Organic Chemistry Unit 5 Organic vs Inorganic History














































- Slides: 144

Organic Chemistry Unit 5

Organic vs Inorganic - History • In the early 19 th century, Swedish chemist Jöns Jacob Berzelius classified compounds into two categories: 1. those obtained from organisms (organic) 2. those obtained from mineral sources (inorganic) • This all changed when Friedrich Wöhler produced an organic compound (urea) from two inorganic compounds.

Organic vs Inorganic - Contemporary • Simply put organic compounds are compounds containing carbon. • However not all carbon molecules are organic. • Carbon oxides, carbonates, carbides and cyanides are considered inorganic. • Organic compounds are covalent compounds and can include other non metals as well.

Organic vs Inorganic - Reason There are millions of organic compounds because: 1. 2. 3. 4. carbon bonds easily to itself carbon bonds easily to H, N, O, and S carbon compounds can form chains or rings carbon can form double or triple bonds

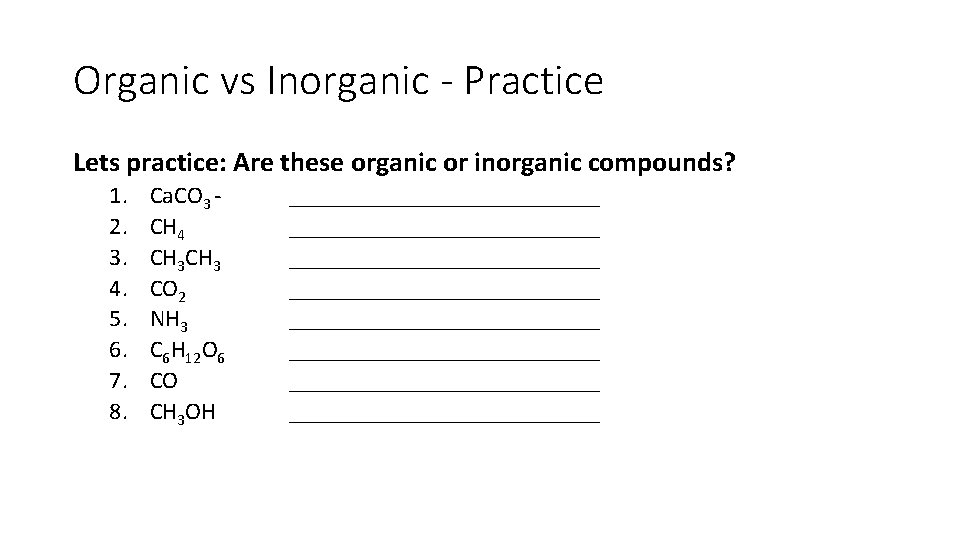
Organic vs Inorganic - Practice Lets practice: Are these organic or inorganic compounds? 1. 2. 3. 4. 5. 6. 7. 8. Ca. CO 3 CH 4 CH 3 CO 2 NH 3 C 6 H 12 O 6 CO CH 3 OH __________________________ __________________________

Organic vs Inorganic – Practice Answers Lets practice: Are these organic or inorganic compounds? 1. 2. 3. 4. 5. 6. 7. 8. Ca. CO 3 CH 4 CH 3 CO 2 NH 3 C 6 H 12 O 6 CO CH 3 OH Inorganic Organic Inorganic Organic

Organic Compounds - Basics All of organic chemistry revolves around carbon and hydrogen bonds. Reminder 1. 2. 3. 4. How many valence electrons does carbon have? Would carbon and hydrogen make covalent or ionic bonds? How many electrons can carbon share? How many bonds does a single carbon want to make?

Organic Compounds - Basics All of organic chemistry revolves around carbon and hydrogen bonds. Reminder 1. 2. 3. 4. How many valence electrons does carbon have? Would carbon and hydrogen make covalent or ionic bonds? How many electrons can carbon share? How many bonds does a single carbon want to make? 4 Covalent 4 4

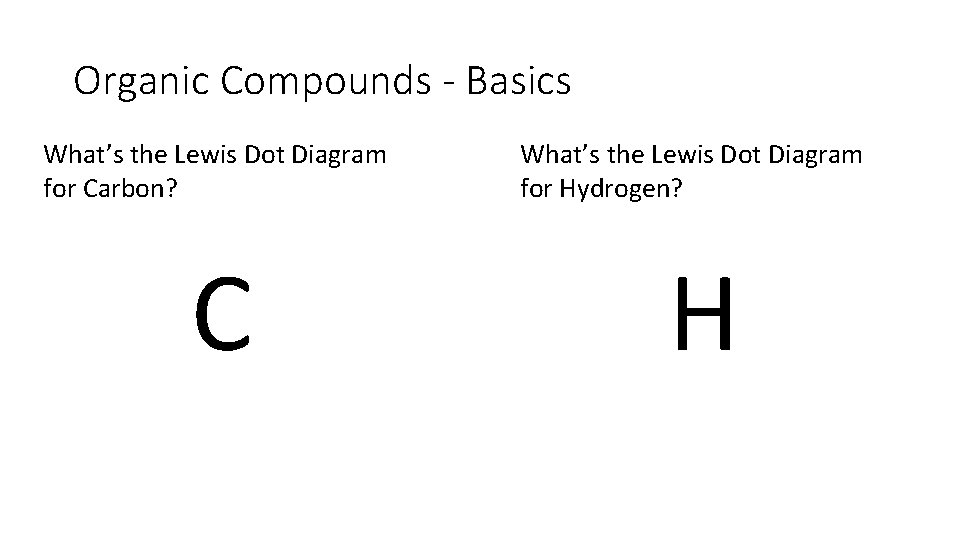
Organic Compounds - Basics What’s the Lewis Dot Diagram for Carbon? C What’s the Lewis Dot Diagram for Hydrogen? H

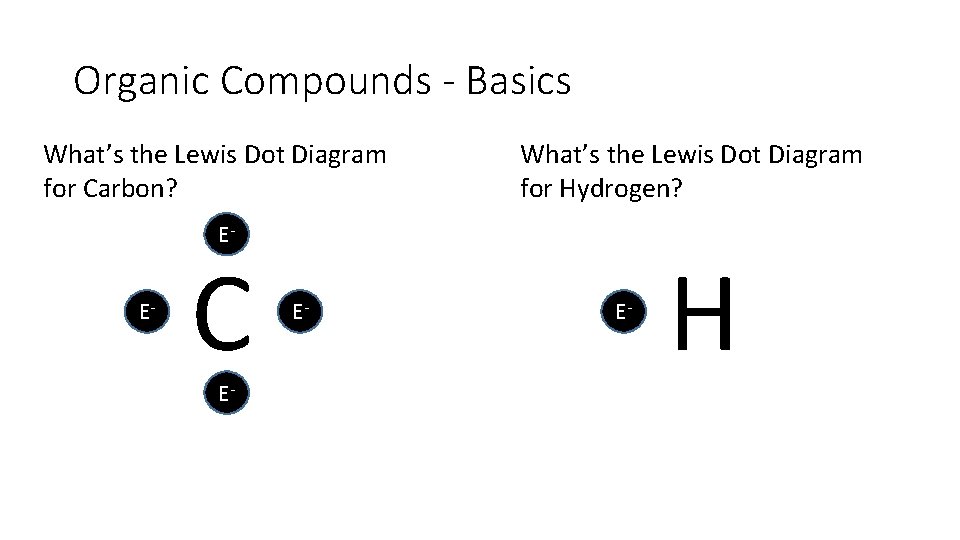
Organic Compounds - Basics What’s the Lewis Dot Diagram for Carbon? What’s the Lewis Dot Diagram for Hydrogen? E E C E E E H

Organic Compounds - Basics How many bonds will the following atoms want to make? (how many electrons can they share) 1. 2. 3. 4. 5. Carbon Hydrogen Oxygen Nitrogen Halides

Organic Compounds - Basics How many bonds will the following atoms want to make? (how many electrons can they share) 1. 2. 3. 4. 5. Carbon Hydrogen Oxygen Nitrogen Halides 4 1 2 3 1

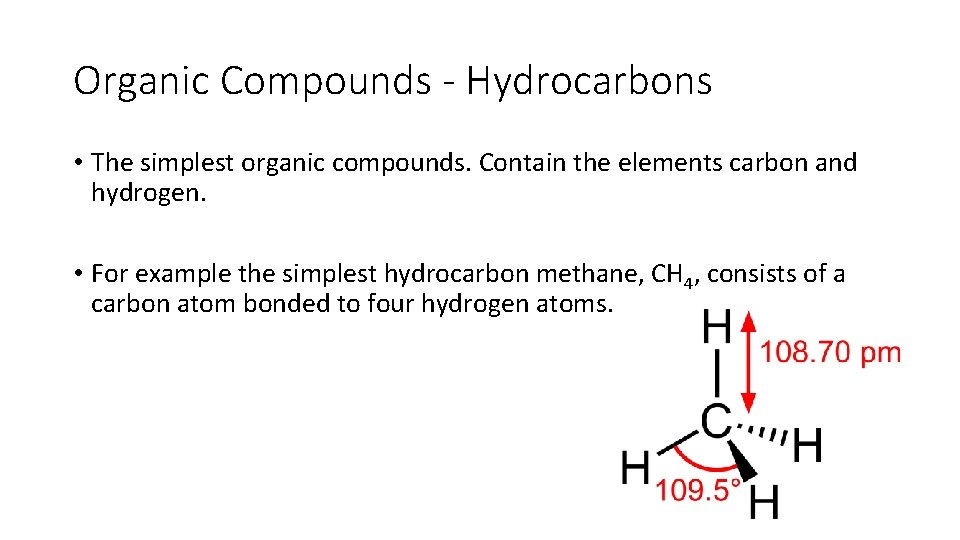
Organic Compounds - Hydrocarbons • The simplest organic compounds. Contain the elements carbon and hydrogen. • For example the simplest hydrocarbon methane, CH 4, consists of a carbon atom bonded to four hydrogen atoms.

Sources of Hydrocarbons There are three major sources of hydrocarbons: 1. Living organisms – you, and all living things produce organic compounds as a result of being alive. Many like enzymes and proteins are incredibly complex 2. Fossil Fuels – Oil, coal and the rest are made from the remains of living things and over the millennia have been distilled into simpler forms. This is the starting point for most organic chemistry. 3. Synthesized – Inorganic materials can be synthesized into organic but this is generally expensive.

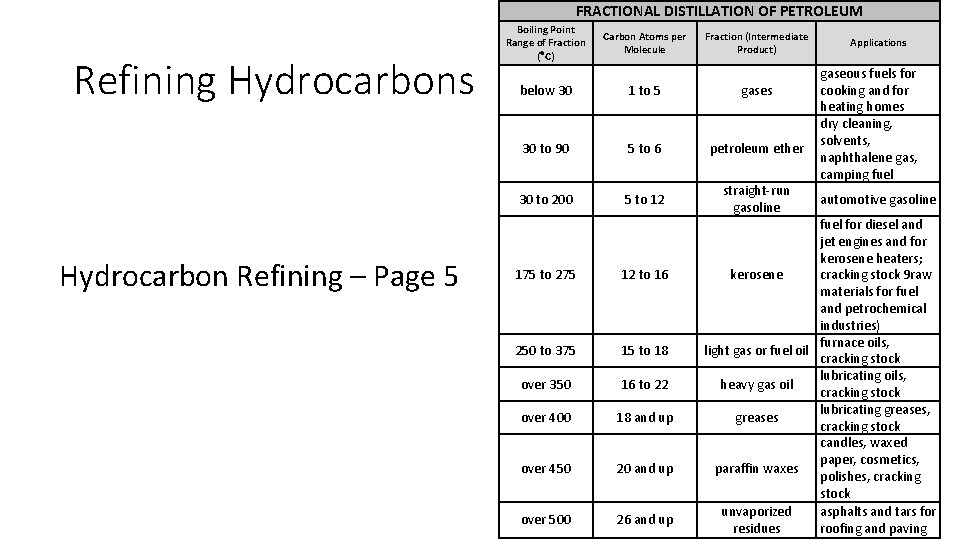
FRACTIONAL DISTILLATION OF PETROLEUM Refining Hydrocarbons Hydrocarbon Refining – Page 5 Boiling Point Range of Fraction ( C) Carbon Atoms per Molecule Fraction (Intermediate Product) below 30 1 to 5 gases 30 to 90 5 to 6 petroleum ether 30 to 200 5 to 12 straight run gasoline 175 to 275 12 to 16 250 to 375 15 to 18 over 350 16 to 22 over 400 18 and up over 450 20 and up over 500 26 and up Applications gaseous fuels for cooking and for heating homes dry cleaning, solvents, naphthalene gas, camping fuel automotive gasoline fuel for diesel and jet engines and for kerosene heaters; cracking stock 9 raw kerosene materials for fuel and petrochemical industries) furnace oils, light gas or fuel oil cracking stock lubricating oils, heavy gas oil cracking stock lubricating greases, greases cracking stock candles, waxed paper, cosmetics, paraffin waxes polishes, cracking stock unvaporized asphalts and tars for residues roofing and paving

Hydrocarbon Structure

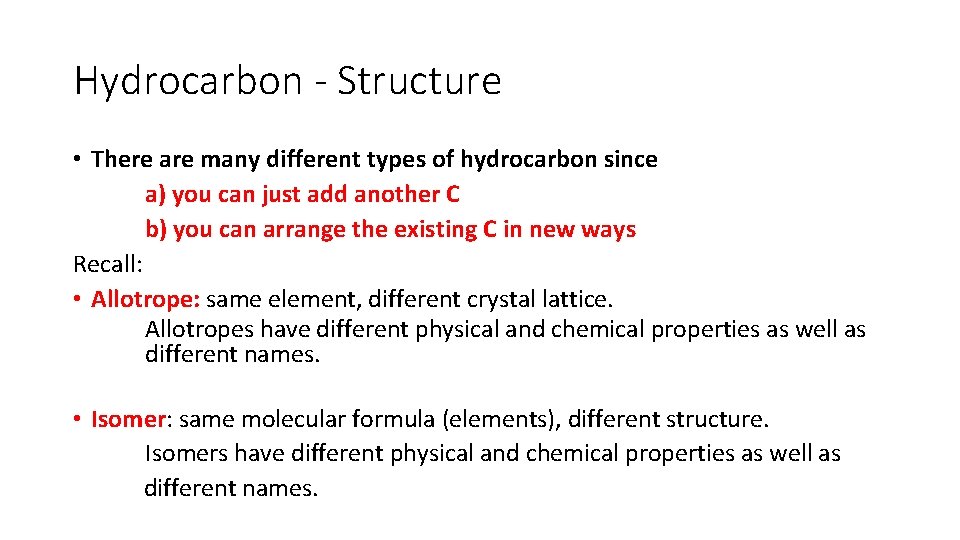
Hydrocarbon - Structure • There are many different types of hydrocarbon since a) you can just add another C b) you can arrange the existing C in new ways Recall: • Allotrope: same element, different crystal lattice. Allotropes have different physical and chemical properties as well as different names. • Isomer: same molecular formula (elements), different structure. Isomers have different physical and chemical properties as well as different names.

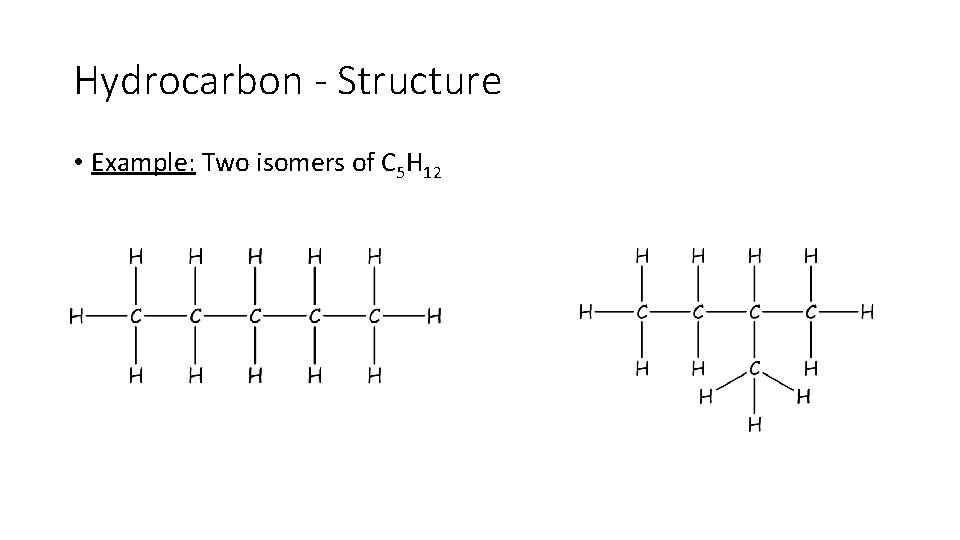
Hydrocarbon - Structure • Example: Two isomers of C 5 H 12

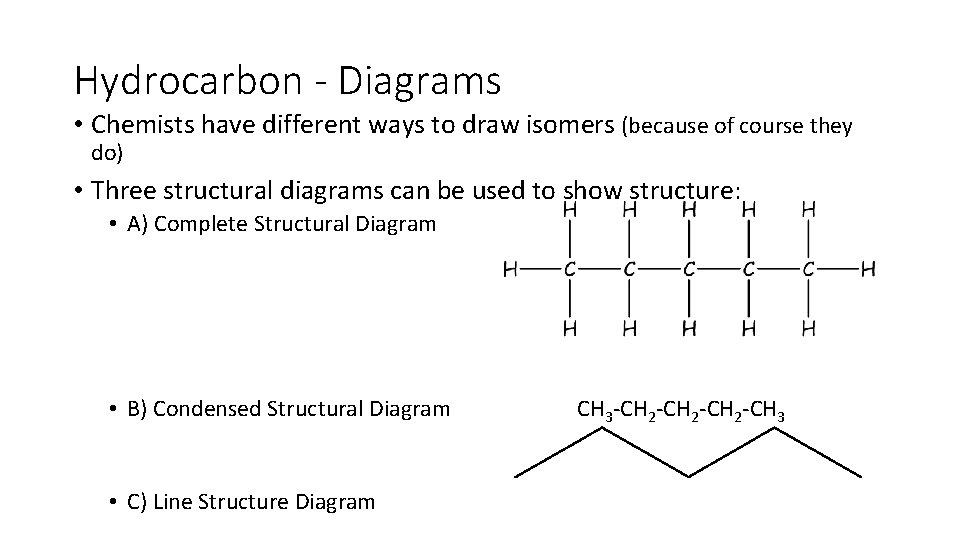
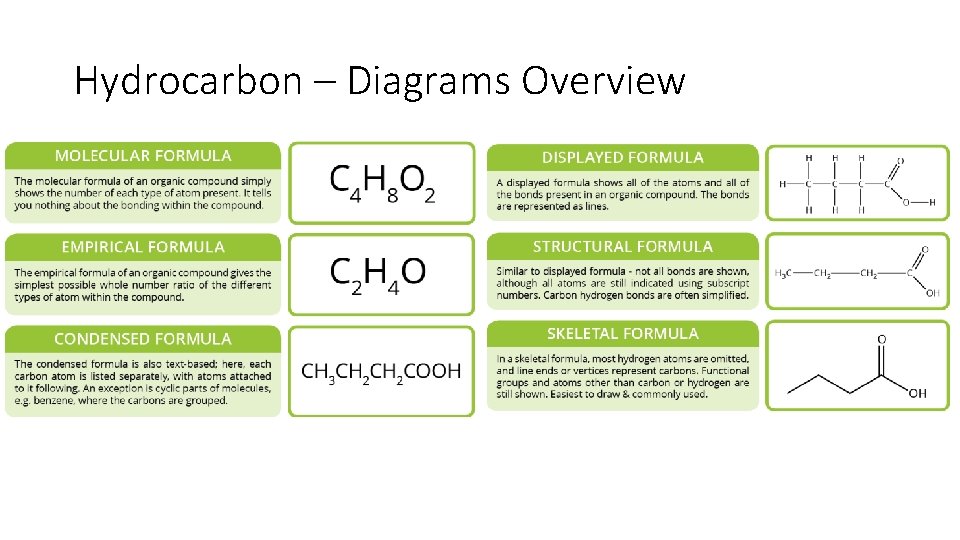
Hydrocarbon - Diagrams • Chemists have different ways to draw isomers (because of course they do) • Three structural diagrams can be used to show structure: • A) Complete Structural Diagram • B) Condensed Structural Diagram • C) Line Structure Diagram CH 3 CH 2 CH 3

Hydrocarbon – Diagrams Overview

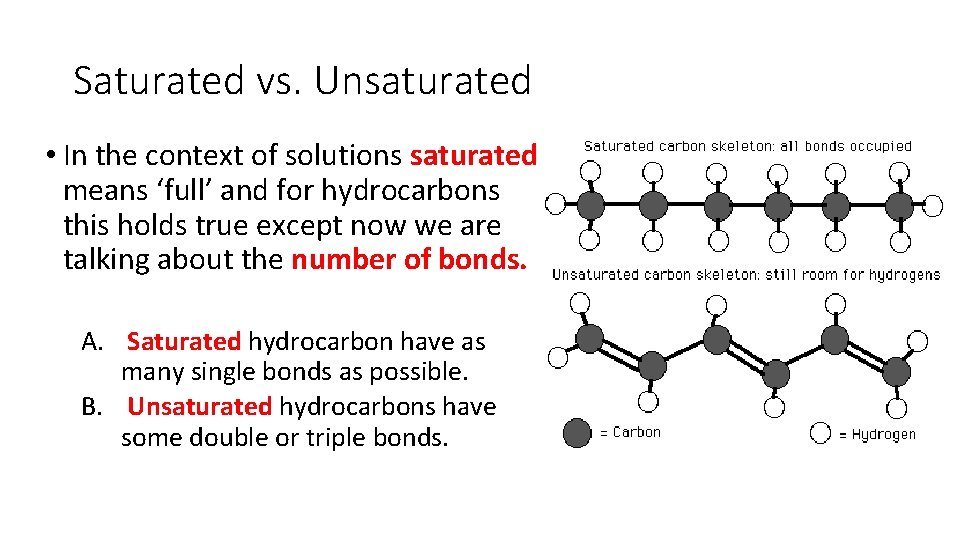
Saturated vs. Unsaturated • In the context of solutions saturated means ‘full’ and for hydrocarbons this holds true except now we are talking about the number of bonds. A. Saturated hydrocarbon have as many single bonds as possible. B. Unsaturated hydrocarbons have some double or triple bonds.

