Organic Chemistry Types of Electronic effects Presented by

Organic Chemistry Types of Electronic effects Presented by Dr: AMIRA Hajri

What is Organic Chemistry?
Organic Chemistry § Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials. § They all contain carbon

Introduction q C is a small atom it forms single, double, and triple bonds, it forms strong bonds with C, H, O, N, and some metals.
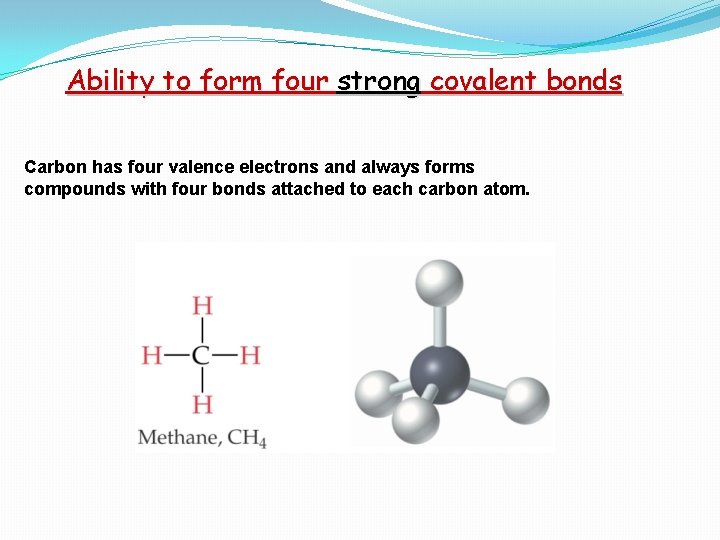
Ability to form four strong covalent bonds Carbon has four valence electrons and always forms compounds with four bonds attached to each carbon atom.
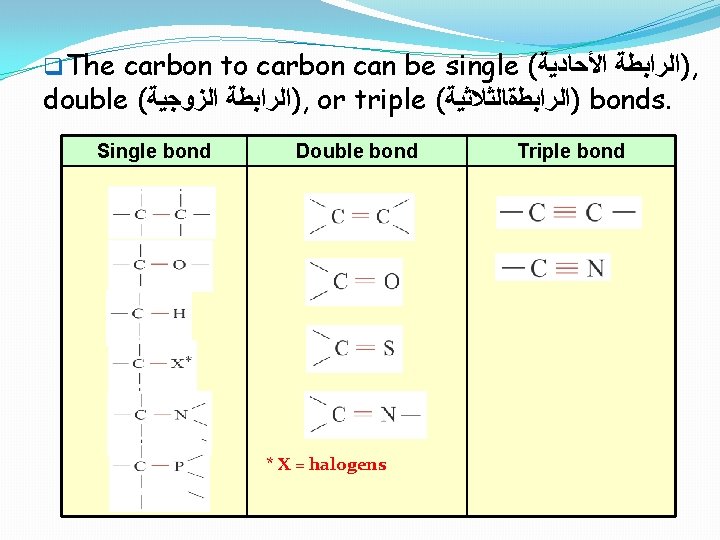
q. The carbon to carbon can be single ( )ﺍﻟﺮﺍﺑﻄﺔ ﺍﻷﺤﺎﺩﻳﺔ , double ( )ﺍﻟﺮﺍﺑﻄﺔ ﺍﻟﺰﻭﺟﻴﺔ , or triple ( )ﺍﻟﺮﺍﺑﻄةﺎﻟﺜﻼﺛﻴﺔ bonds. Single bond Double bond * X = halogens Triple bond
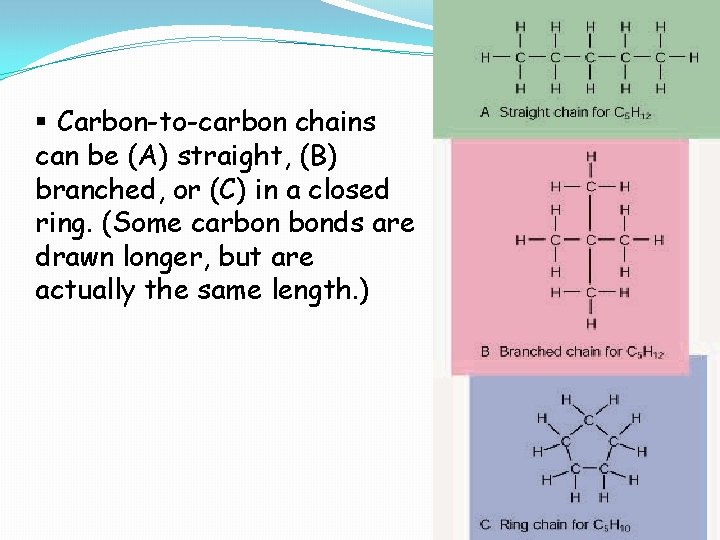
§ Carbon-to-carbon chains can be (A) straight, (B) branched, or (C) in a closed ring. (Some carbon bonds are drawn longer, but are actually the same length. )

