Organic Chemistry Topic 11 Hw P 197 Q
















































- Slides: 79

Organic Chemistry Topic 11 Hw P 197 Q 1 to 18 The study of Carbon and its compounds

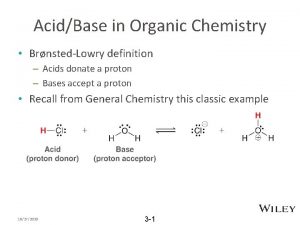
ORGANIC CHEMISTRY • The study of compounds containing Carbon atoms. • Carbon has 4 valence electrons, always draw it with 4 covalent bonds around it. • When it forms all single bonds the shape of around the carbon is TETRAHEDRAL.

Properties • Solubility – Most nonpolar (like dissolves like) – Most compounds are insoluble in water – Soluble in non-polar solvents O H O

Properties • Most are non-electrolytes – Covalent, no conductivity – (an exception is organic acids)

Properties • Low Melting/ Boiling Points

Properties • Rate of Reaction – Slower than inorganic compounds – High activation energy

Properties • Bonding – Nonpolar covalent – Carbon has 4 valance electrons- tetrahedron – Carbon can bond with itself indefinitely (in dif. shapes, many variations) Always make 4 bonds C

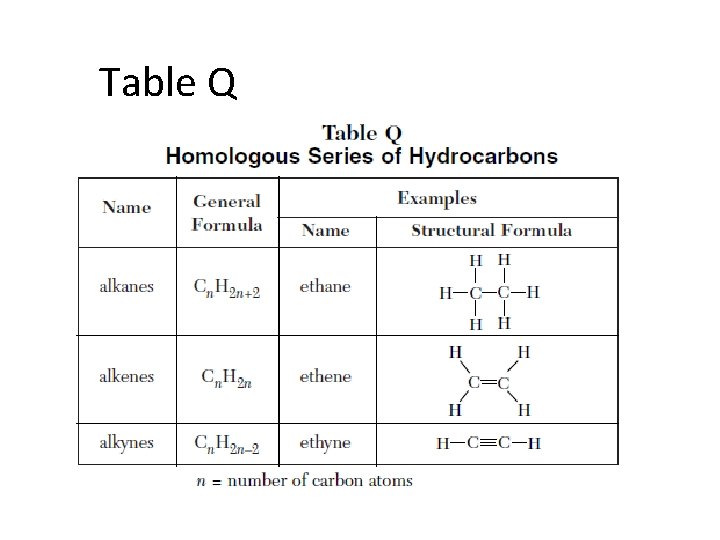
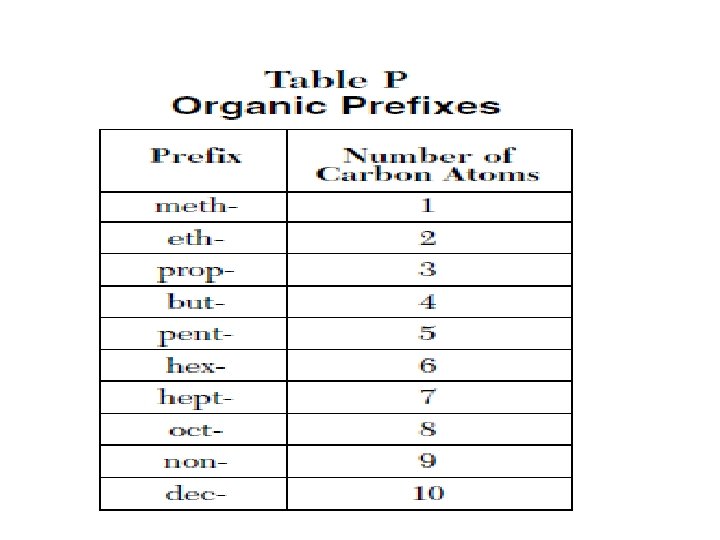
HYDROCARBONS • • • Compounds containing only C and H. 3 open chain families ALKANES ALKENES ALKYNES