Hydrocarbon Naming

Alkanes • Alkanes are hydrocarbons that contain only single carbon bonds • Examples: Methane, Ethane, and Propane • general formula: Cn. H 2 n+2

Naming Straight Chain Alkanes • The current procedure for naming organic compounds is based on a set of rules formulated by the International Union of Pure and Applied Chemistry (IUPAC). • The rules involve choosing a main or parent chain and then using a series of: a) prefixes (beginning of name) b) suffixes (or endings) to name the structure.

Naming Straight Chain Alkanes • For straight chain alkanes the prefix is determined by the number of carbons in the molecule. To the prefix the suffix “–ane” is added.

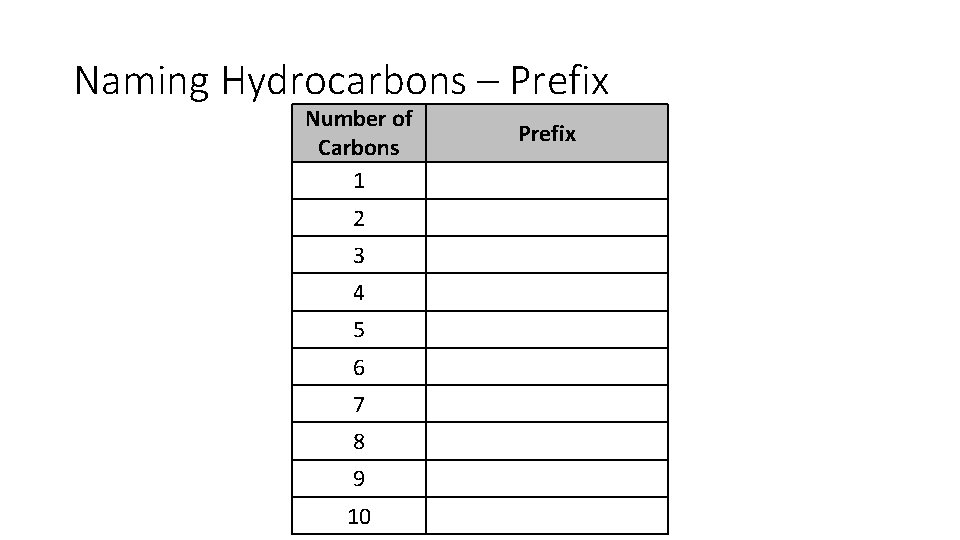
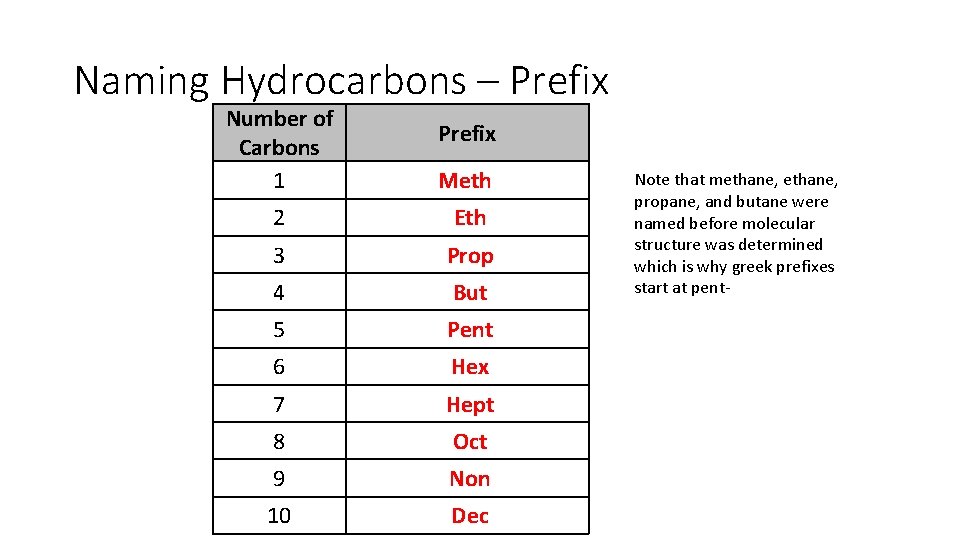
Naming Hydrocarbons – Prefix Number of Carbons 1 2 3 4 5 6 7 8 9 10 Prefix

Naming Hydrocarbons – Prefix Number of Carbons 1 Meth 2 Eth 3 Prop 4 But 5 Pent 6 Hex 7 Hept 8 Oct 9 Non 10 Dec Prefix Note that methane, propane, and butane were named before molecular structure was determined which is why greek prefixes start at pent

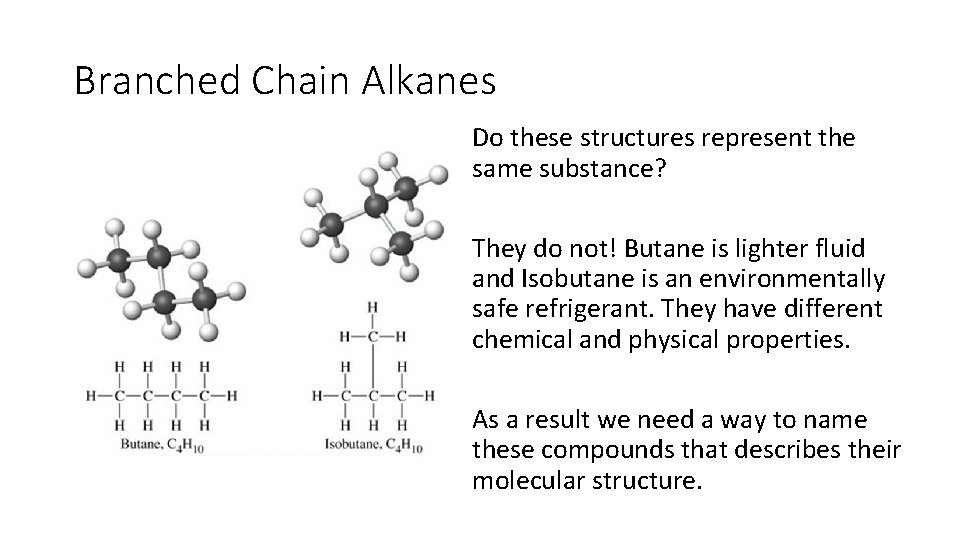
Branched Chain Alkanes Do these structures represent the same substance? They do not! Butane is lighter fluid and Isobutane is an environmentally safe refrigerant. They have different chemical and physical properties. As a result we need a way to name these compounds that describes their molecular structure.

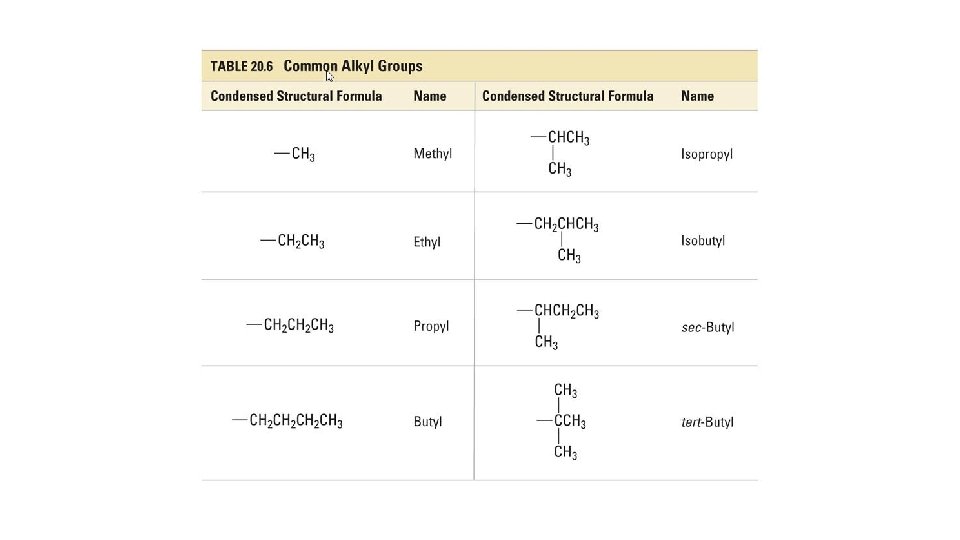
Alkyl Groups • When naming branched chain alkanes, the longest chain of carbon atoms is called the parent chain. • All side branches are called substituent groups because they appear to substitute for a hydrogen atom in the straight chain. • Basically, alkyl groups are alkanes that replace a hydrogen on the parent. • They are named with the suffix “-yl” instead of “-ane”


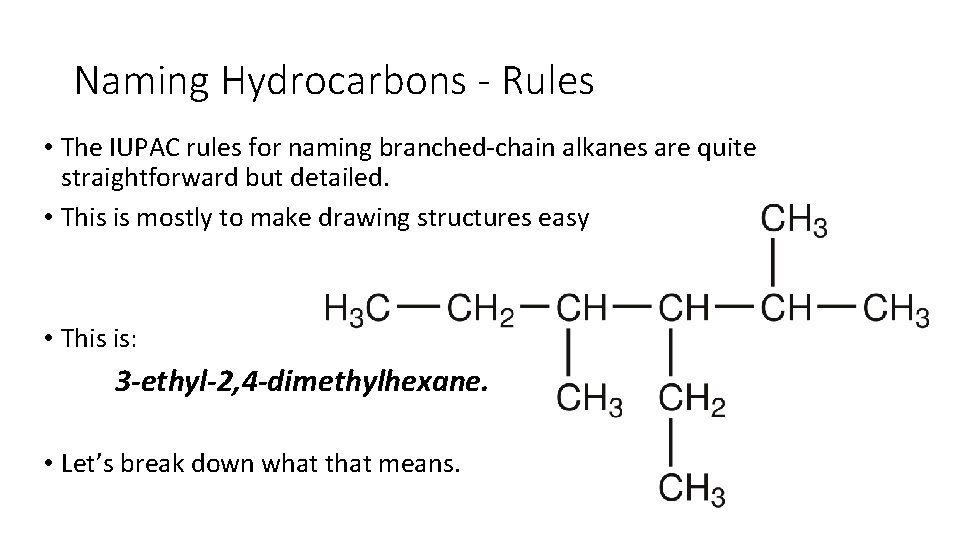
Naming Hydrocarbons - Rules • The IUPAC rules for naming branched chain alkanes are quite straightforward but detailed. • This is mostly to make drawing structures easy • This is: 3 -ethyl-2, 4 -dimethylhexane. • Let’s break down what that means.

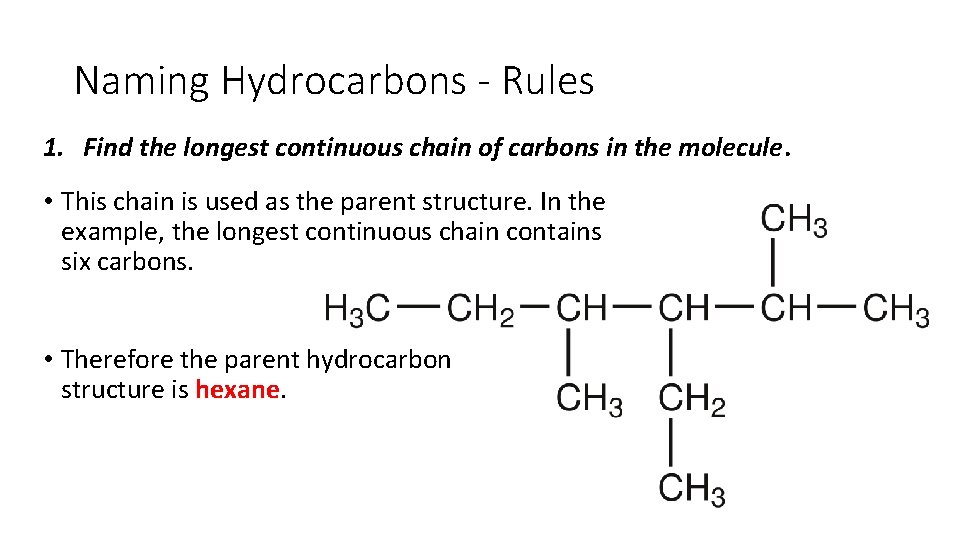
Naming Hydrocarbons - Rules 1. Find the longest continuous chain of carbons in the molecule. • This chain is used as the parent structure. In the example, the longest continuous chain contains six carbons. • Therefore the parent hydrocarbon structure is hexane.

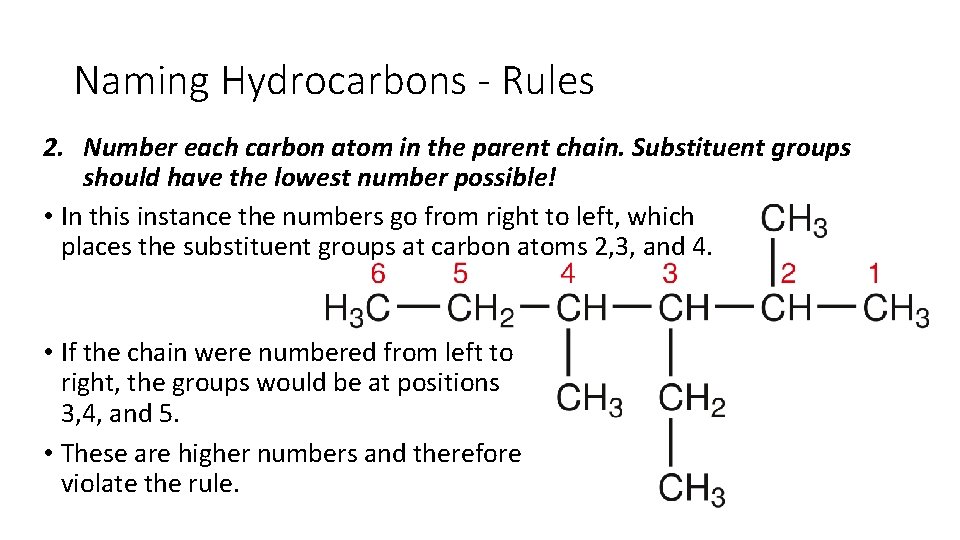
Naming Hydrocarbons - Rules 2. Number each carbon atom in the parent chain. Substituent groups should have the lowest number possible! • In this instance the numbers go from right to left, which places the substituent groups at carbon atoms 2, 3, and 4. • If the chain were numbered from left to right, the groups would be at positions 3, 4, and 5. • These are higher numbers and therefore violate the rule.

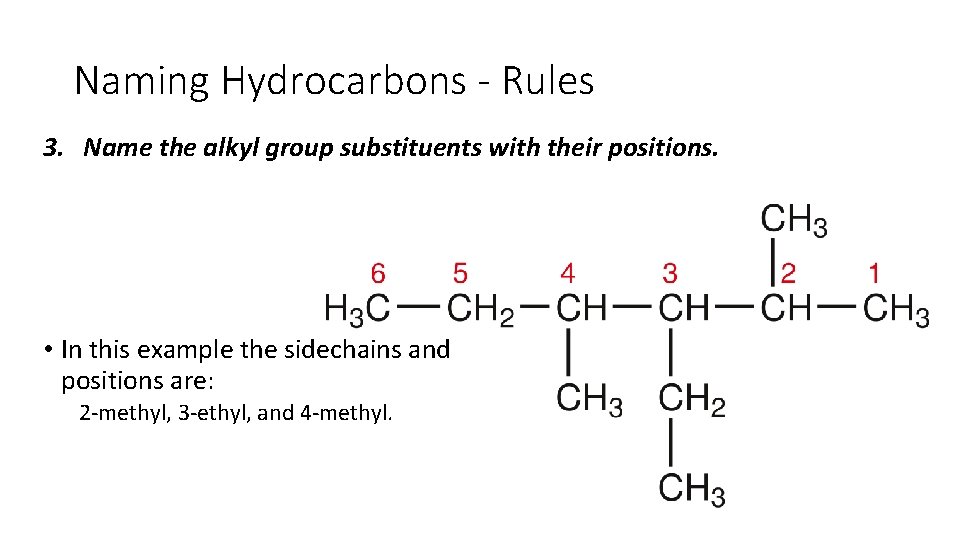
Naming Hydrocarbons - Rules 3. Name the alkyl group substituents with their positions. • In this example the sidechains and positions are: 2 methyl, 3 ethyl, and 4 methyl.

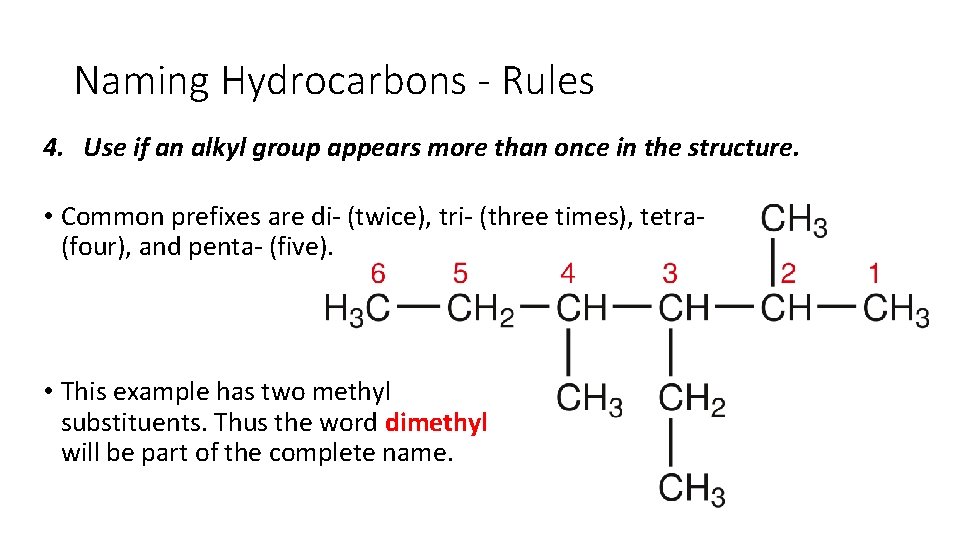
Naming Hydrocarbons - Rules 4. Use if an alkyl group appears more than once in the structure. • Common prefixes are di (twice), tri (three times), tetra (four), and penta (five). • This example has two methyl substituents. Thus the word dimethyl will be part of the complete name.

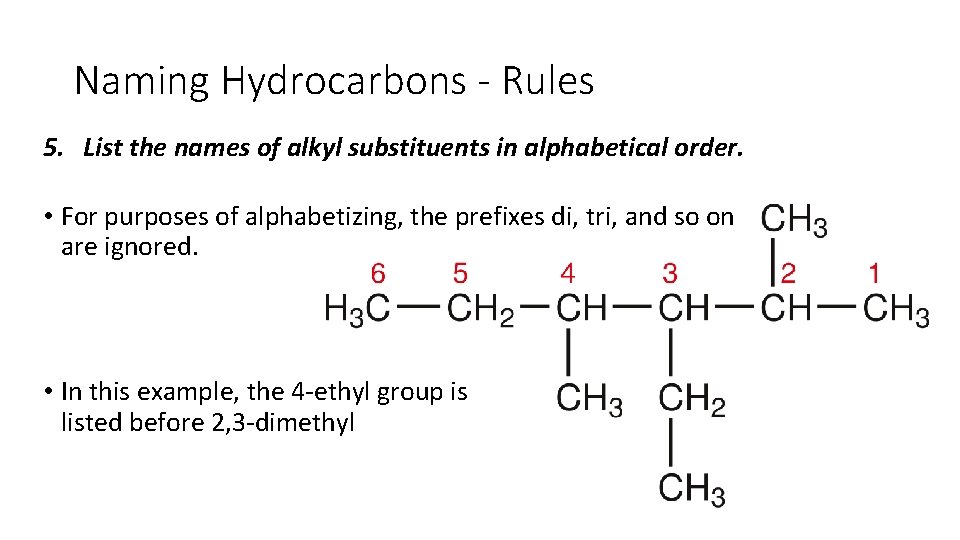
Naming Hydrocarbons - Rules 5. List the names of alkyl substituents in alphabetical order. • For purposes of alphabetizing, the prefixes di, tri, and so on are ignored. • In this example, the 4 ethyl group is listed before 2, 3 dimethyl

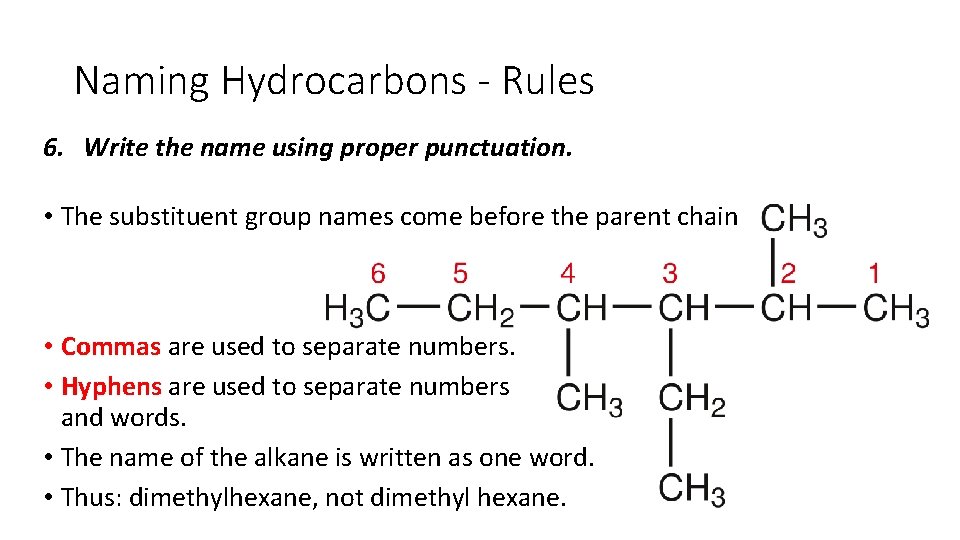
Naming Hydrocarbons - Rules 6. Write the name using proper punctuation. • The substituent group names come before the parent chain • Commas are used to separate numbers. • Hyphens are used to separate numbers and words. • The name of the alkane is written as one word. • Thus: dimethylhexane, not dimethyl hexane.