Electrophiles and Nucleophiles Electrophiles • electron-deficient species that tend to accept electron(s) • possess an empty orbital to receive the electron pair • cations or free radicals seeking electronrich centres
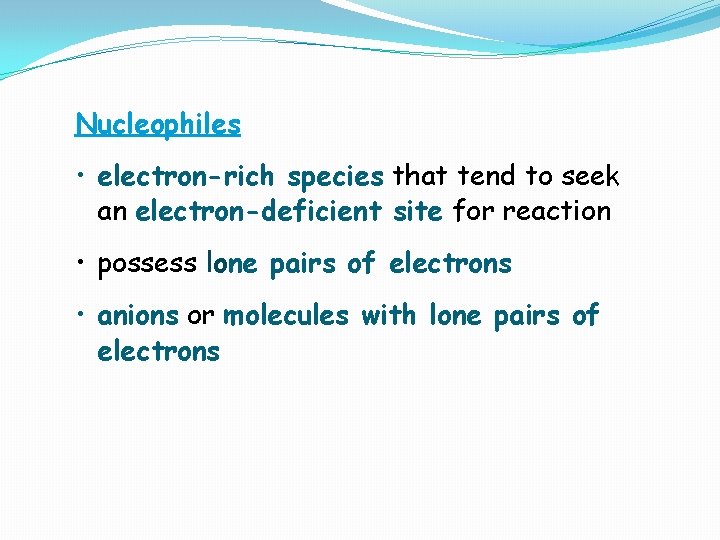
Nucleophiles • electron-rich species that tend to seek an electron-deficient site for reaction • possess lone pairs of electrons • anions or molecules with lone pairs of electrons
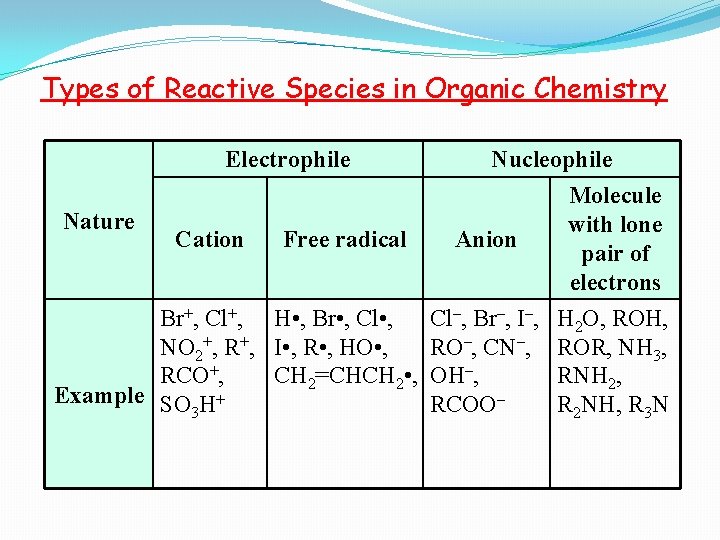
Types of Reactive Species in Organic Chemistry Electrophile Nature Cation Free radical Br+, Cl+, H • , Br • , Cl • , NO 2+, R+, I • , R • , HO • , RCO+, CH 2=CHCH 2 • , Example SO H+ 3 Nucleophile Molecule with lone Anion pair of electrons Cl–, Br–, I–, RO–, CN–, OH–, RCOO– H 2 O, ROH, ROR, NH 3, RNH 2, R 2 NH, R 3 N