Homologous Series or families Group of related compounds in which each member differs from the next by one carbon and 2 hydrogens

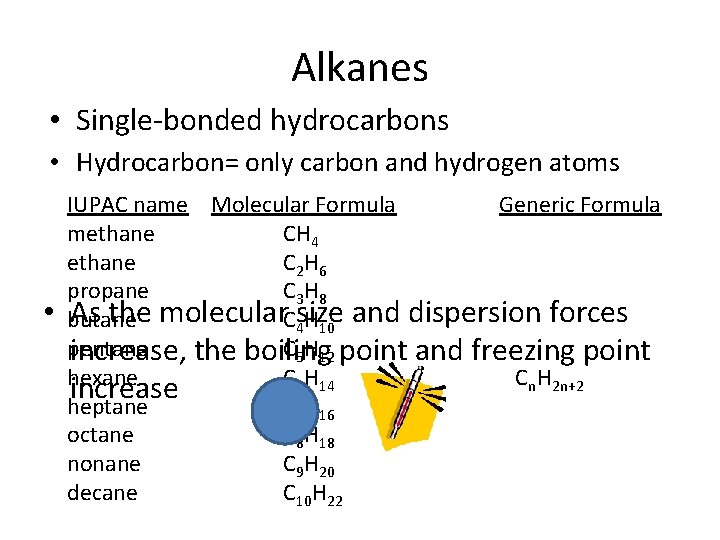
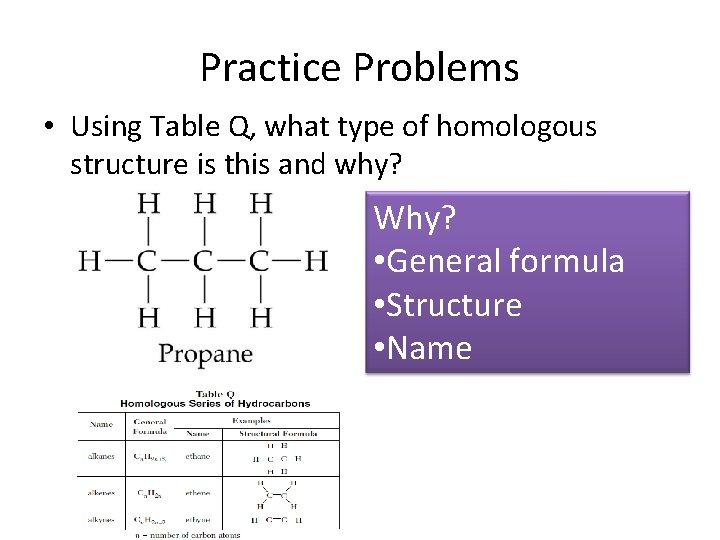
Alkanes • Single-bonded hydrocarbons • Hydrocarbon= only carbon and hydrogen atoms • IUPAC name Molecular Formula Generic Formula methane CH 4 ethane C 2 H 6 propane C 3 H 8 As the molecular size and dispersion forces butane C 4 H 10 pentane C 5 H 12 increase, the boiling point and freezing point hexane C 6 H 14 Cn. H 2 n+2 increase heptane C 7 H 16 octane C 8 H 18 nonane C 9 H 20 decane C 10 H 22

Table Q


Alkenes • have one Double-bonded hydrocarbon • Unsaturated • Same prefix as alkanes, with suffix -ene Dien e doub s contai n alke le bonds TWO nes! , and are not

Alkynes • one Triple-bonded hydrocarbon • Unsaturated • Same prefix as alkanes, and alkenes, with suffix yne