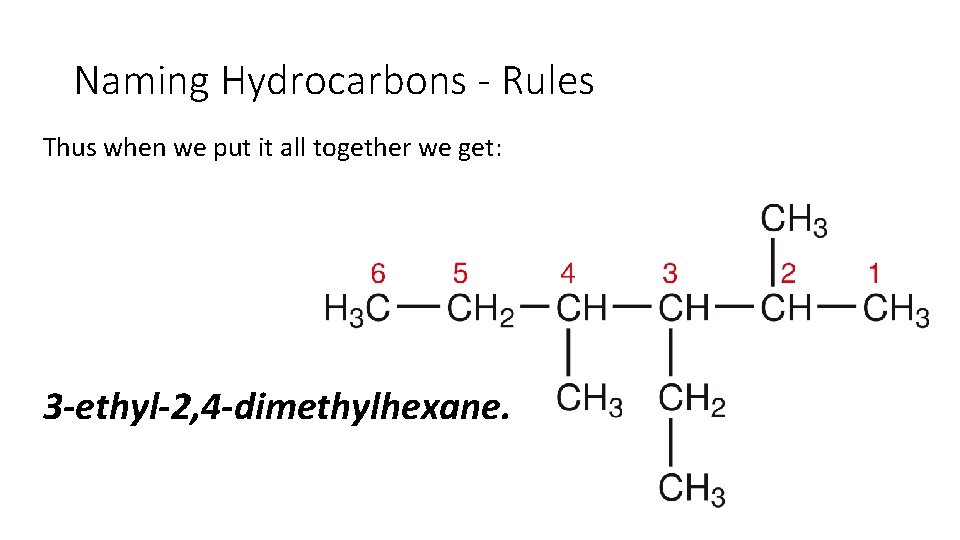
Naming Hydrocarbons - Rules Thus when we put it all together we get: 3 -ethyl-2, 4 -dimethylhexane.

Naming Hydrocarbons – Rules Summary 1) 2) 3) 4) 5) 6) Find the longest chain. Number the carbons, giving substituent groups lowest #’s possible. List the side chains by number and their composition. Use prefixes to mention doubles, triples, etc. List the side chains in alphabetical order. Use – and , marks to separate numbers and letters.

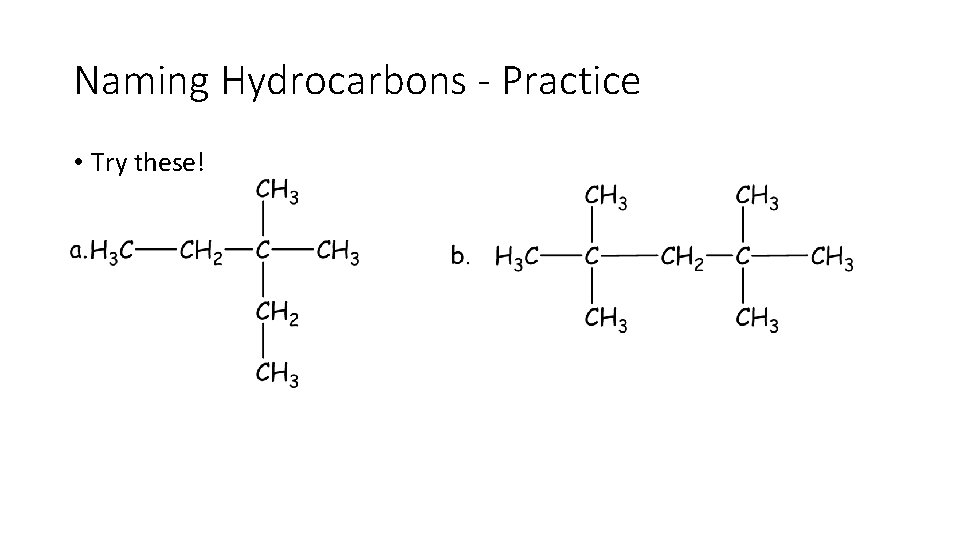
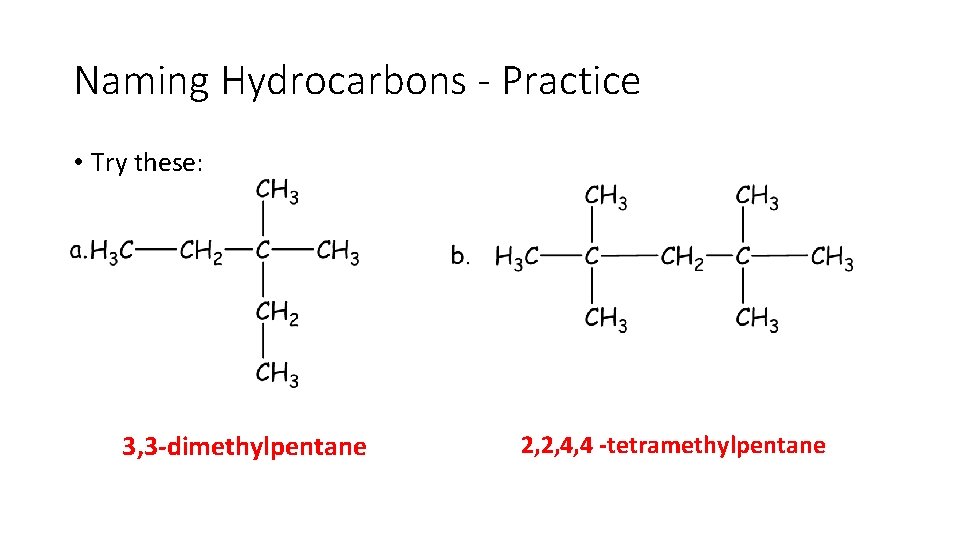
Naming Hydrocarbons - Practice • Try these!

Naming Hydrocarbons - Practice • Try these: 3, 3 -dimethylpentane 2, 2, 4, 4 -tetramethylpentane

Drawing Hydrocarbons With an alkane name and knowledge of the IUPAC rules, it is easy to reconstruct the structural formula. 1. Find the root word (ending in ane) in the hydrocarbon name. Then write the longest carbon chain to create the parent structure. 2. Number the carbons on this parent carbon chain. 3. Identify the substituent groups. Attach the substituents to the numbered parent chain at the proper position. 4. Add hydrogens as needed.

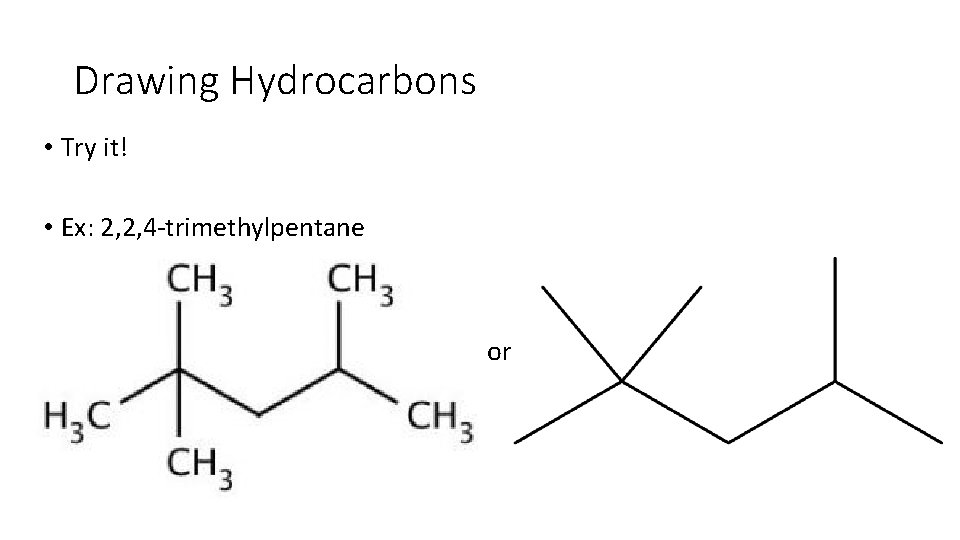
Drawing Hydrocarbons • Try it! • Ex: 2, 2, 4 trimethylpentane

Drawing Hydrocarbons • Try it! • Ex: 2, 2, 4 trimethylpentane or

Drawing Hydrocarbons - Exercise • Practice

Cycloalkanes • One of the reasons that such a variety of organic compounds exists is that carbon can form ring structures. An organic compound that contains a hydrocarbon ring is called a cyclic hydrocarbon. • To indicate that a hydrocarbon has a ring structure, the prefix “cyclo-“ is used with the hydrocarbon name. Cycloalkanes have only single carbon bonds. • ex. ) cyclohexane C 6 H 12

Naming Substituted Cycloalkanes • We will utilize the same rules as with naming straight chain alkanes, but with a few modifications. 1. The ring is considered to be the parent chain 2. The carbons are numbered such that the substituent groups have the lowest numbers possible. • Since it is a ring numbering can start anywhere on the ring, and can be numbered in clockwise or counterclockwise orientation. If there is only one substituent group, no number is needed. Ties are settled the same way.

Try This

Alkenes • Recall that alkanes are saturated hydrocarbons because they contain only single carbon bonds. Unsaturated hydrocarbons contain at least one double or triple bond. • Unsaturated hydrocarbons that contain one or more double bonds between carbon atoms in a chain are called alkenes. • When naming alkenes the suffix “-ene” is utilized • Alkene general formula: Cn. H 2 n + (2 2 x) x = number of double bonds

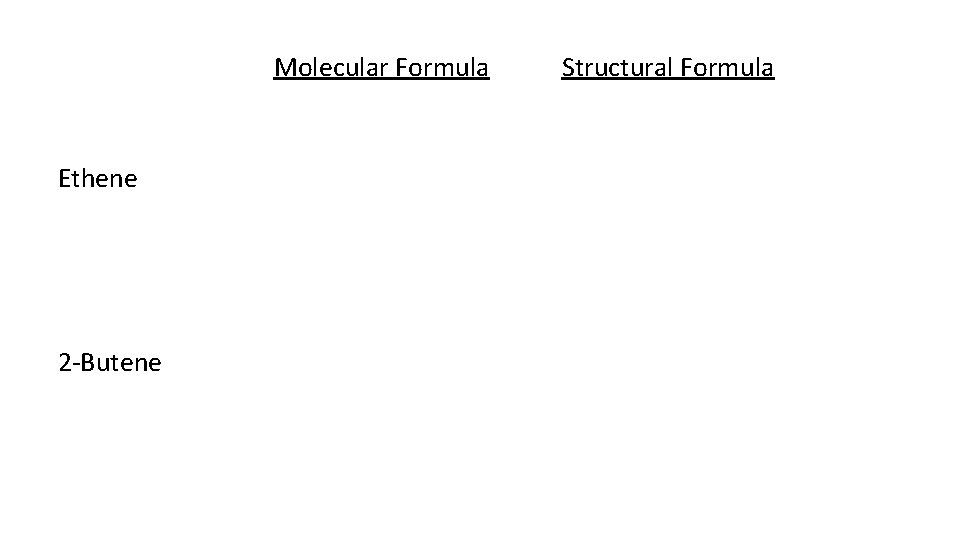
Molecular Formula Ethene 2 Butene Structural Formula

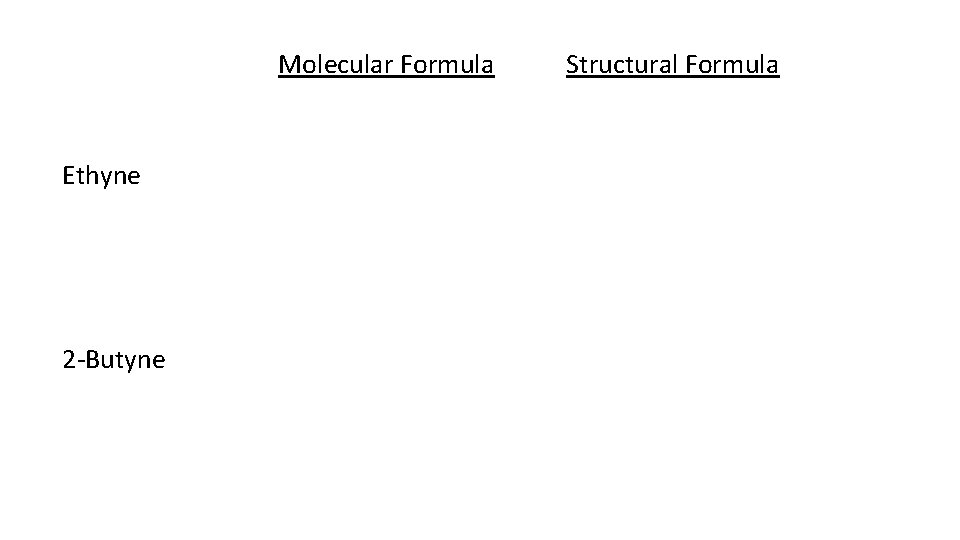
Alkynes • Unsaturated hydrocarbons that contain one or more triple bonds between carbon atoms in a chain are called alkynes. • When naming alkynes the suffix “-yne” is utilized • Alkyne general formula: Cn. H 2 n + (2 4 x) x = number of triple bonds

Molecular Formula Ethyne 2 Butyne Structural Formula

Naming Alkenes and Alkynes • The same naming rules for alkanes will be utilized with a few modifications 1. The parent chain must contain the double and/or triple bonds, even if not the longest chain! 2. The position of the double and/or triple bond determines how the parent chain is numbered. The double and/or triple bonds must be given the lowest numbers possible. • 3. If there is more than one double or triple bond the number is shown with a prefix (di-, tri-, and so on) added before the suffix

Hydrocarbon Isomers

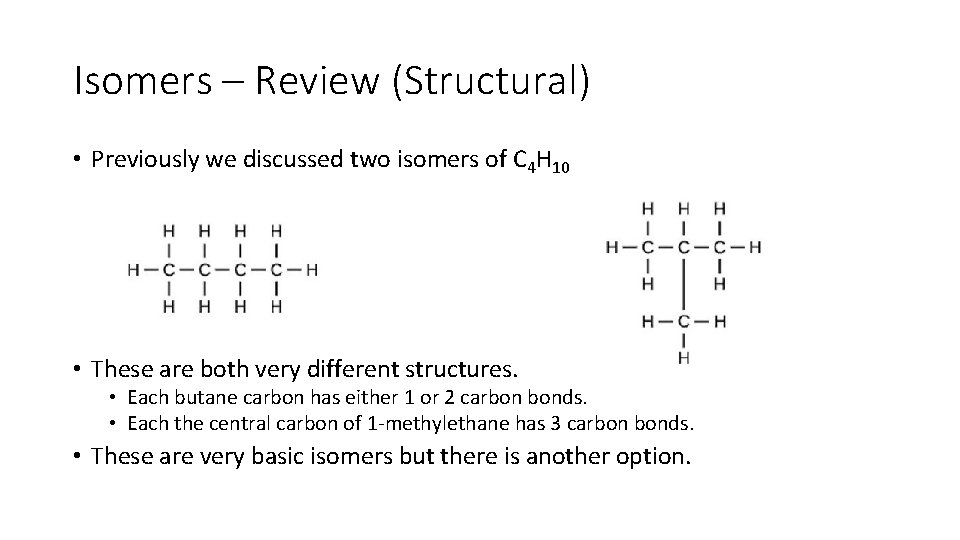
Isomers – Review (Structural) • Previously we discussed two isomers of C 4 H 10 • These are both very different structures. • Each butane carbon has either 1 or 2 carbon bonds. • Each the central carbon of 1 methylethane has 3 carbon bonds. • These are very basic isomers but there is another option.

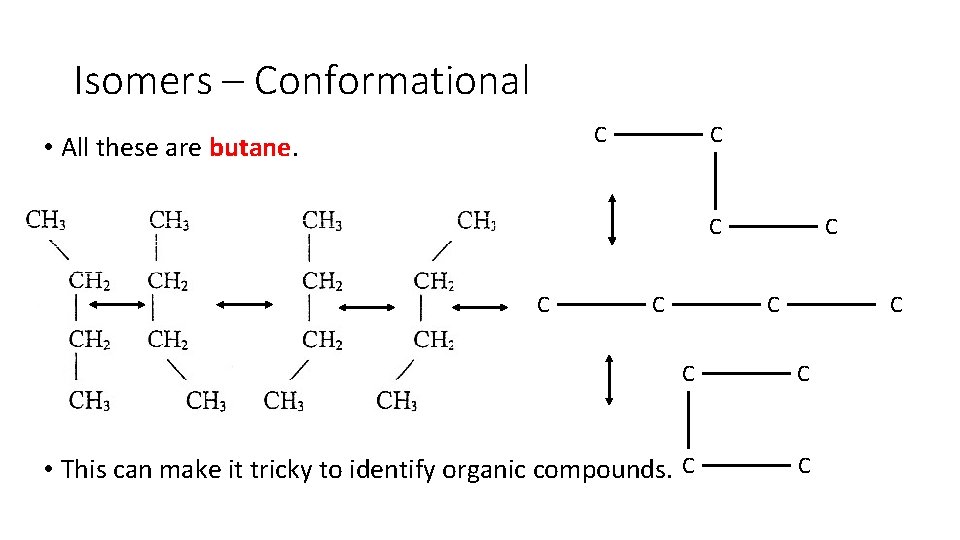
Isomers – Conformational • All particles are in constant motion, including those in molecules. • This means carbon atoms rotate about their bonds. • This rotating motion results in different conformations (shapes) but are actually all the same chemical. • Conformations convert rapidly from one to another, and are just different perspectives of the same structural formula. • What may look like a different structure, is actually the same.

Isomers – Conformational C • All these are butane. C C C C C • This can make it tricky to identify organic compounds. C C

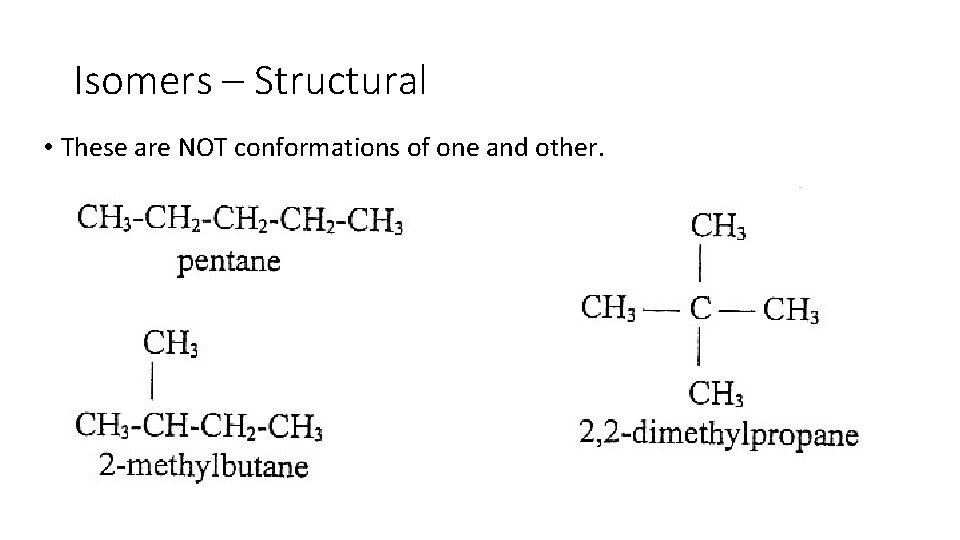
Isomers – Structural • These are NOT conformations of one and other.

Isomers – Structural • If you cannot rotate carbon to get the same structure then you have a structural isomer. • Structural isomers are compounds that have the same molecular formula (in this case C 5 H 12), but different structural arrangements. • Structural isomers have different properties from one another. • This helps distinguish one isomer from another, as they would both have the same molar mass.