Check Point -1 Identify the following chemical species as electrophiles, nucleophiles, or one that could act as both an electrophile and a nucleophile. (a) Cl– (c) NH 3 (b) C 2 H 5+(d) NO 2+ (a) Nucleophile (b) Electrophile (c) Nucleophile (d) Electrophile Answer

Inductive Effect Due to the difference in electronegativity between two atoms linked up by bonds, the bonding electrons will displace towards the more electronegative atom. The atom exhibits a partial negative charge. The electronic effect of a group that is transmitted by the polarization of electrons in bonds is called an inductive effect.
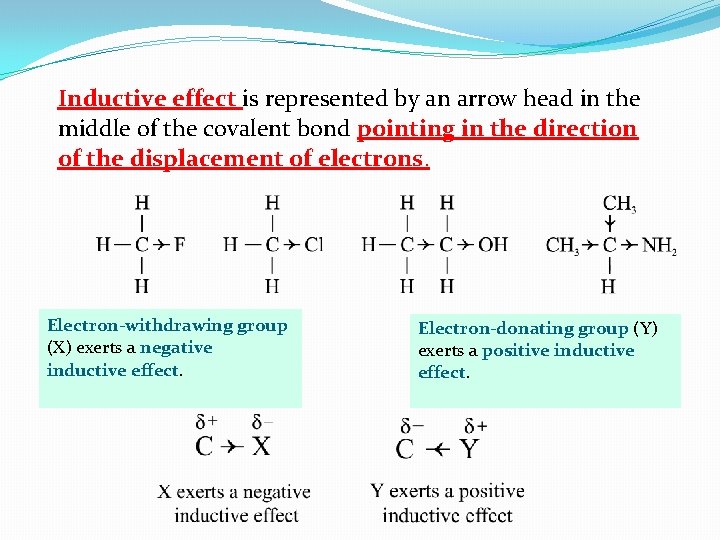
Inductive effect is represented by an arrow head in the middle of the covalent bond pointing in the direction of the displacement of electrons. Electron-withdrawing group (X) exerts a negative inductive effect. Electron-donating group (Y) exerts a positive inductive effect.
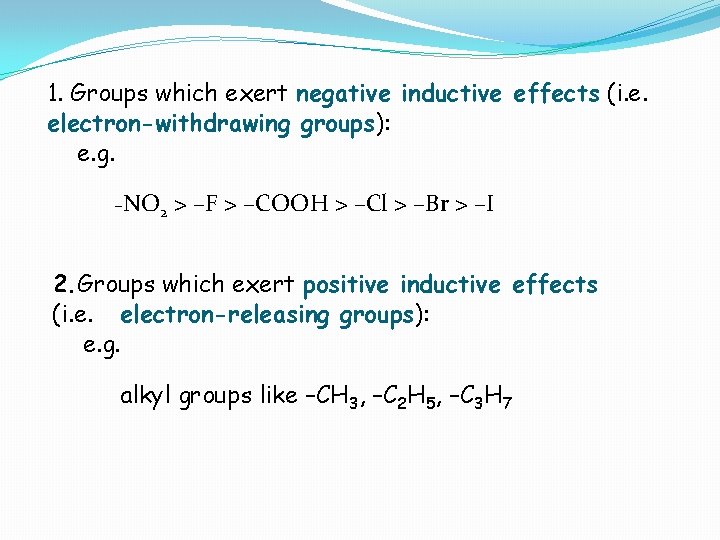
1. Groups which exert negative inductive effects (i. e. electron-withdrawing groups): e. g. –NO 2 > –F > –COOH > –Cl > –Br > –I 2. Groups which exert positive inductive effects (i. e. electron-releasing groups): e. g. alkyl groups like –CH 3, –C 2 H 5, –C 3 H 7
Functional groups Organic compounds are classified by the presence of characteristic functional groups. – A way to classify organic compounds into families. – They determine the chemical and physical properties of a compound. – They undergo the same types of chemical reactions. – A way to name organic compounds.
Functional groups �The symbol “R” is used to represent any carbon chains or rings �The Table I, shows some of the major categories, and their functional groups -
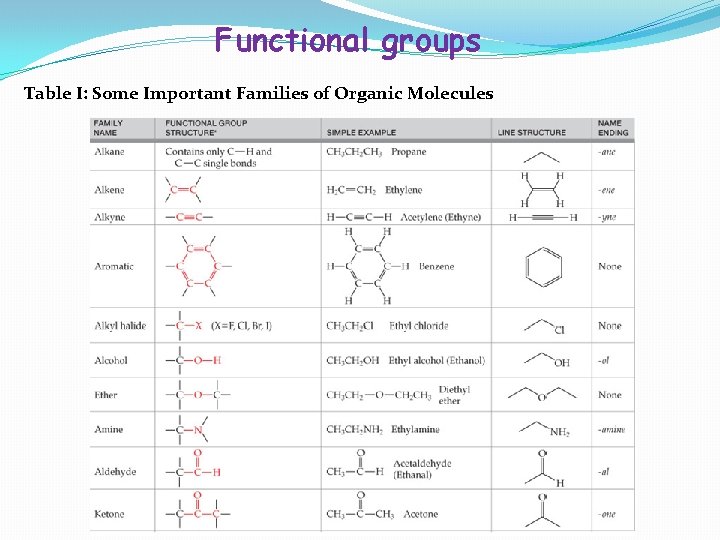
Functional groups Table I: Some Important Families of Organic Molecules

