Food § Carbohydrates, proteins, and lipids are all organic compounds § Examples: sugar, fruits, vegetables, etc § Cons: Americans throw away ¼ to ½ of food (26 millions tons/year), jams up landfills, and hugely contributes to greenhouse gas emissions (methane) § What you can do: freeze, can, donate food, start a compost pile

Life § Life on Earth depends on carbon § ALL life contains carbon

Bonding in Organic Compounds § Covalent bonds § 2 or more atoms SHARE valence electrons § Gain a full octet Valence electrons: electrons in outermost energy level Octet: atoms want 8 electrons (full outer shell) to be stable

Structures formed by carbon compounds § Carbon is unique because it can form: § Chains § Branched chains § Rings § single, double, and triple bonds Alkanes: all single bonds Alkenes: contains at least one double bond Alkynes: at least one triple bond present

Saturated vs. Unsaturated § Alkenes: saturated hydrocarbons/contain as many hydrogens as possible § Alkenes and Alkynes: unsaturated hydrocarbons/contain less carbons because double or triple bonds are present

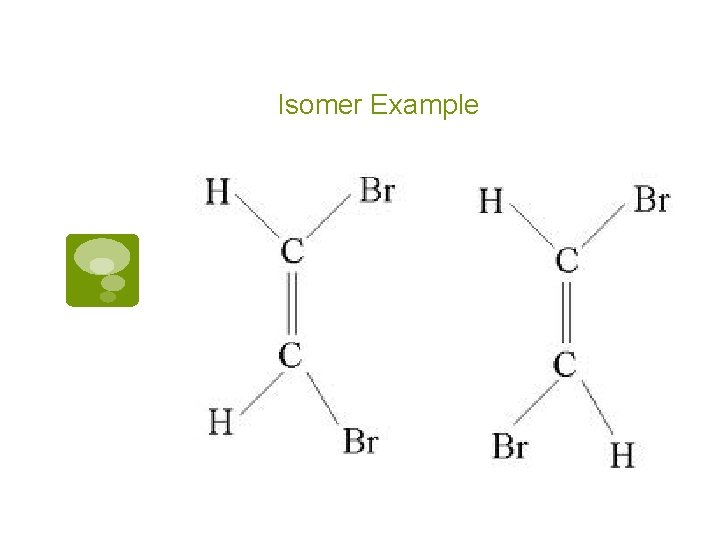
Isomers § Carbon structures with identifcal chemical formulas (made of the same stuff) § Different structures (shaped differently) § Different chemical and physical reactivities

Isomer Example