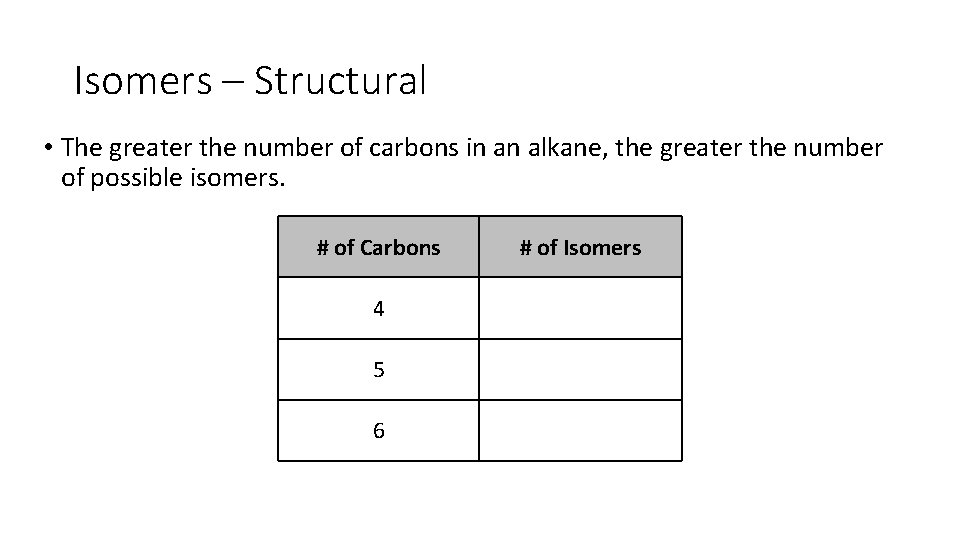
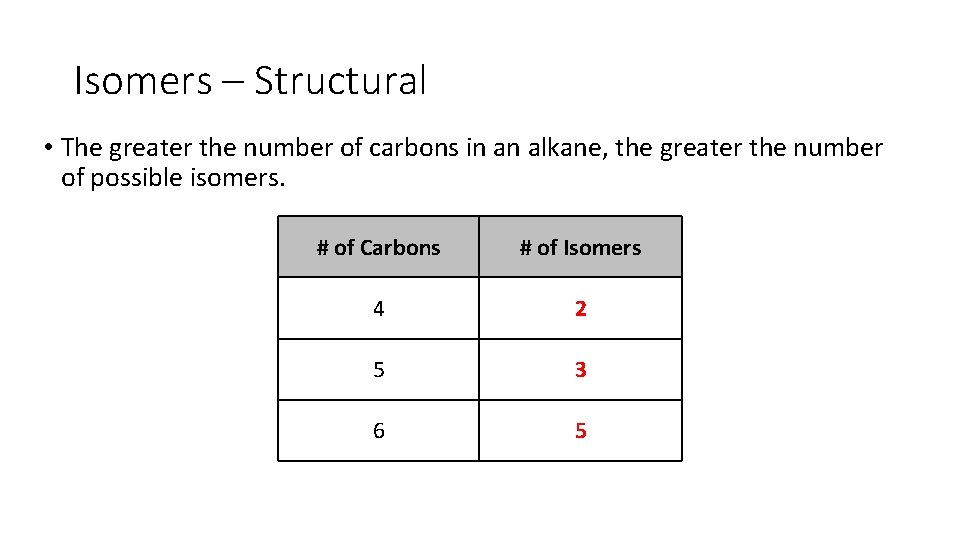
Isomers – Structural • The greater the number of carbons in an alkane, the greater the number of possible isomers. # of Carbons 4 5 6 # of Isomers

Isomers – Structural • The greater the number of carbons in an alkane, the greater the number of possible isomers. # of Carbons # of Isomers 4 2 5 3 6 5

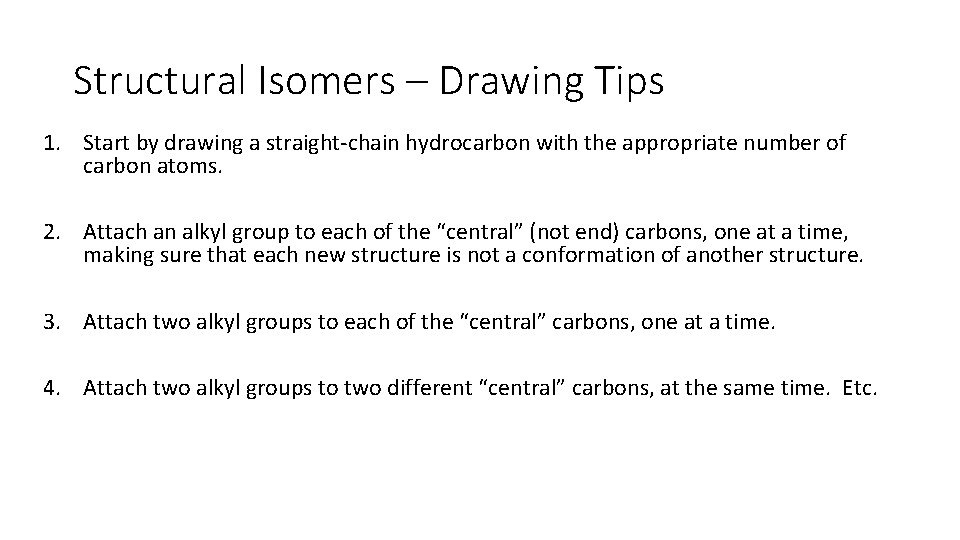
Structural Isomers – Drawing Tips 1. Start by drawing a straight chain hydrocarbon with the appropriate number of carbon atoms. 2. Attach an alkyl group to each of the “central” (not end) carbons, one at a time, making sure that each new structure is not a conformation of another structure. 3. Attach two alkyl groups to each of the “central” carbons, one at a time. 4. Attach two alkyl groups to two different “central” carbons, at the same time. Etc.

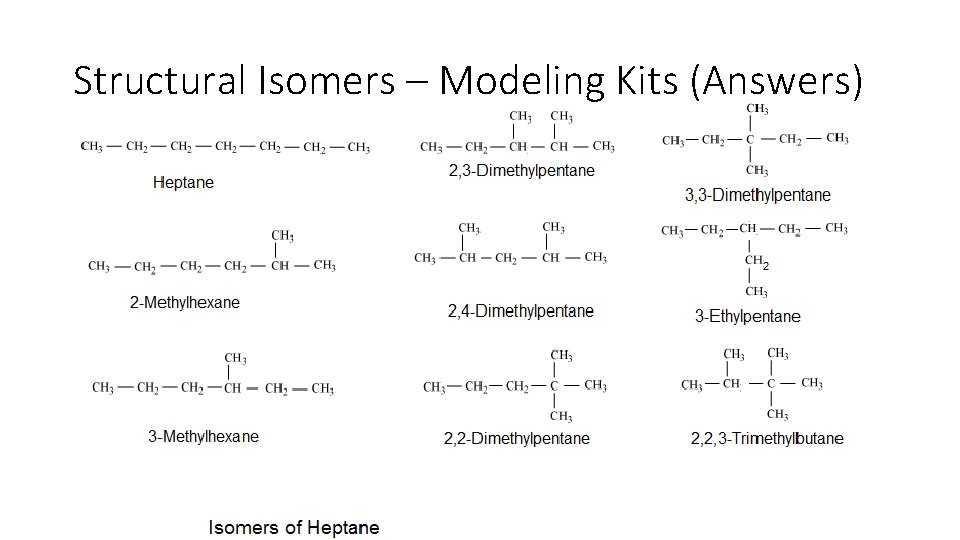
Structural Isomers – Modeling Kits • Heptane Isomers – Page 25 • Answers next slide!

Structural Isomers – Modeling Kits (Answers)

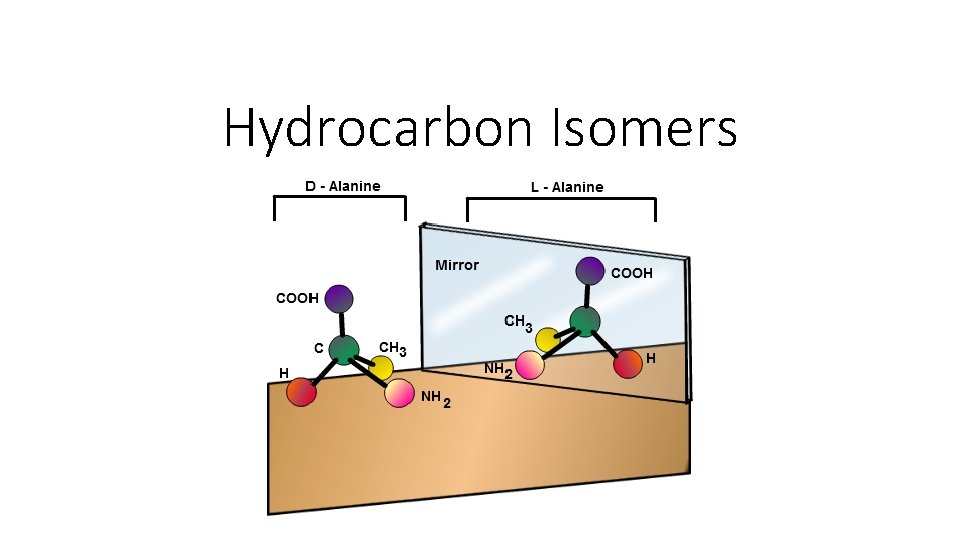
Isomers – Stereoisomers • We’ve had: 1. Conformational Isomers – same formula, rotated structure 2. Structural Isomers – Same formula, different structure So we now add: Stereoisomers. • Stereoisomers are molecules where the atoms are joined in the same order, but the positions of the atoms in space are different. • These can be either geometric isomers or optical isomers.

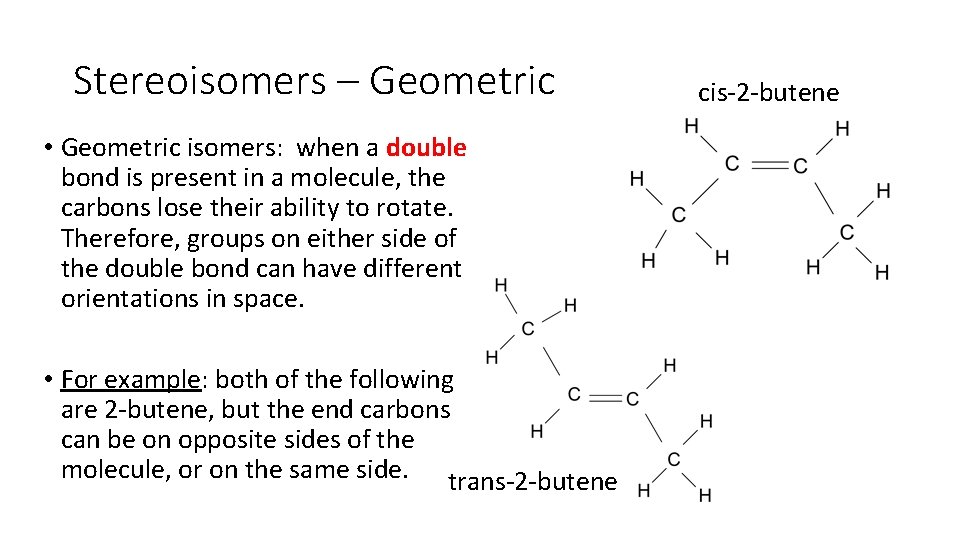
Stereoisomers – Geometric • Geometric isomers: when a double bond is present in a molecule, the carbons lose their ability to rotate. Therefore, groups on either side of the double bond can have different orientations in space. • For example: both of the following are 2 butene, but the end carbons can be on opposite sides of the molecule, or on the same side. trans 2 butene cis 2 butene

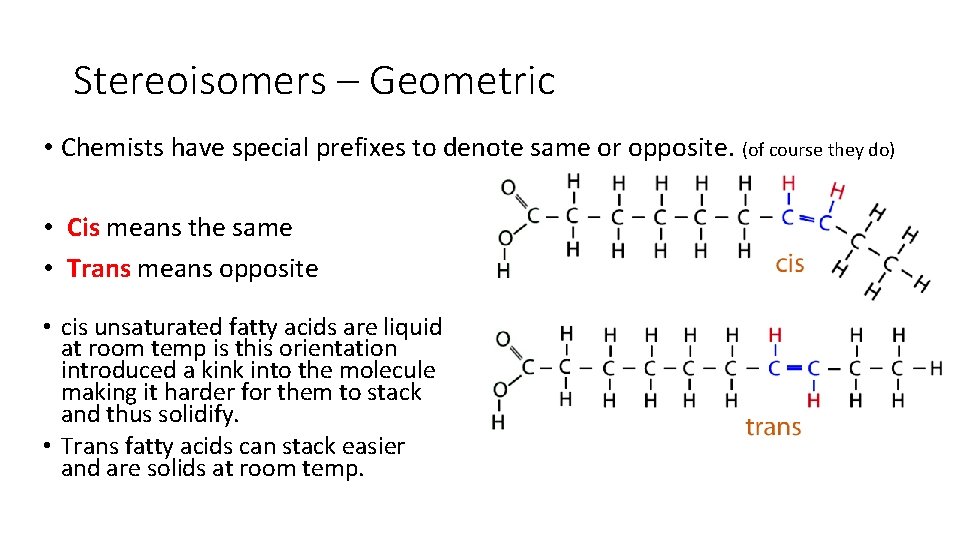
Stereoisomers – Geometric • Chemists have special prefixes to denote same or opposite. (of course they do) • Cis means the same • Trans means opposite • cis unsaturated fatty acids are liquid at room temp is this orientation introduced a kink into the molecule making it harder for them to stack and thus solidify. • Trans fatty acids can stack easier and are solids at room temp.

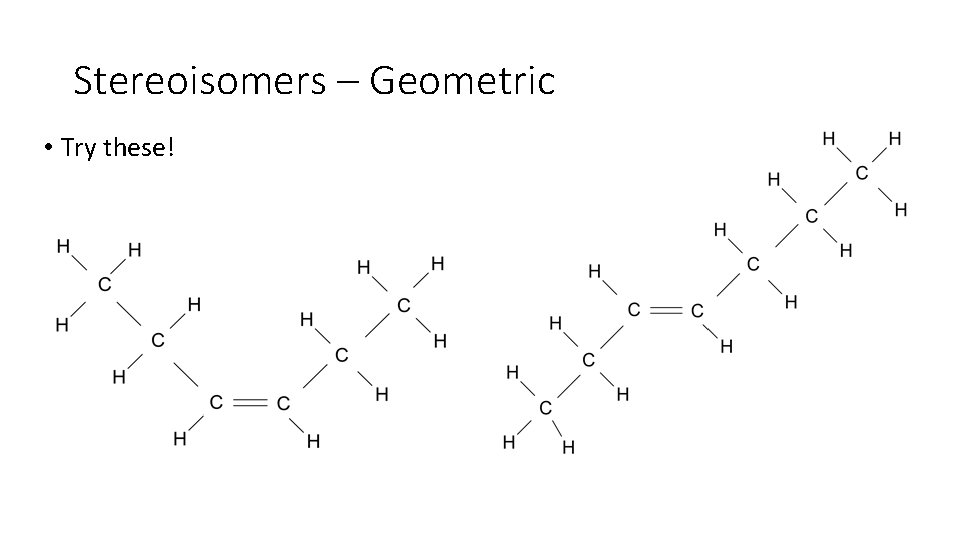
Stereoisomers – Geometric • Try these!

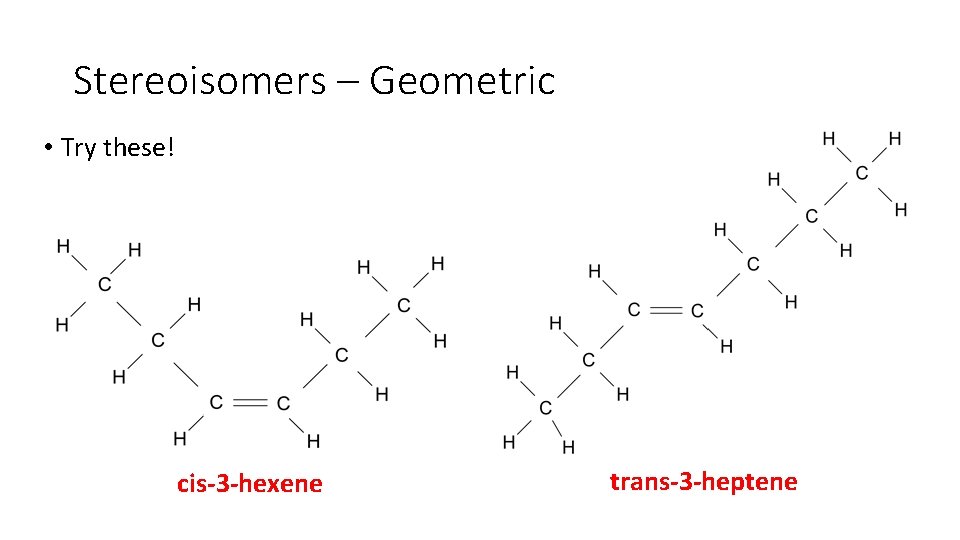
Stereoisomers – Geometric • Try these! cis-3 -hexene trans-3 -heptene

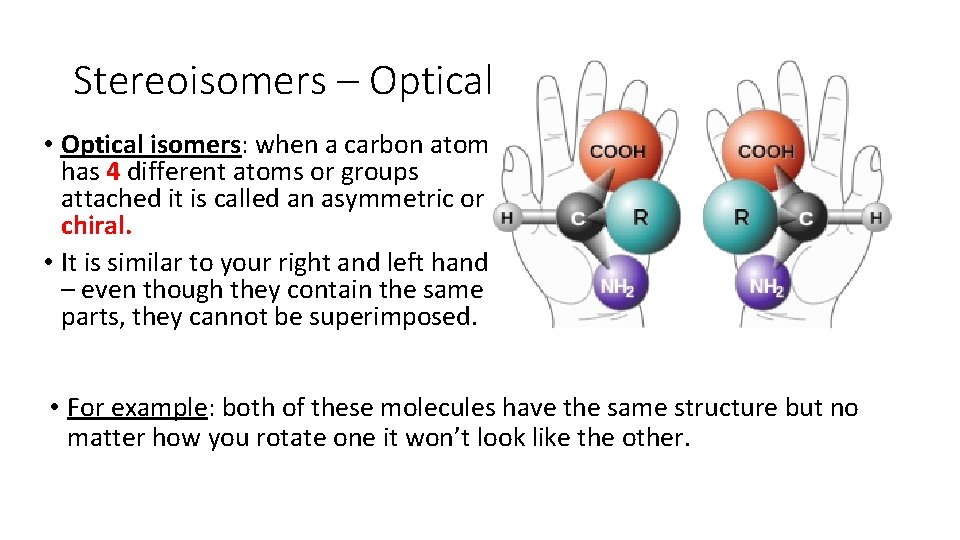
Stereoisomers – Optical • Optical isomers: when a carbon atom has 4 different atoms or groups attached it is called an asymmetric or chiral. • It is similar to your right and left hand – even though they contain the same parts, they cannot be superimposed. • For example: both of these molecules have the same structure but no matter how you rotate one it won’t look like the other.

Aromatic Hydrocarbon

Aromatic Hydrocarbons • Previously we studied straight chains with or without branches. Chemists call these Aliphatic. • However the most wildly studied organic compounds are actually rings which we call Aromatic because they often have an odour. • The most common aromatic is benzene (C 6 H 6) which has a truly unique structure. (If benzene were aliphatic what type would it be? Alkane, Alkene, Alkyne? )

Aromatic Hydrocarbons • The C – C bonds of benzene are all the wrong size. (1. 40 A) • Single bonds are 1. 54 A making benzene too short. • Double bonds are 1. 34 A making benzene too long. • This lead chemists to conclude the aromatic ring is sharing electrons in a unique way. • This is called a hybrid structure and we draw it like this:

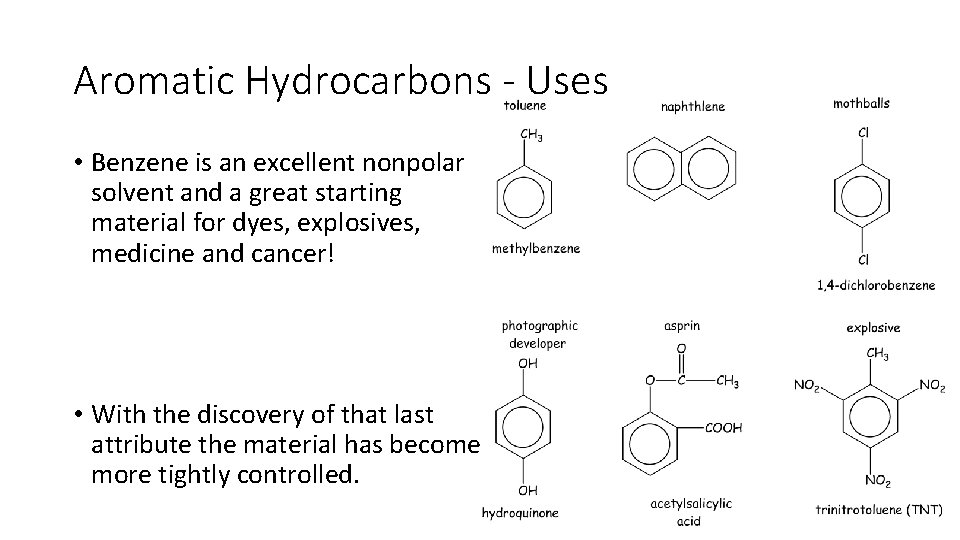
Aromatic Hydrocarbons - Uses • Benzene is an excellent nonpolar solvent and a great starting material for dyes, explosives, medicine and cancer! • With the discovery of that last attribute the material has become more tightly controlled.