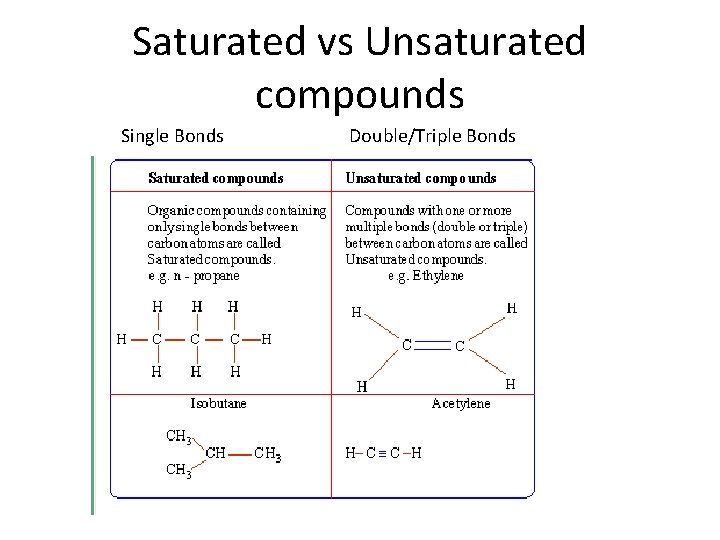
Saturated vs Unsaturated compounds Single Bonds Double/Triple Bonds

Cyclic Hydrocarbons Benzene • 6 carbon atoms in a ring Aromatic Hydrocarbon Only structure really needs to be known for the regents.

May 30 • How to draw and name different organic compounds? • ISOMERS • HW : RB PG 198 Q 19 TO 33

Isomers Although these structures look • Compounds with: different, they both have the same • the same molecular formulas molecular formula of C • different structural formulas 4 H 10 C 4 H 10 Normal Butane 2 -Methyl Propane

ISOMERS • Same molecular formula but different structural formula. Have different chemical and physical properties.

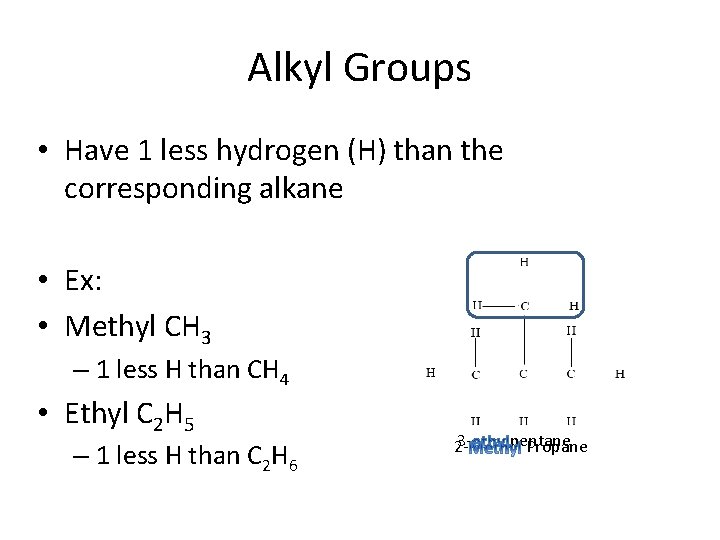
Alkyl Groups • Have 1 less hydrogen (H) than the corresponding alkane • Ex: • Methyl CH 3 – 1 less H than CH 4 • Ethyl C 2 H 5 – 1 less H than C 2 H 6 32 - pentane Propane