• In Table I, the first four families are hydrocarbons – organic compounds that contain only carbon and hydrogen. - Alkanes have only single bonds. - Alkenes contain a carbon-carbon double bond functional group. - Alkynes contain a all functional group. - Aromatic compounds contain a sixmembered ring of carbon atoms with three alternating double bonds.
• • In Table I, the next four families have functional groups that contain only single bonds and have a carbon atom bonded to an electronegative atom. - Alkyl halides have a carbon-halogen bond (the “halogens are” F, Cl, Br, and I); - Alcohols have a carbon-oxygen (-OH) bond; - Ethers have two carbons bonded to the same oxygen atom (R-O-R’); and - Amines have a carbon-nitrogen bond. The remaining families have functional groups that contain a carbon-oxygen double bond; aldehydes, ketones, carboxylic acids, anhydrides, esters, and amides.
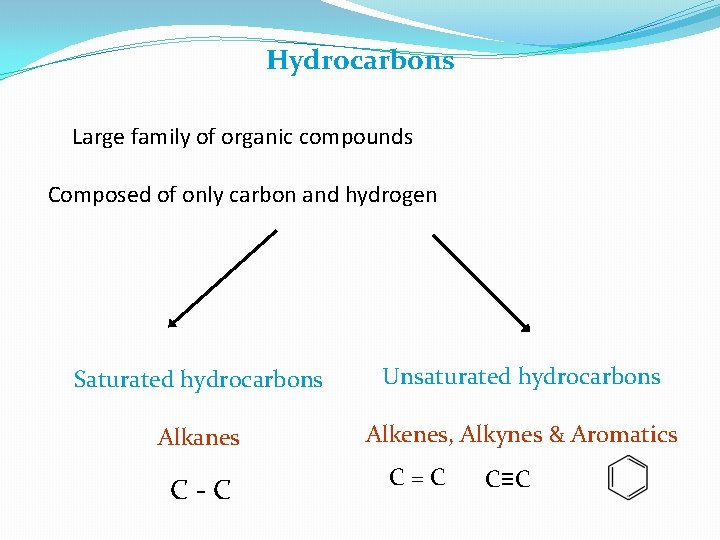
Hydrocarbons Large family of organic compounds Composed of only carbon and hydrogen Saturated hydrocarbons Unsaturated hydrocarbons Alkanes Alkenes, Alkynes & Aromatics C-C C=C C≡C
- Slides: 21