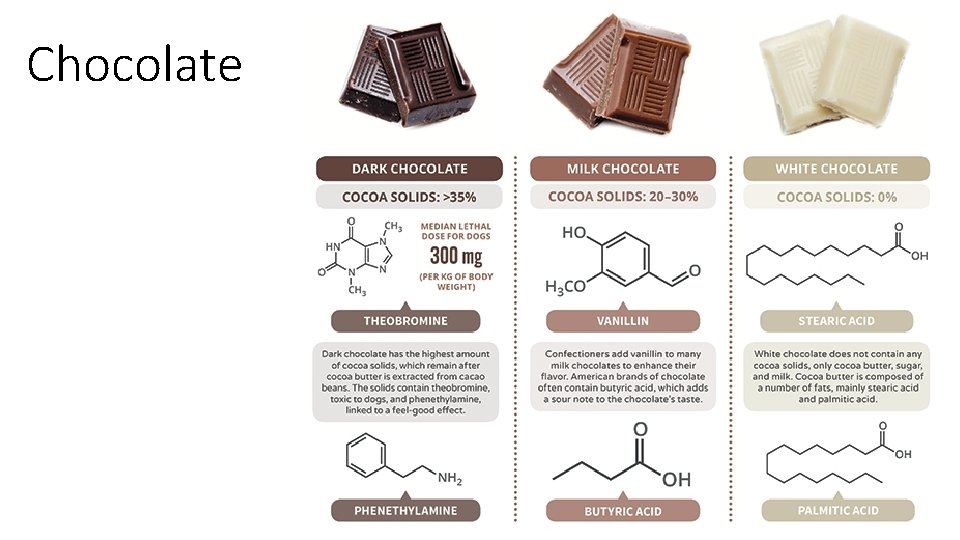
Chocolate

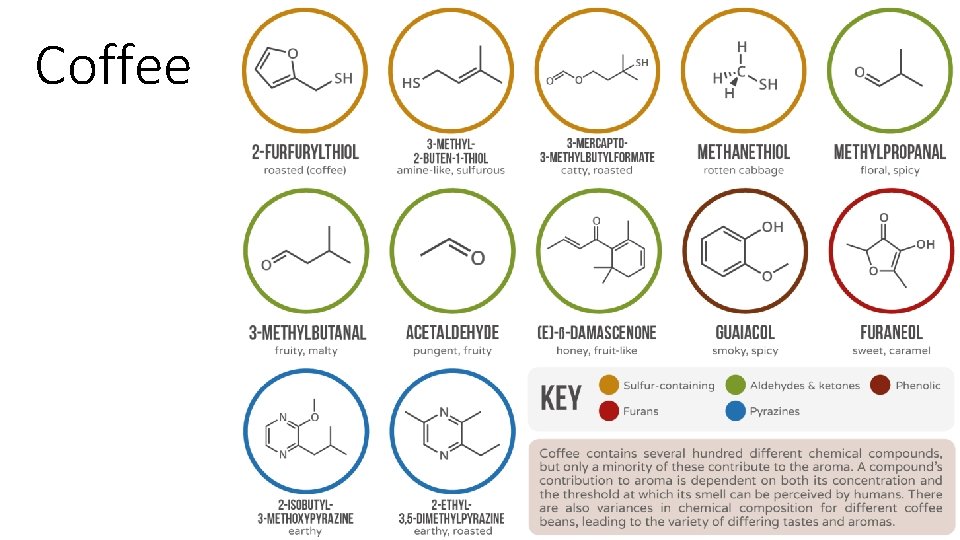
Coffee

Aromatic Hydrocarbons – Naming Rules • Naming rules for hydrocarbons with a benzene ring depend on if the benzene is the main ‘chain’ or a ‘branch’. • Case 1 – Benzene Substituent: Benzene is a branch on an existing chain • Case 2 – Benzene Backbone: Other branches are on a benzene ring • Aromatic rings still use all the rules for aliphatic chains, though there are some complications since it is a ring. • (I. E. Where do you start and end)

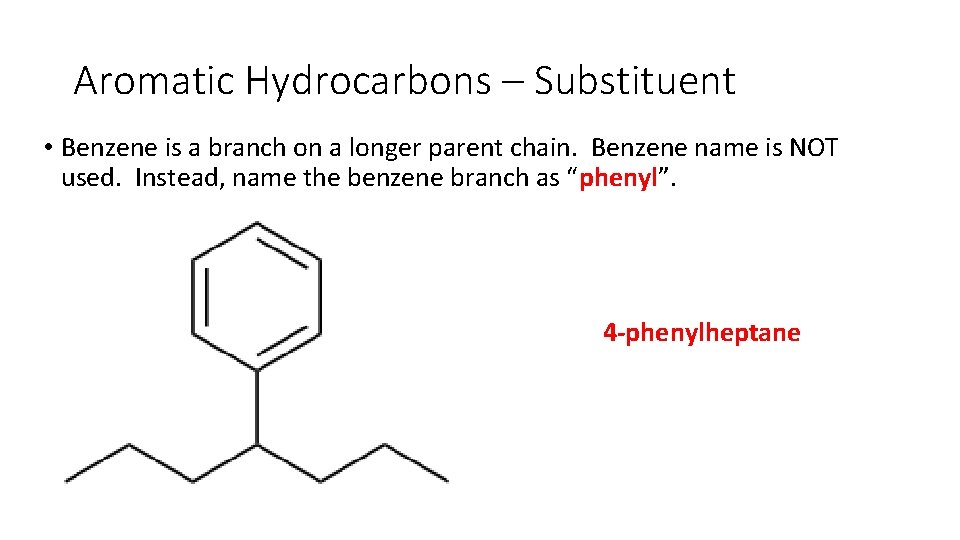
Aromatic Hydrocarbons – Substituent • Benzene is a branch on a longer parent chain. Benzene name is NOT used. Instead, name the benzene branch as “phenyl”.

Aromatic Hydrocarbons – Substituent • Benzene is a branch on a longer parent chain. Benzene name is NOT used. Instead, name the benzene branch as “phenyl”. 4 -phenylheptane

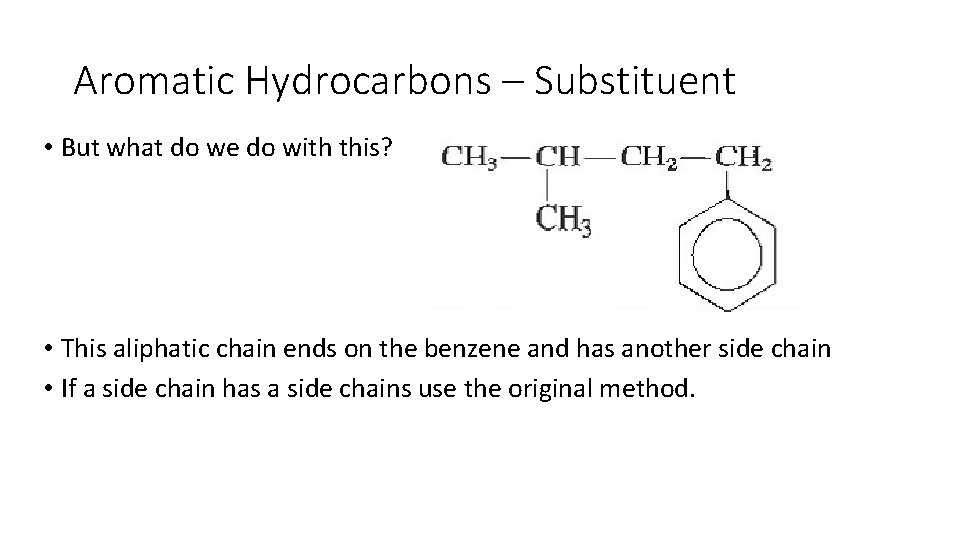
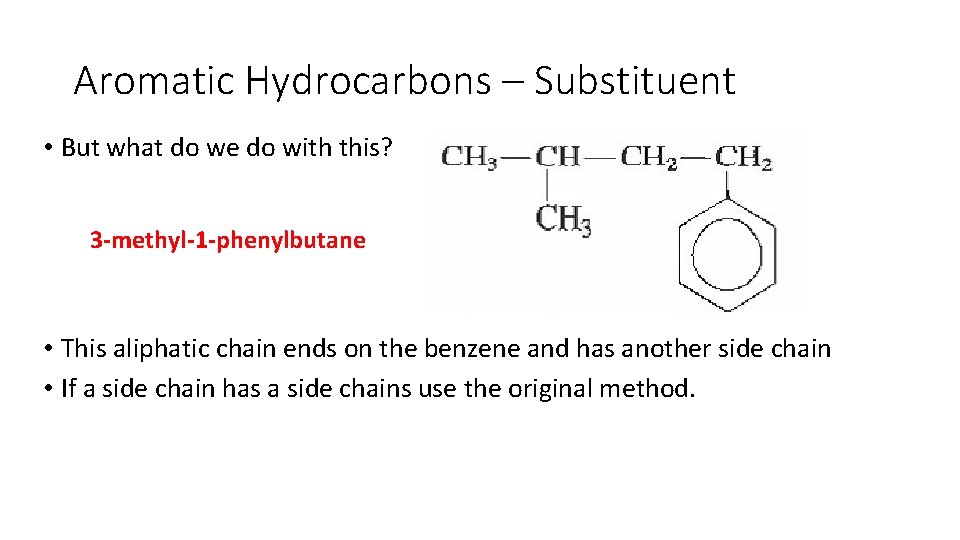
Aromatic Hydrocarbons – Substituent • But what do we do with this? • This aliphatic chain ends on the benzene and has another side chain • If a side chain has a side chains use the original method.

Aromatic Hydrocarbons – Substituent • But what do we do with this? 3 -methyl-1 -phenylbutane • This aliphatic chain ends on the benzene and has another side chain • If a side chain has a side chains use the original method.

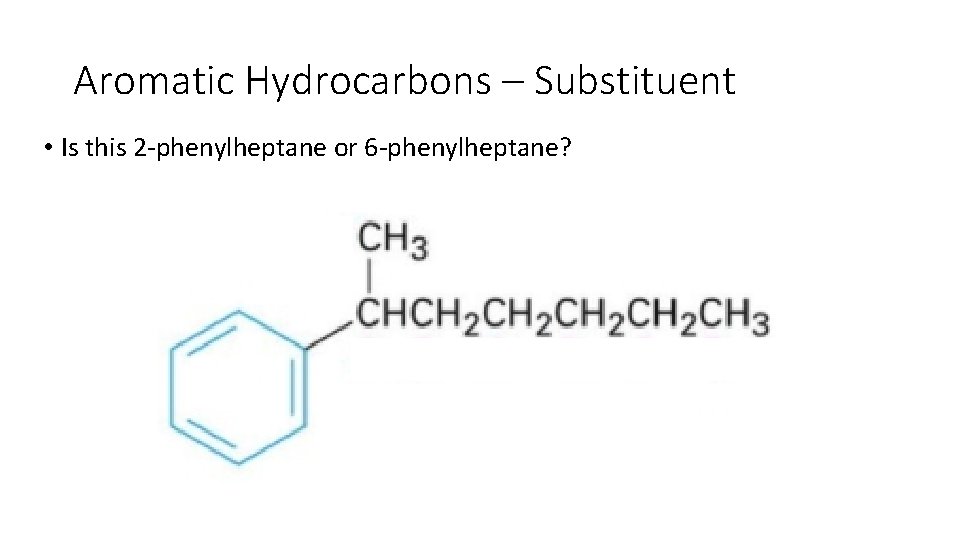
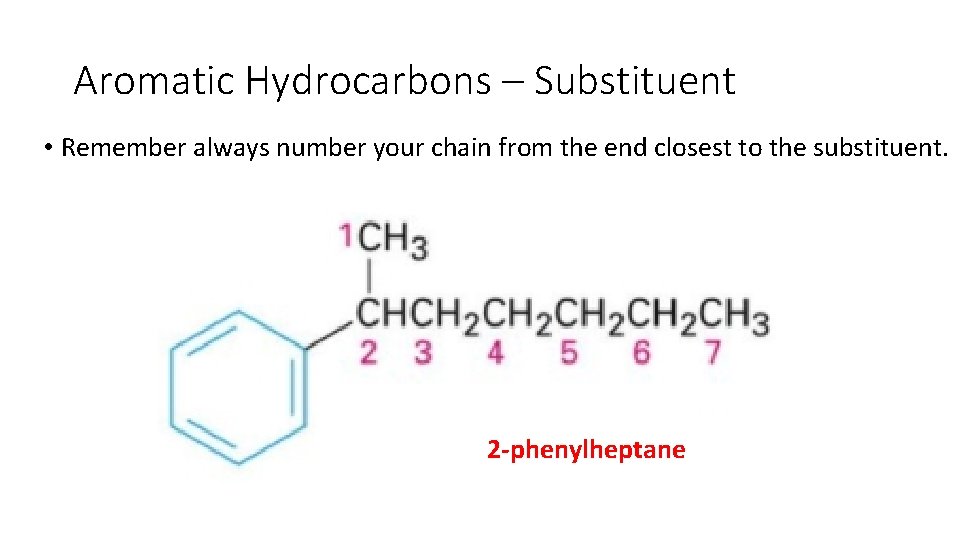
Aromatic Hydrocarbons – Substituent • Is this 2 phenylheptane or 6 phenylheptane?

Aromatic Hydrocarbons – Substituent • Remember always number your chain from the end closest to the substituent. 2 -phenylheptane

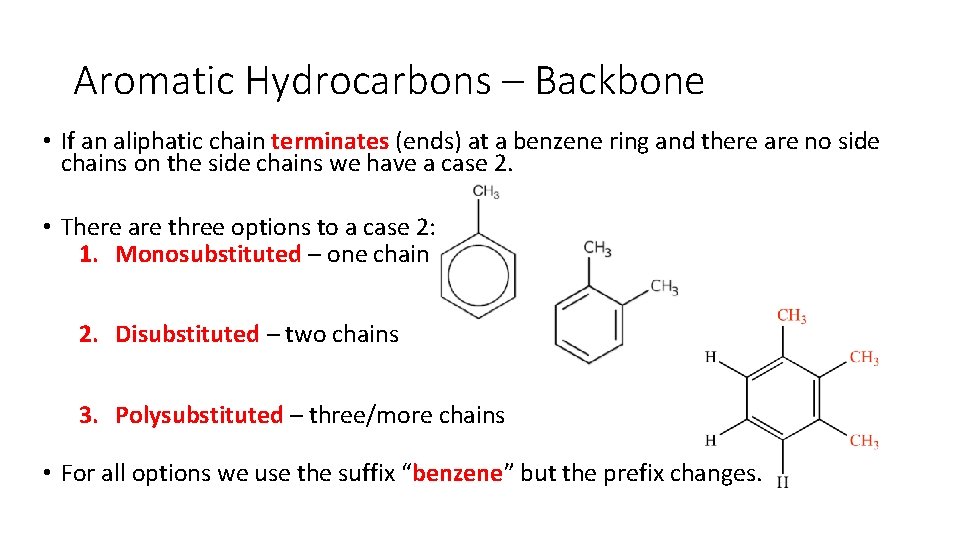
Aromatic Hydrocarbons – Backbone • If an aliphatic chain terminates (ends) at a benzene ring and there are no side chains on the side chains we have a case 2. • There are three options to a case 2: 1. Monosubstituted – one chain 2. Disubstituted – two chains 3. Polysubstituted – three/more chains • For all options we use the suffix “benzene” but the prefix changes.

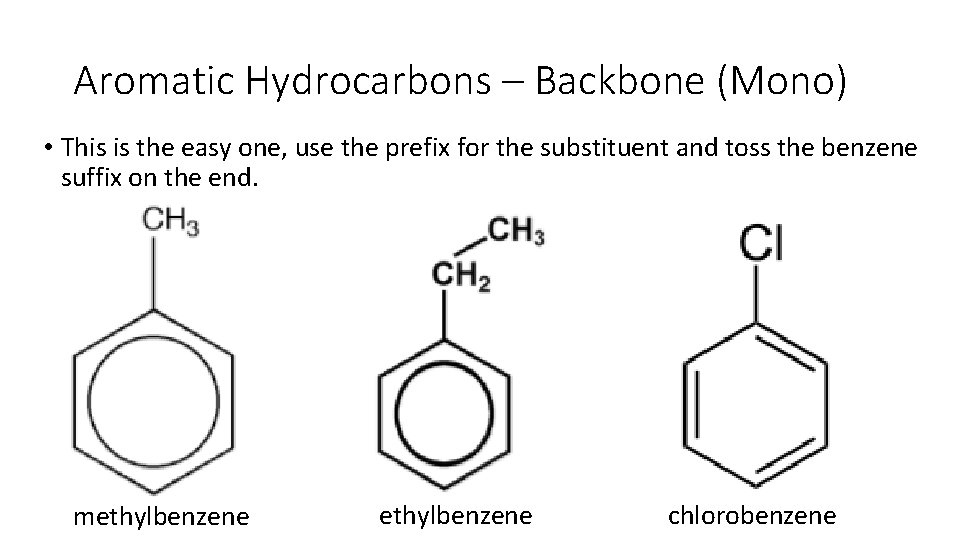
Aromatic Hydrocarbons – Backbone (Mono) • This is the easy one, use the prefix for the substituent and toss the benzene suffix on the end. methylbenzene chlorobenzene

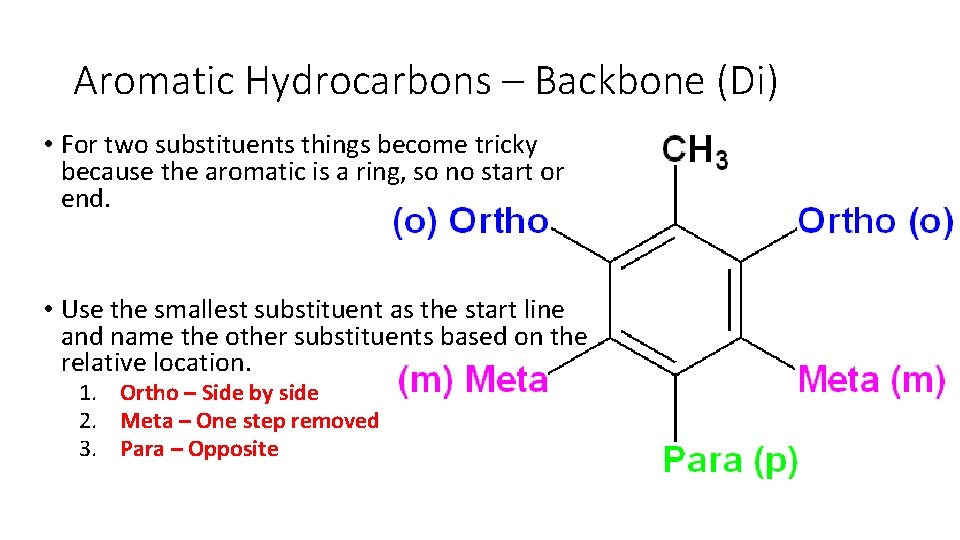
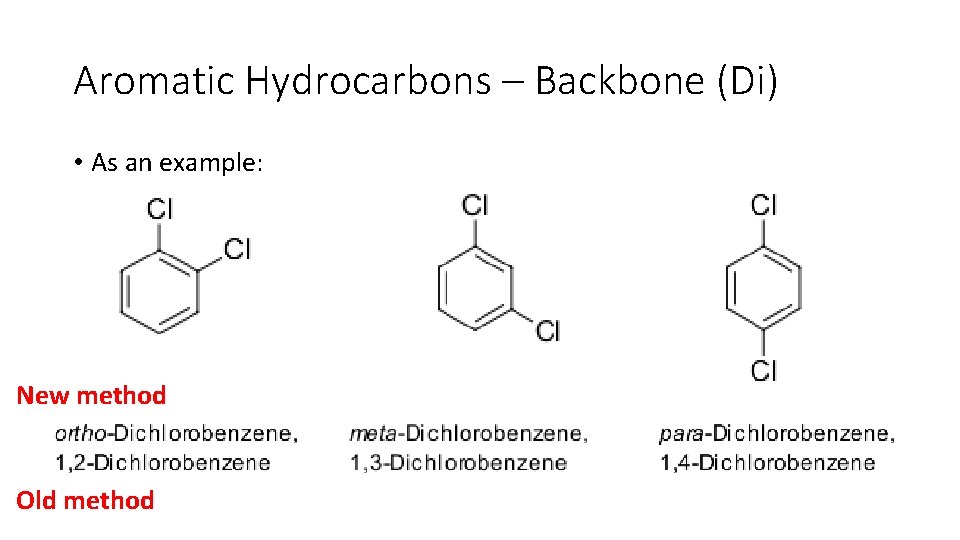
Aromatic Hydrocarbons – Backbone (Di) • For two substituents things become tricky because the aromatic is a ring, so no start or end. • Use the smallest substituent as the start line and name the other substituents based on the relative location. 1. Ortho – Side by side 2. Meta – One step removed 3. Para – Opposite

Aromatic Hydrocarbons – Backbone (Di) • As an example: New method Old method

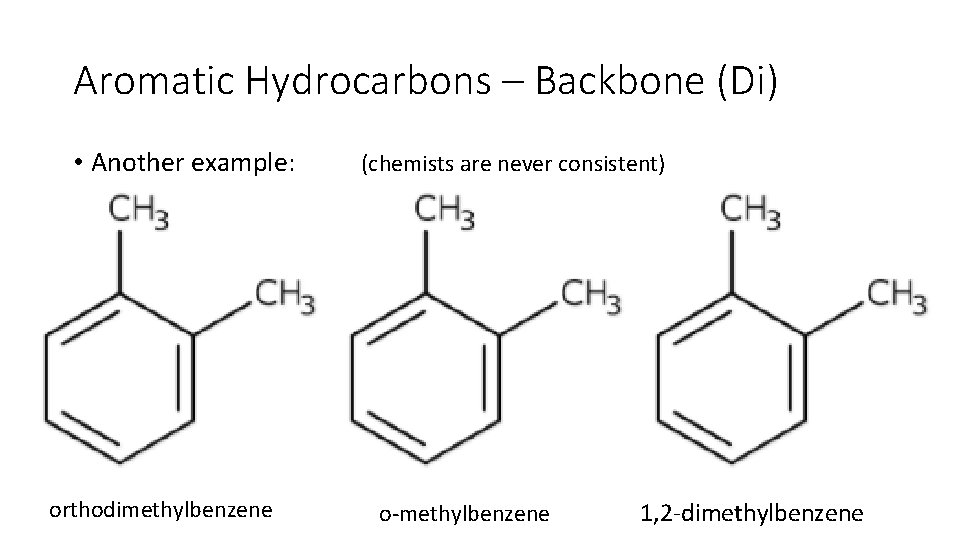
Aromatic Hydrocarbons – Backbone (Di) • Another example: orthodimethylbenzene (chemists are never consistent) o methylbenzene 1, 2 dimethylbenzene

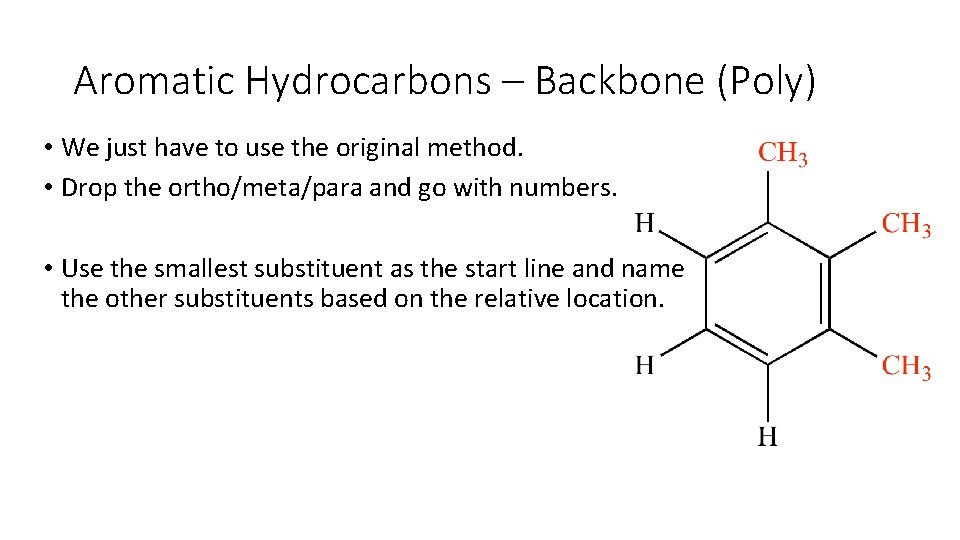
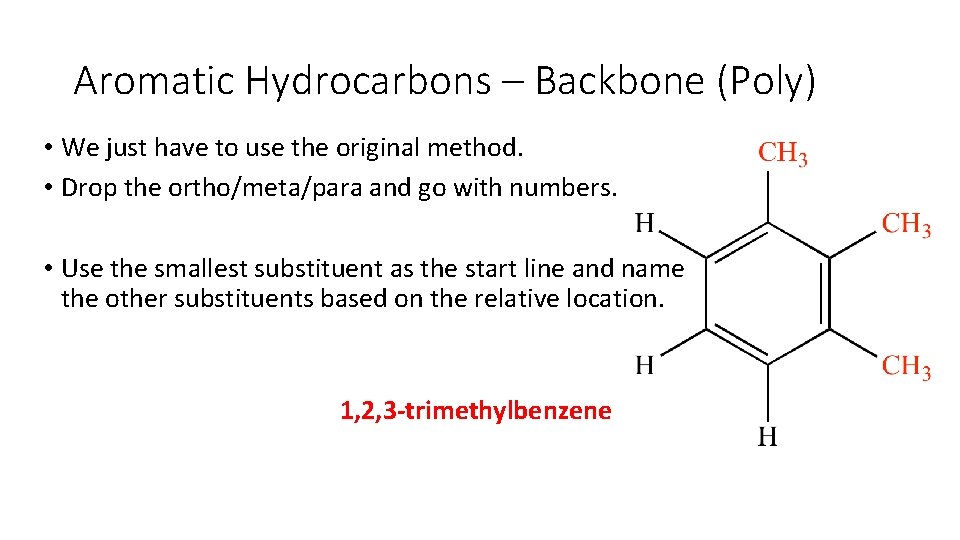
Aromatic Hydrocarbons – Backbone (Poly) • We just have to use the original method. • Drop the ortho/meta/para and go with numbers. • Use the smallest substituent as the start line and name the other substituents based on the relative location.

Aromatic Hydrocarbons – Backbone (Poly) • We just have to use the original method. • Drop the ortho/meta/para and go with numbers. • Use the smallest substituent as the start line and name the other substituents based on the relative location. 1, 2, 3 -trimethylbenzene

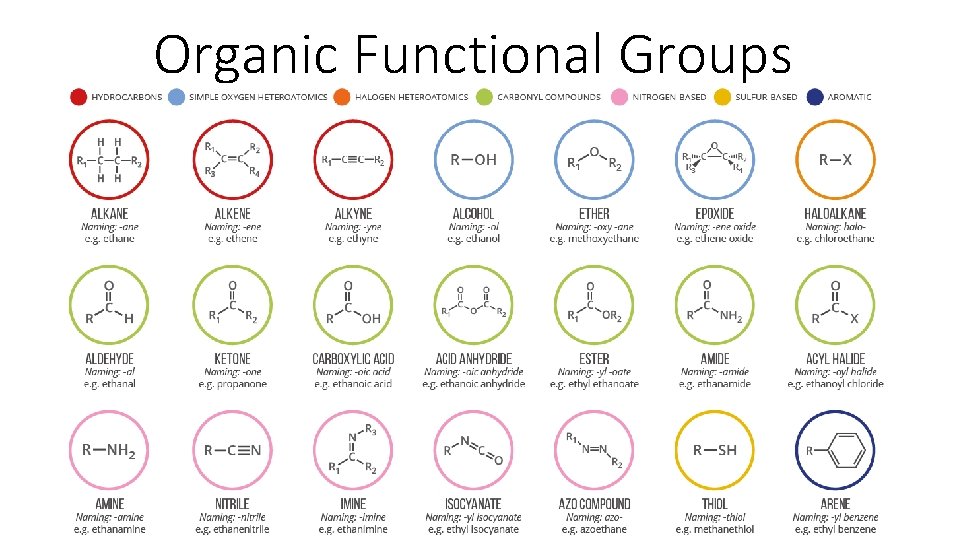
Organic Functional Groups

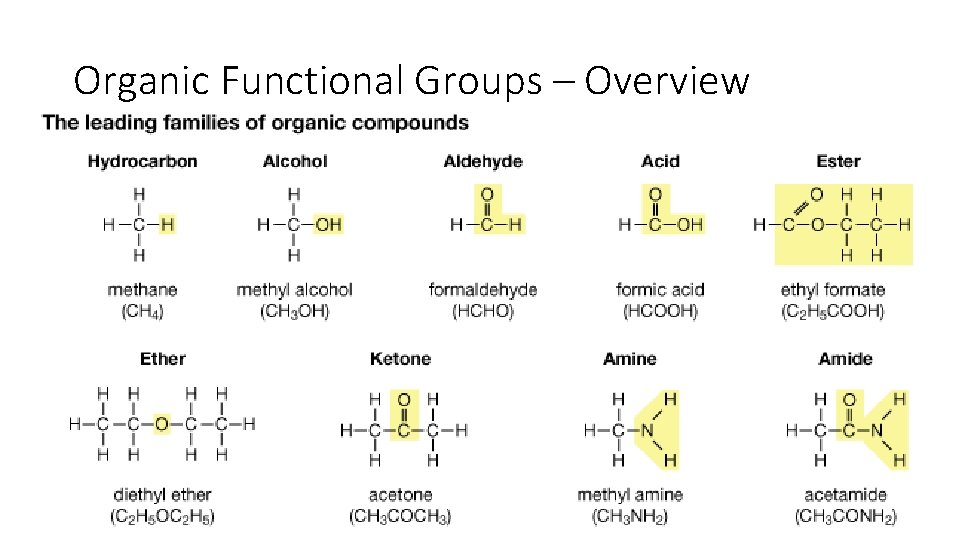
Organic Functional Groups – Overview • Up until now we’ve spoken about substituents of more carbon, but organic chemistry also involves oxygen and nitrogen. • We call repeating structures of oxygen/nitrogen & carbon/hydrogen functional groups. • Functional groups have consistent structure that we can use to classify compounds into ‘families’. • If you understand the behavior of a particular functional group, you will know a great deal about the general properties of that class of compounds.

Organic Functional Groups – Overview

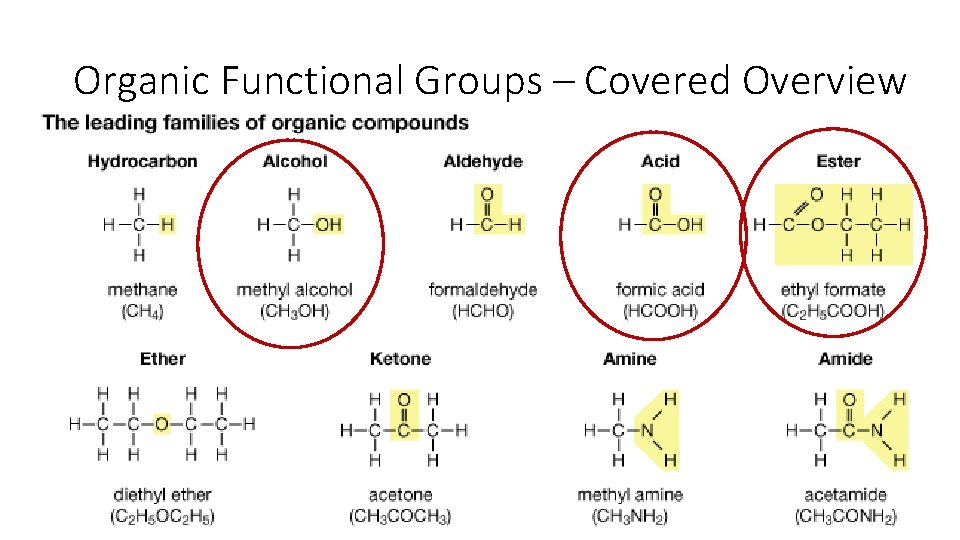
Organic Functional Groups – Covered Overview

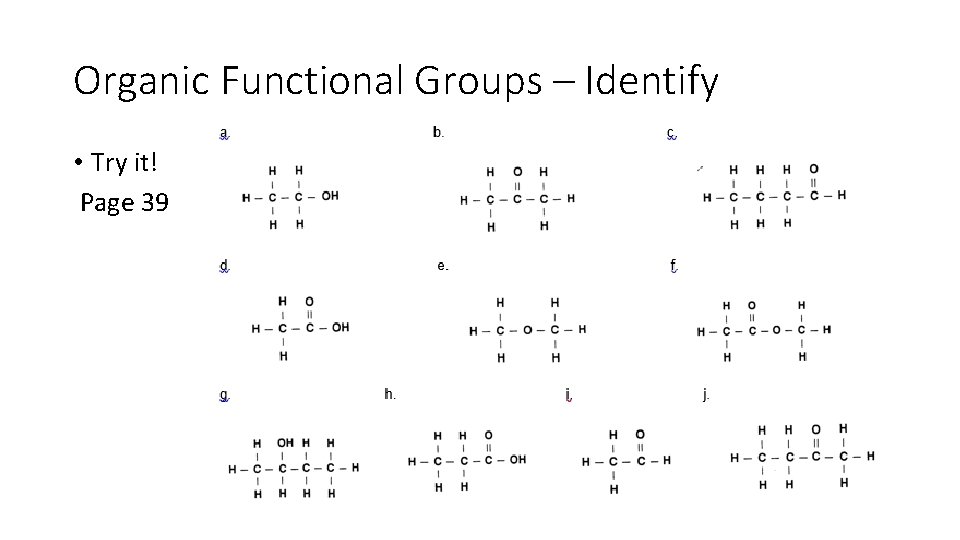
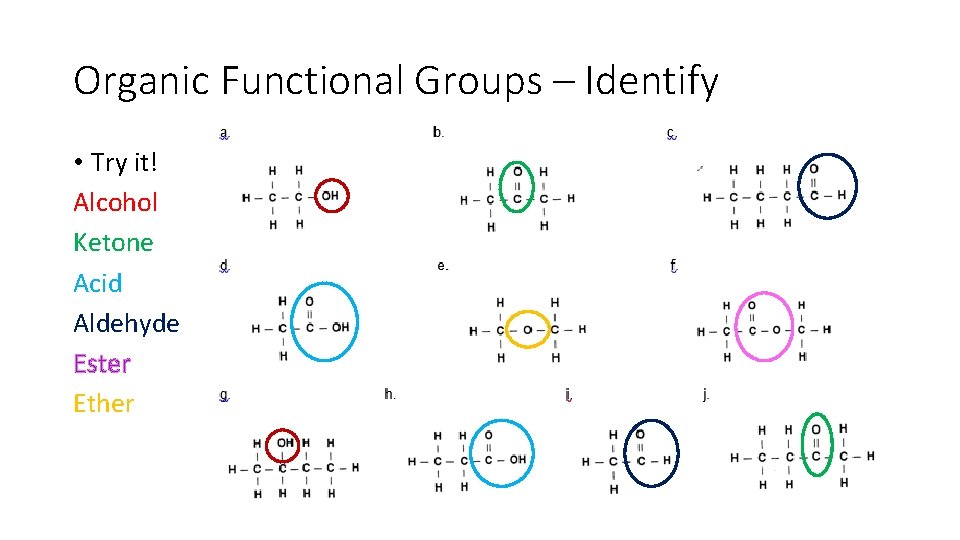
Organic Functional Groups – Identify • Try it! Page 39

Organic Functional Groups – Identify • Try it! Alcohol Ketone Acid Aldehyde Ester Ether

Functional Groups – Alcohol Methane • Organic alcohols contain the hydroxyl OH group. • They are neither acidic nor basic. • The names of this family are formed from the alkane. • The ‘e’ is substituted for the suffix -ol • ex. methane becomes methanol. • Alcohols are similar to alkanes with one hydrogen atom replaced by an OH group. Molecular Formula CH 3 OH Methanol

Functional Groups – Alcohol (Uses) 1. Methanol • Used to make formaldehyde, and then: plastics, medicine and explosives. • Methanol is highly toxic causing blindness, nerve damage and death. • Methanol is produced from carbon monoxide and hydrogen. 3. Propanol • Used as a solvent and often called ‘rubbing alcohol’ (so you don’t drink it) • Propanol is toxic causing loss of consciousness and respiratory failure.

Functional Groups – Alcohol (Uses) 2. Ethanol • Mostly used to make alcoholic beverages but is also an organic solvent for making: diethyl ether, chloroform, acetic acid, perfumes, dyes and synthetic rubber. • Ethanol is somewhat toxic (it’s why a drinking too much feels bad, you were poisoned) and at lethal levels it causes respiratory failure, hypothermia and nerve damage. • Ethanol is made from fermenting yeast with cane sugar. • The yeast enzyme invertase breaks sucrose into two isomeric sugars (glucose and fructose) • Other enzymes removes carbon dioxide from the sugar to produce alcohol.
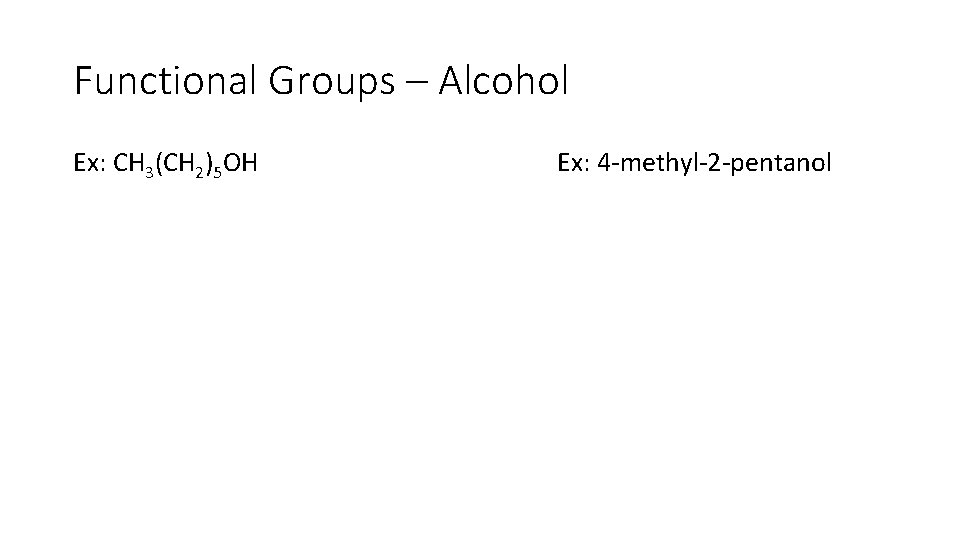
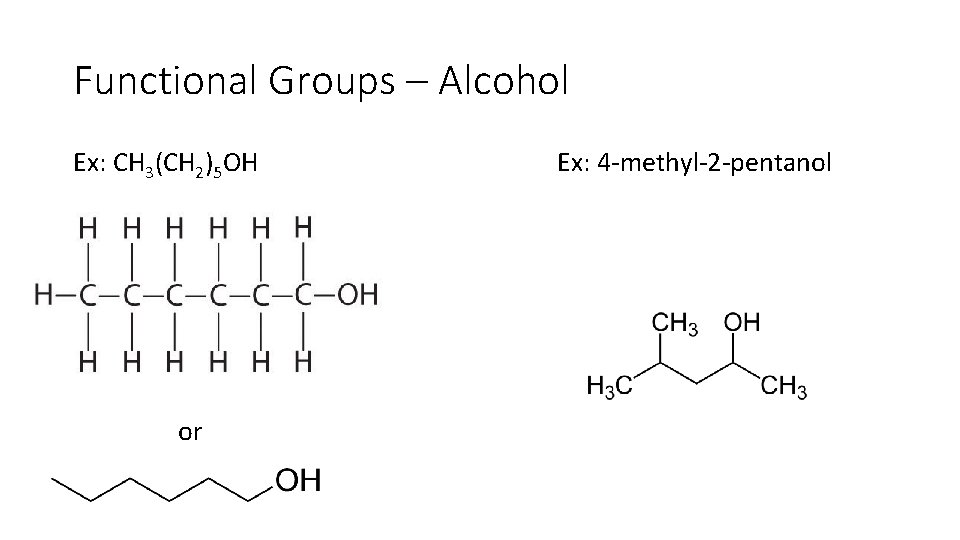
Functional Groups – Alcohol Ex: CH 3(CH 2)5 OH Ex: 4 methyl 2 pentanol

Functional Groups – Alcohol Ex: CH 3(CH 2)5 OH or Ex: 4 methyl 2 pentanol

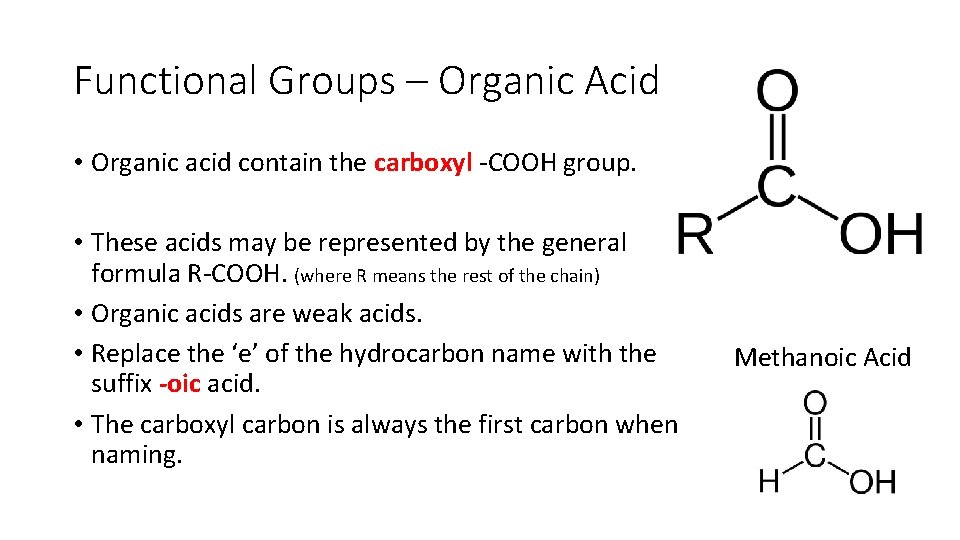
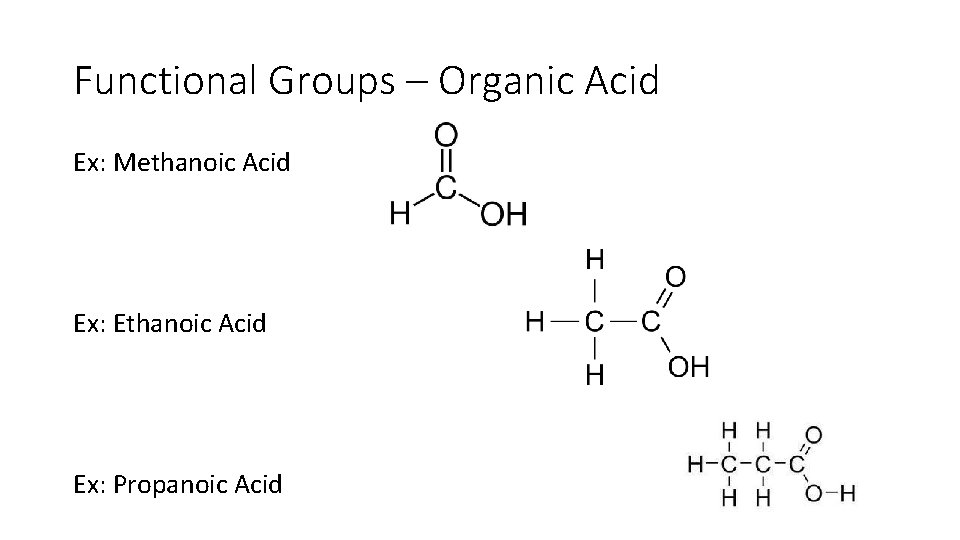
Functional Groups – Organic Acid • Organic acid contain the carboxyl COOH group. • These acids may be represented by the general formula R COOH. (where R means the rest of the chain) • Organic acids are weak acids. • Replace the ‘e’ of the hydrocarbon name with the suffix -oic acid. • The carboxyl carbon is always the first carbon when naming. Methanoic Acid

Functional Groups – Organic Acid Ex: Methanoic Acid Ex: Ethanoic Acid Ex: Propanoic Acid

Functional Groups – Esters • Organic acids react with alcohols to remove water from the reactants and form esters. • Esters are responsible for: 1. Tastes and odours in food. Fats are triesters made from glycerol and three fatty acids. 2. Drugs aspirin. Acetic acid, ethanoic acid and salicylic acid. Uses of aspirin reduces pain (analgesic), reduces temperature (antipyretic) and reduces inflammation. 3. Clothing polyester a synthetic fabric made from a long chain of ester molecules. 4. Adhesives and paints ethenyl ethanoate or vinyl acetate.

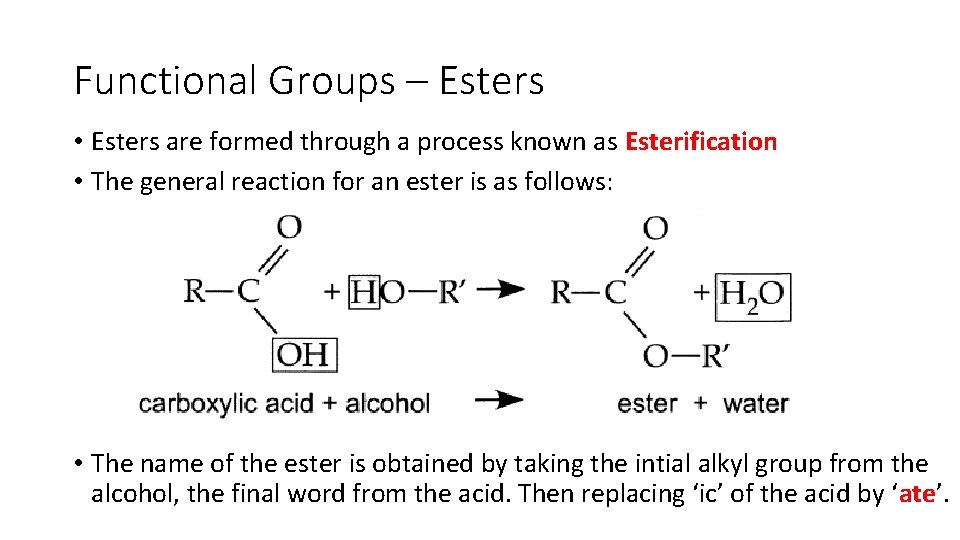
Functional Groups – Esters • Esters are formed through a process known as Esterification • The general reaction for an ester is as follows: • The name of the ester is obtained by taking the intial alkyl group from the alcohol, the final word from the acid. Then replacing ‘ic’ of the acid by ‘ate’.

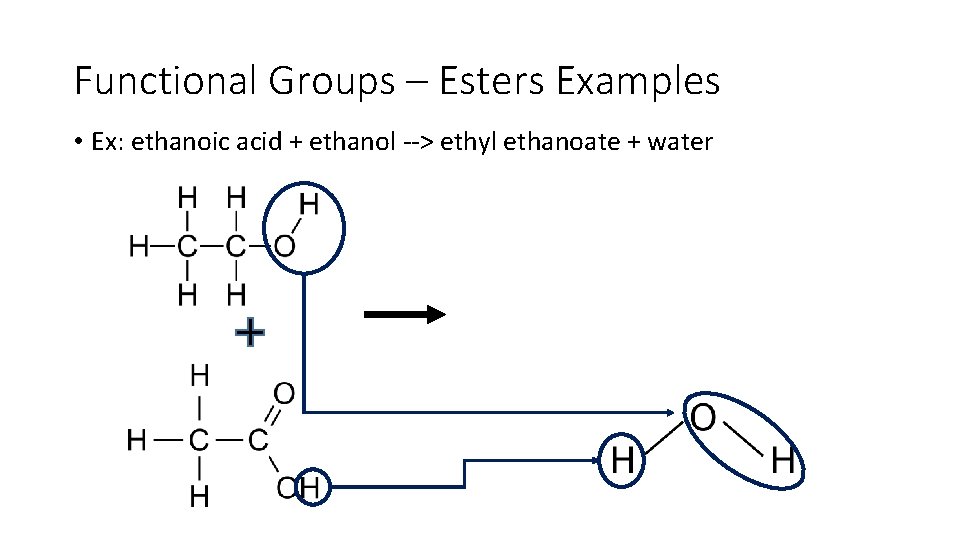
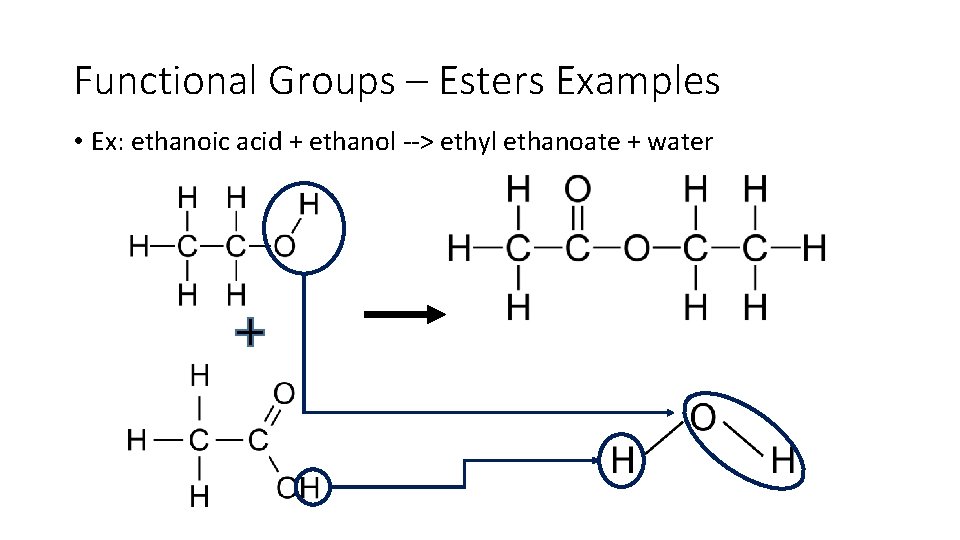
Functional Groups – Esters Examples • Ex: ethanoic acid + ethanol > ethyl ethanoate + water

Functional Groups – Esters Examples • Ex: ethanoic acid + ethanol > ethyl ethanoate + water

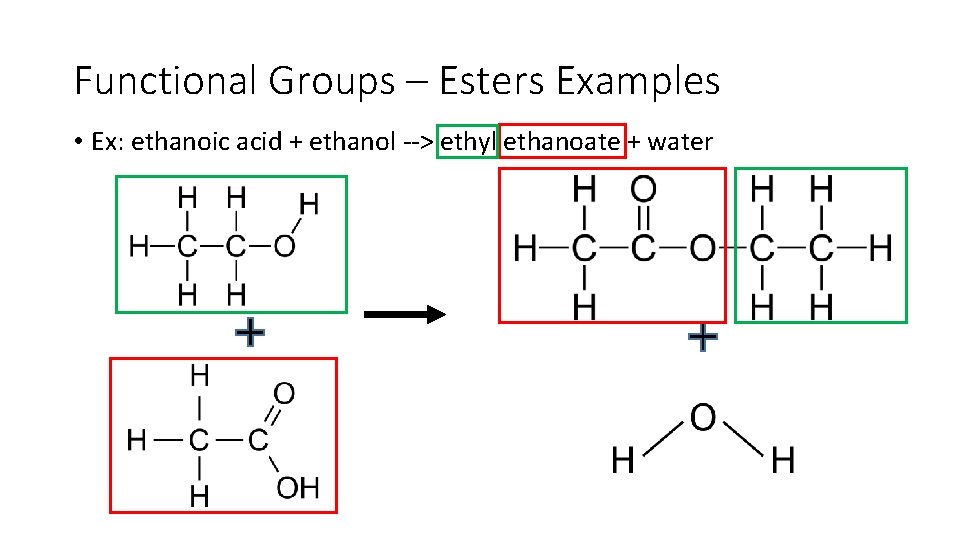
Functional Groups – Esters Examples • Ex: ethanoic acid + ethanol > ethyl ethanoate + water

Functional Groups – Esters Examples • Ex: ethanoic acid + ethanol > ethyl ethanoate + water

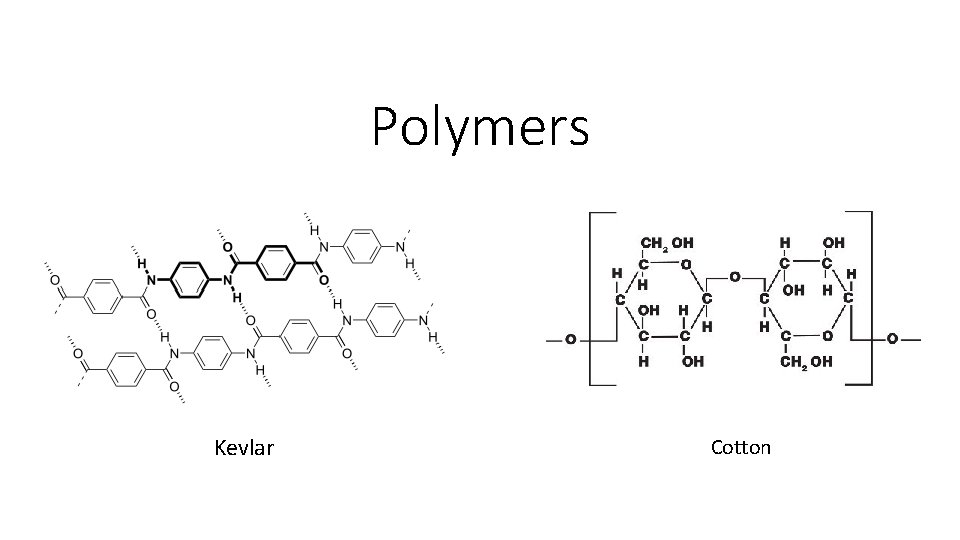
Polymers Kevlar Cotton

Polymers • A Polymer is a large molecule chain made of small fragments called monomers. • Polymerization is the formation of polymers from these small units. • Polymers can occur naturally (proteins, carbohydrates) and can be synthesized (nylon, Teflon, polyethylene). • Starch is a polymer of glucose molecules. Proteins are polymers of amino acids.

Organic Chemistry Review Use your white boards to answer each of the following questions. You have 60 seconds to answer the question.

Question 1 • What is the name of the process used to separate hydrocarbons into smaller groups of alkanes?

Answer to Question 1 • What is the name of the process used to separate hydrocarbons into smaller groups of alkanes? • Cracking

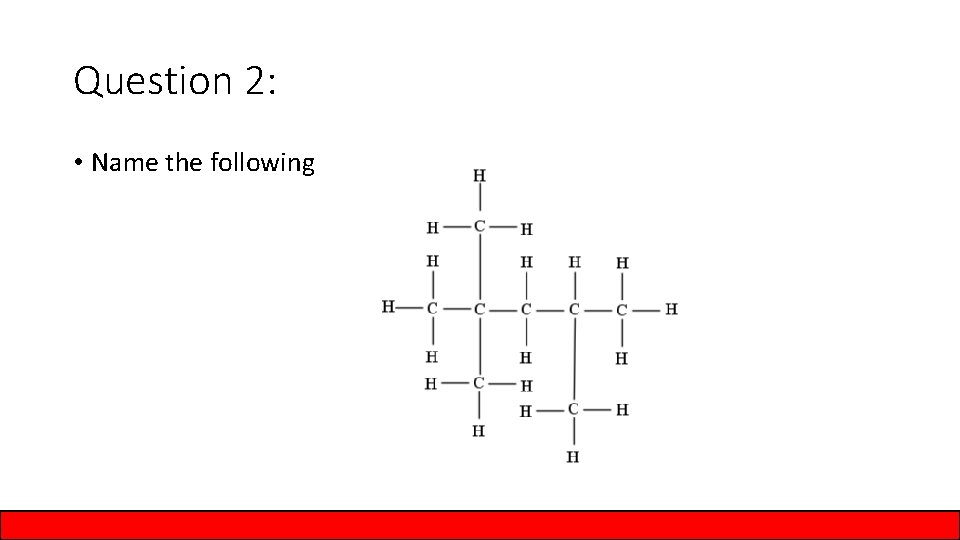
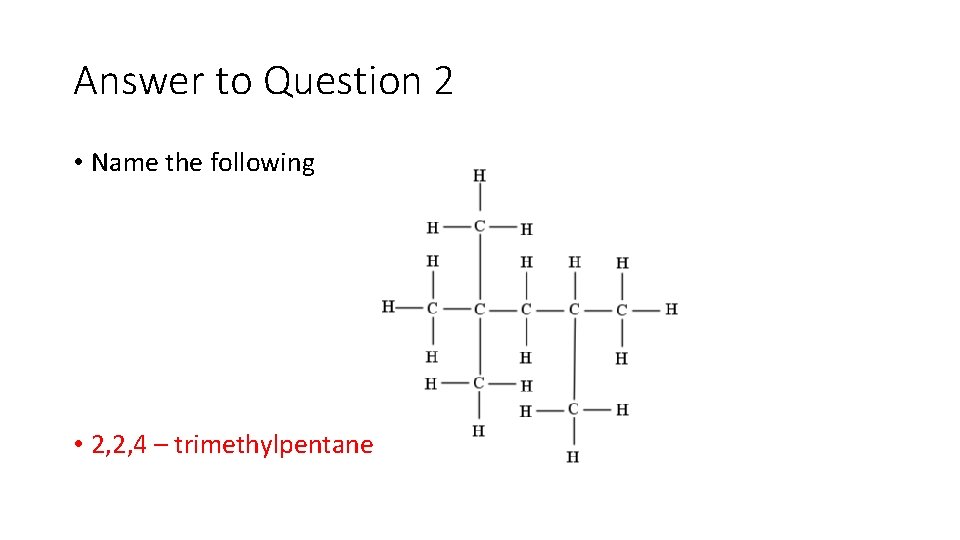
Question 2: • Name the following

Answer to Question 2 • Name the following • 2, 2, 4 – trimethylpentane

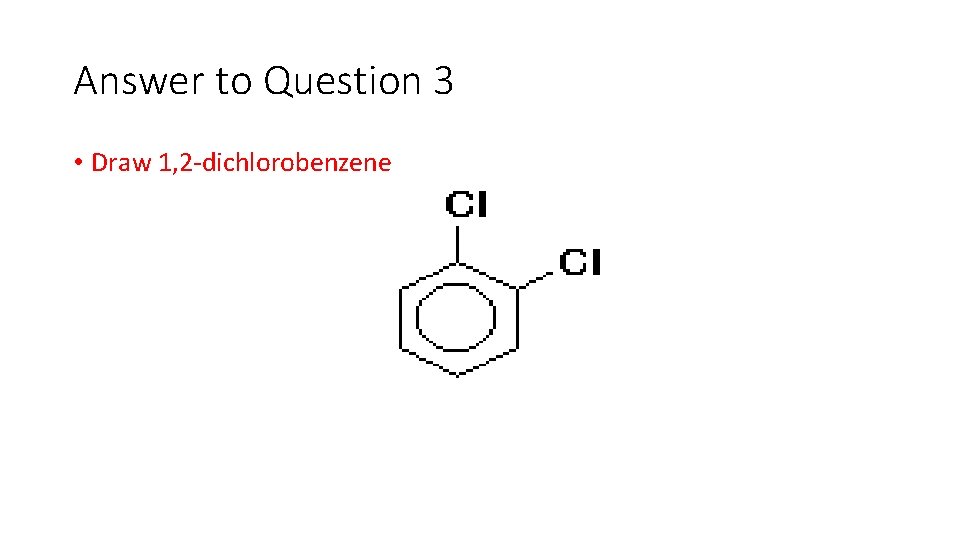
Question 3 • Draw 1, 2 dichlorobenzene

Answer to Question 3 • Draw 1, 2 dichlorobenzene

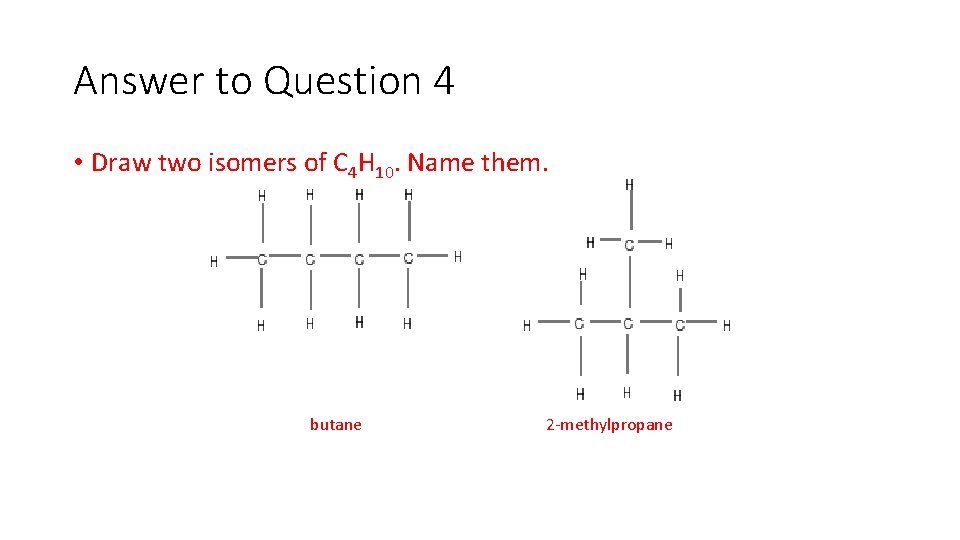
Question 4 • Draw two isomers of C 4 H 10. Name them.

Answer to Question 4 • Draw two isomers of C 4 H 10. Name them. butane 2 methylpropane

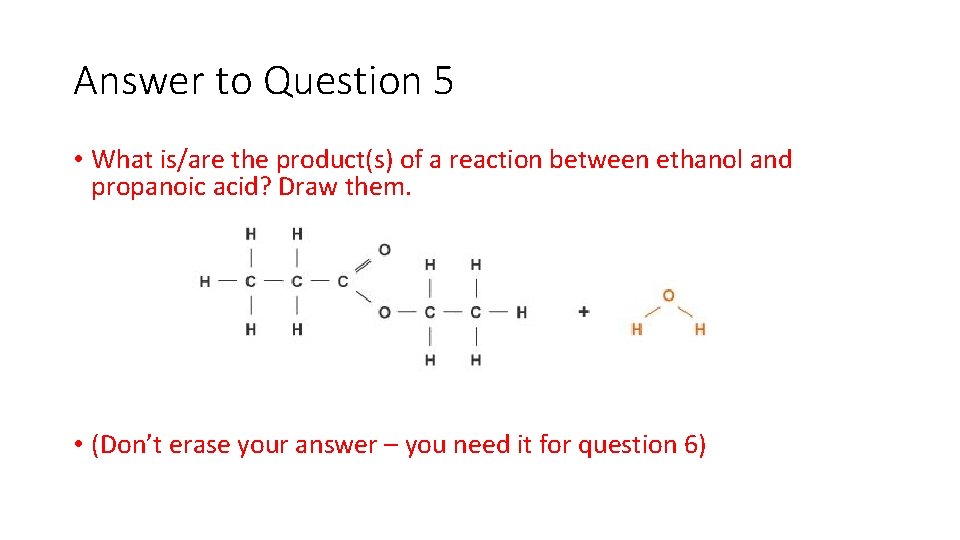
Question 5 • What is/are the product(s) of a reaction between ethanol and propanoic acid? Draw them.

Answer to Question 5 • What is/are the product(s) of a reaction between ethanol and propanoic acid? Draw them. • (Don’t erase your answer – you need it for question 6)

Question 6 • Name the ester produced in question 5. • What is the process to make the ester called?

Answer to Question 6 • Name the ester produced in question 5. Ethyl propanoate • What is the process to make the ester called? Esterification

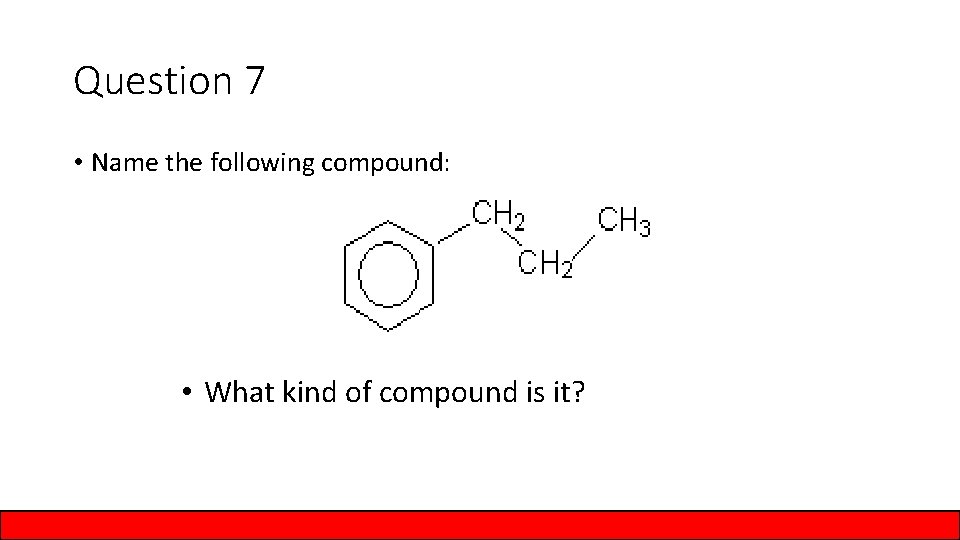
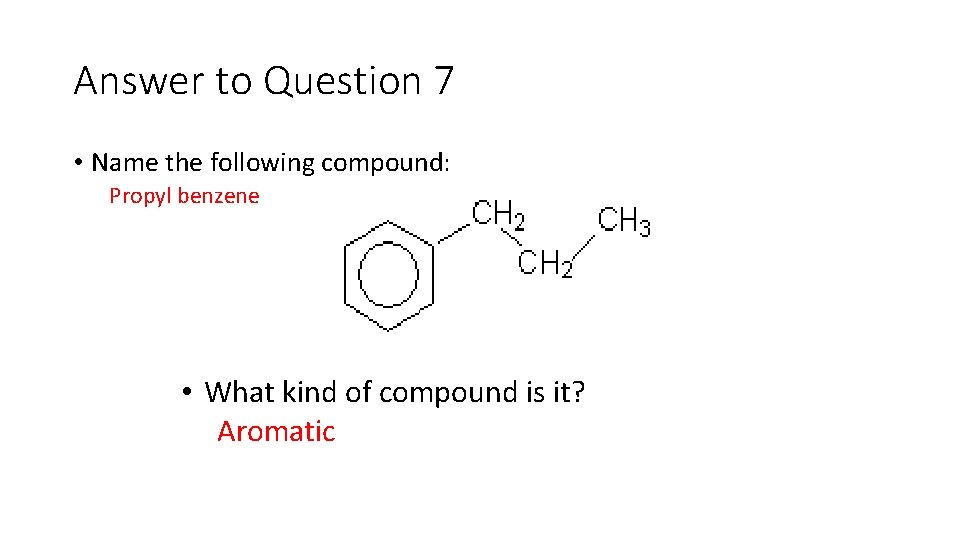
Question 7 • Name the following compound: • What kind of compound is it?

Answer to Question 7 • Name the following compound: Propyl benzene • What kind of compound is it? Aromatic

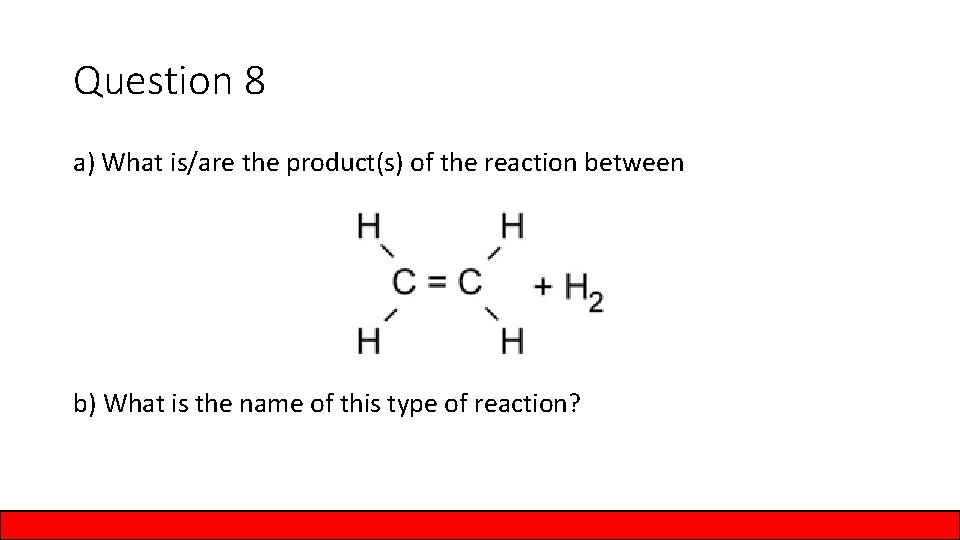
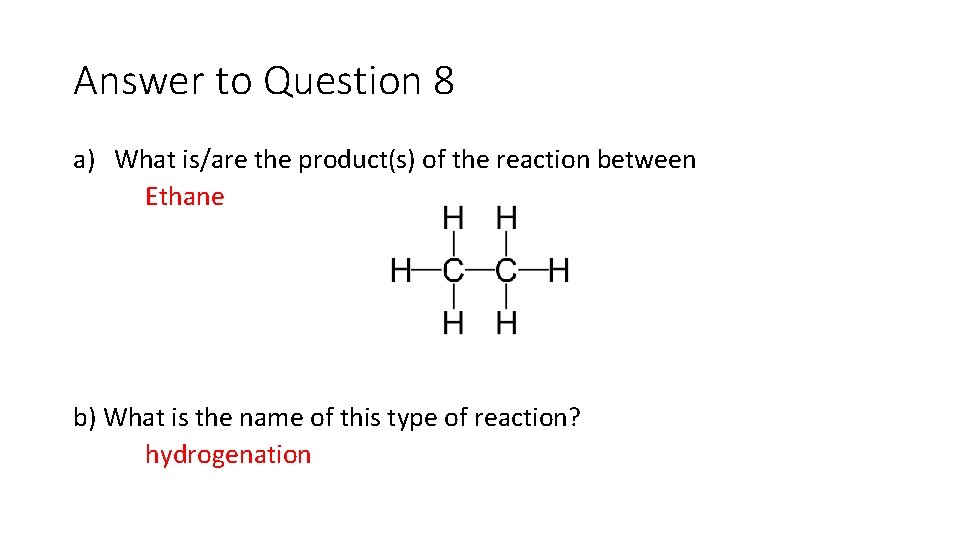
Question 8 a) What is/are the product(s) of the reaction between b) What is the name of this type of reaction?

Answer to Question 8 a) What is/are the product(s) of the reaction between Ethane b) What is the name of this type of reaction? hydrogenation

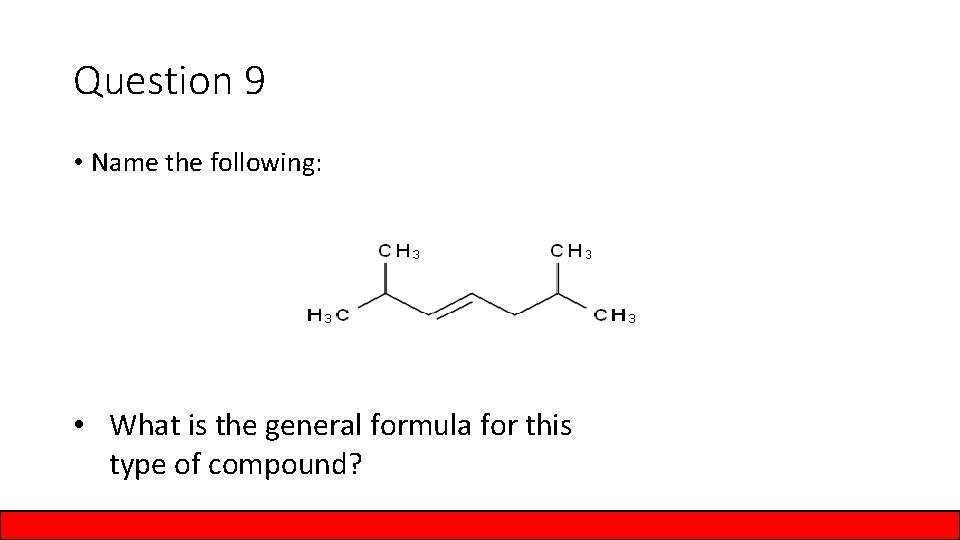
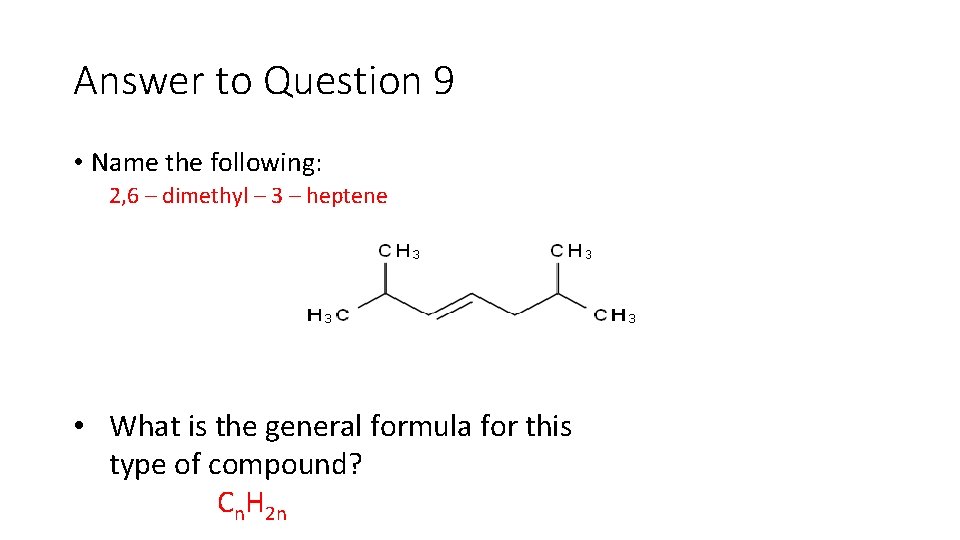
Question 9 • Name the following: • What is the general formula for this type of compound?

Answer to Question 9 • Name the following: 2, 6 – dimethyl – 3 – heptene • What is the general formula for this type of compound? Cn. H 2 n

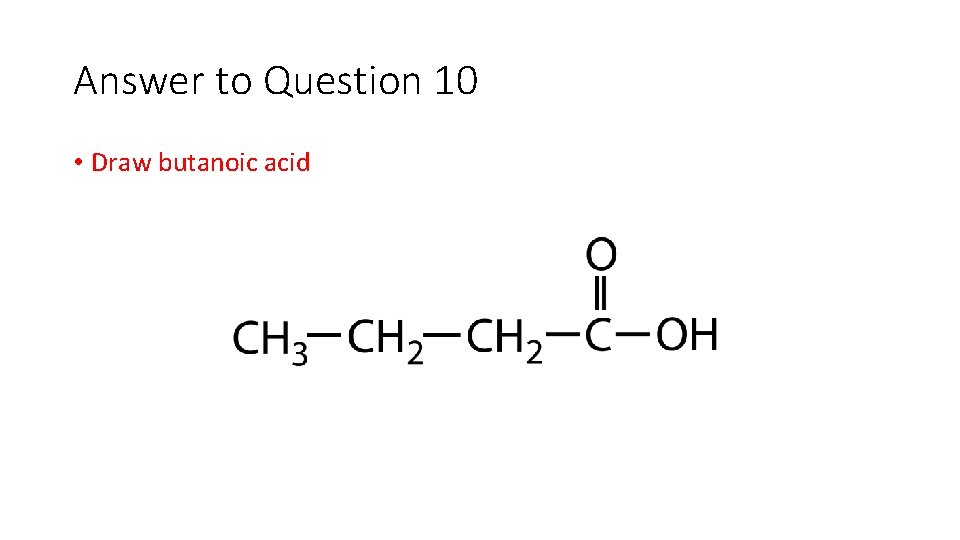
Question 10 • Draw butanoic acid

Answer to Question 10 • Draw butanoic acid

Question 11 • What is the general formula for an alkane? • What is a use for an alkane?

Answer to Question 11 • What is the general formula for an alkane? Cn. H 2 n+2 • What is a use for an alkane? Fuels

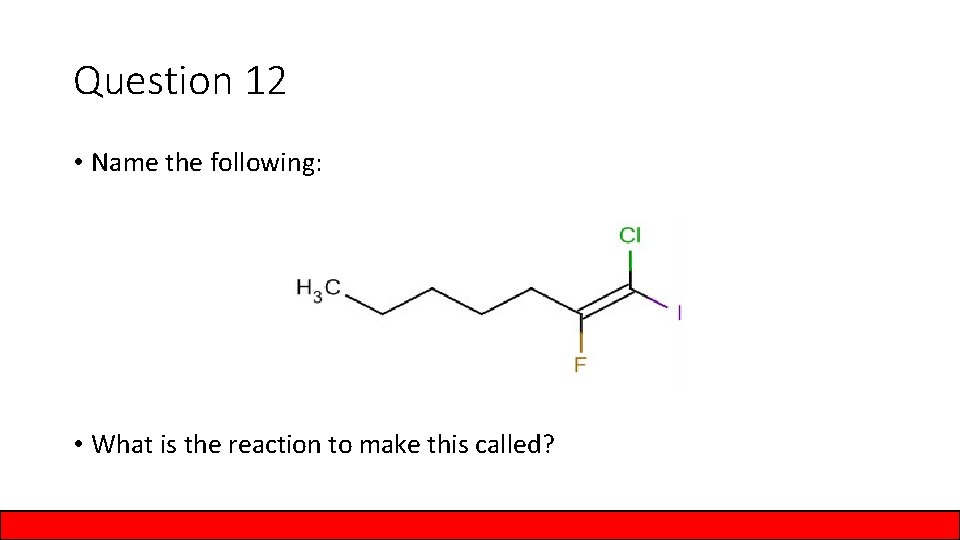
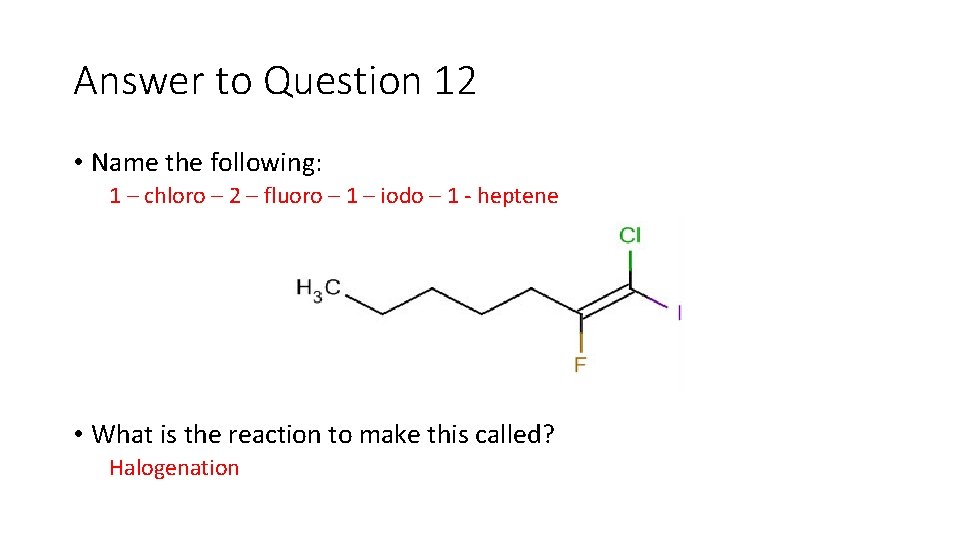
Question 12 • Name the following: • What is the reaction to make this called?

Answer to Question 12 • Name the following: 1 – chloro – 2 – fluoro – 1 – iodo – 1 heptene • What is the reaction to make this called? Halogenation

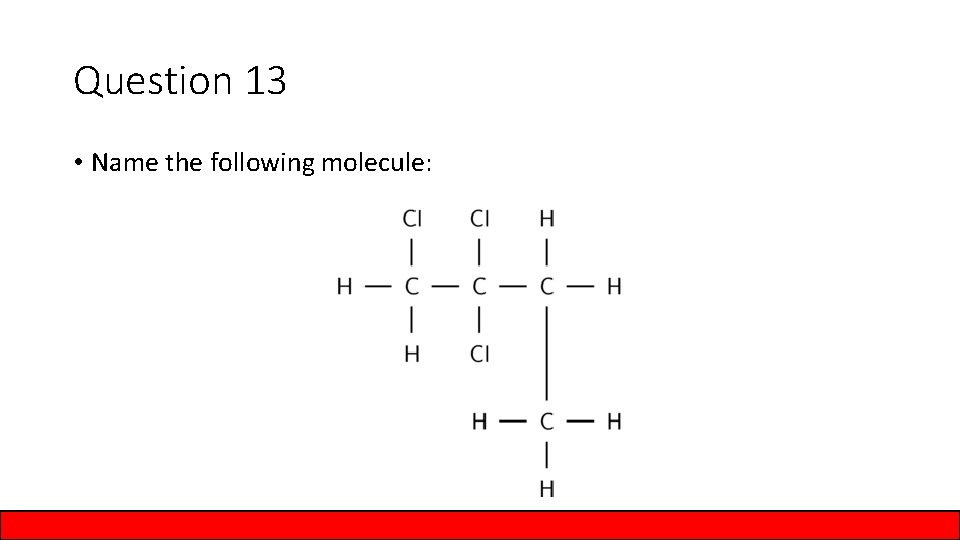
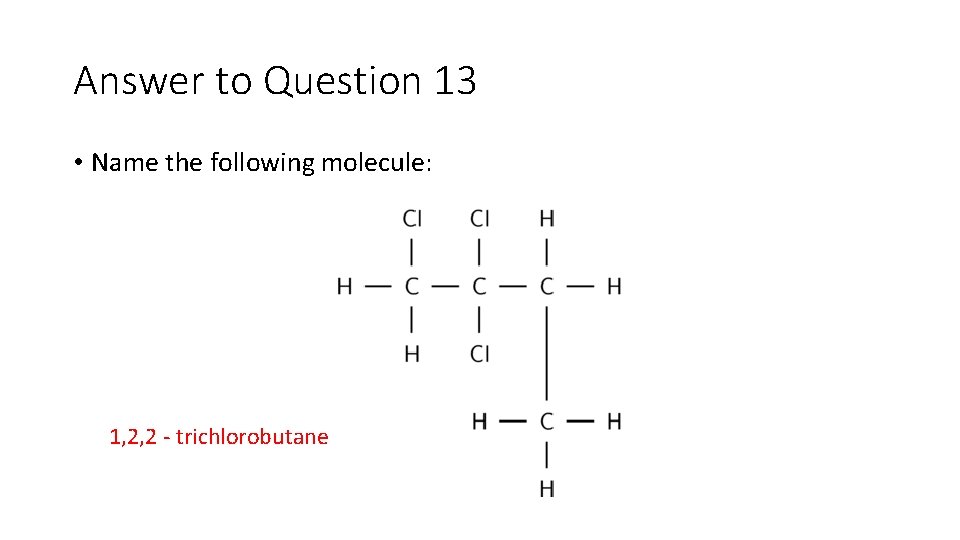
Question 13 • Name the following molecule:

Answer to Question 13 • Name the following molecule: 1, 2, 2 trichlorobutane

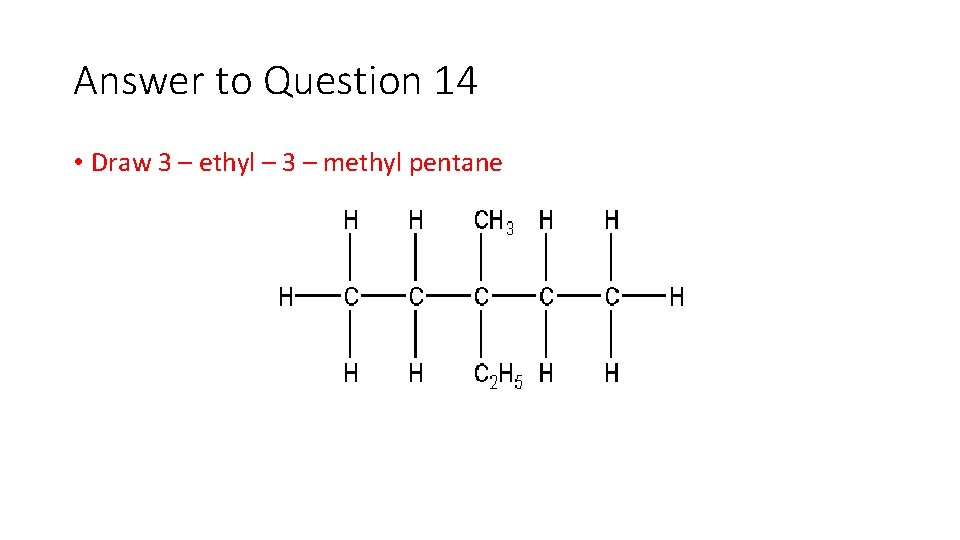
Question 14 • Draw 3 – ethyl – 3 – methyl pentane

Answer to Question 14 • Draw 3 – ethyl – 3 – methyl pentane

Question 15 • How do you tell the difference between an organic and inorganic compound? • Give an example of each.

Answer to Question 15 • An organic compound must contain carbon and hydrogen. An inorganic compound usually doesn’t contain carbon but if it does will not contain hydrogen. • Organic: CH 4 (there are lots of examples) • Inorganic: Na. Cl, CO 2 (there are lots of examples).

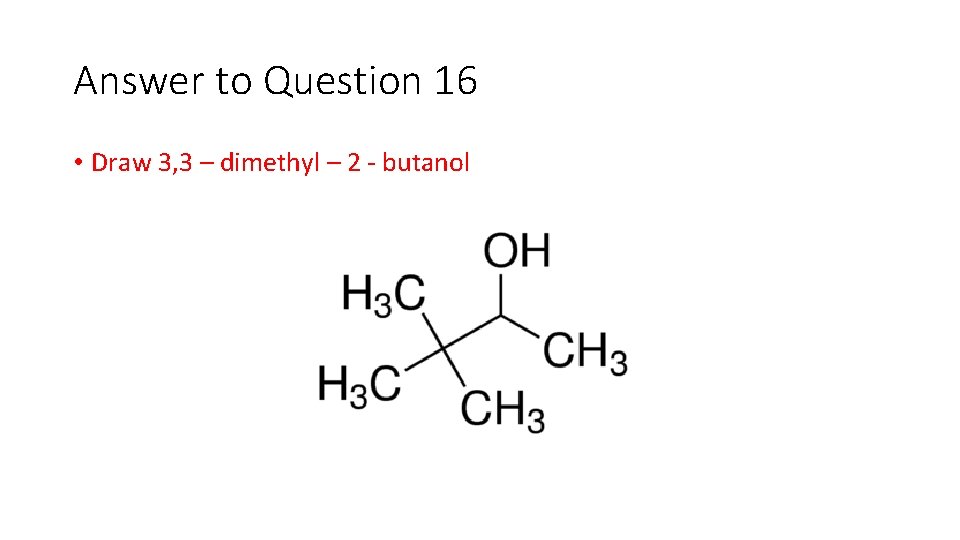
Question 16 • Draw 3, 3 – dimethyl – 2 butanol

Answer to Question 16 • Draw 3, 3 – dimethyl – 2 butanol