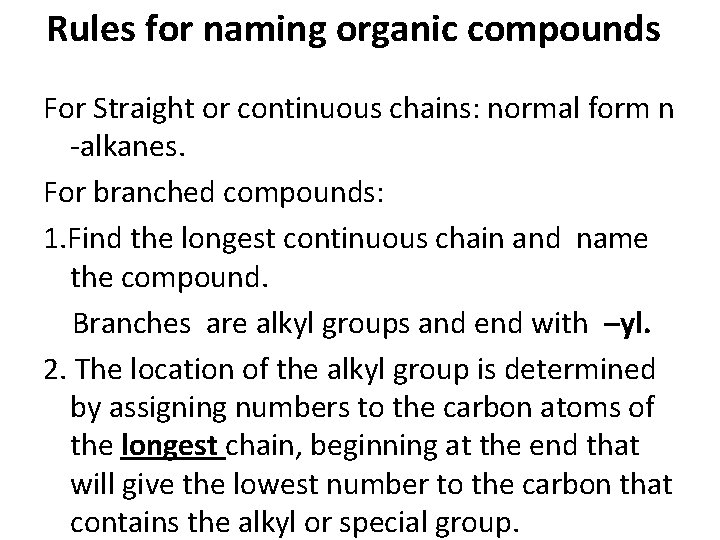
Rules for naming organic compounds For Straight or continuous chains: normal form n -alkanes. For branched compounds: 1. Find the longest continuous chain and name the compound. Branches are alkyl groups and end with –yl. 2. The location of the alkyl group is determined by assigning numbers to the carbon atoms of the longest chain, beginning at the end that will give the lowest number to the carbon that contains the alkyl or special group.

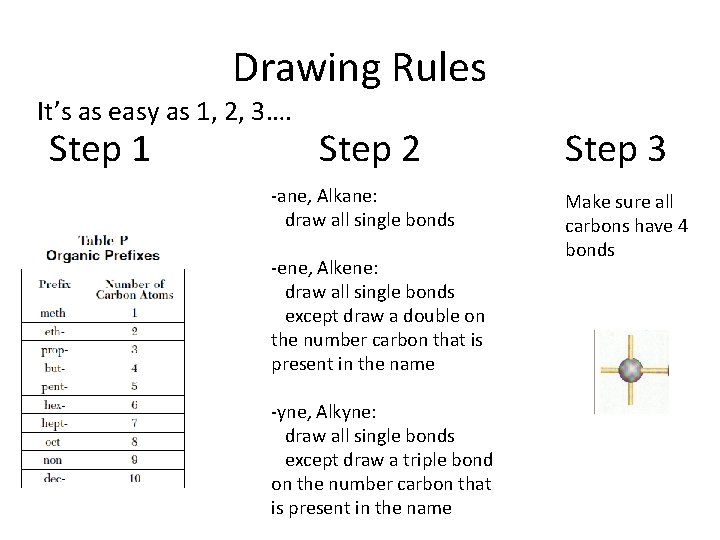
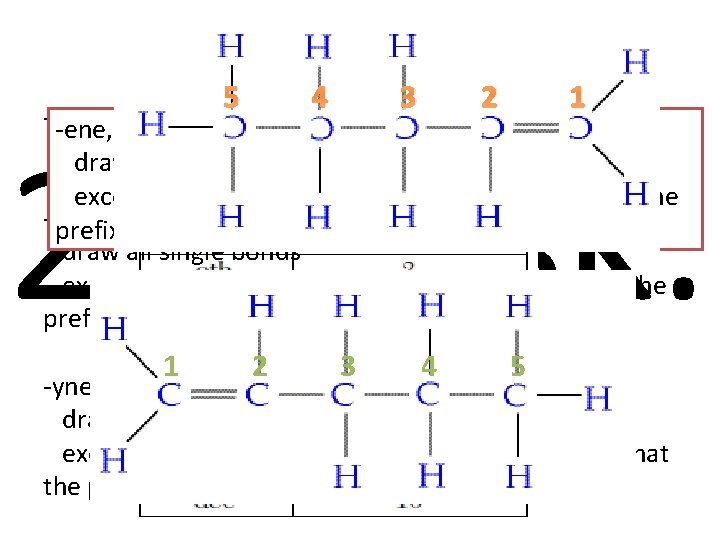
Drawing Rules It’s as easy as 1, 2, 3…. Step 1 Step 2 -ane, Alkane: draw all single bonds -ene, Alkene: draw all single bonds except draw a double on the number carbon that is present in the name -yne, Alkyne: draw all single bonds except draw a triple bond on the number carbon that is present in the name Step 3 Make sure all carbons have 4 bonds

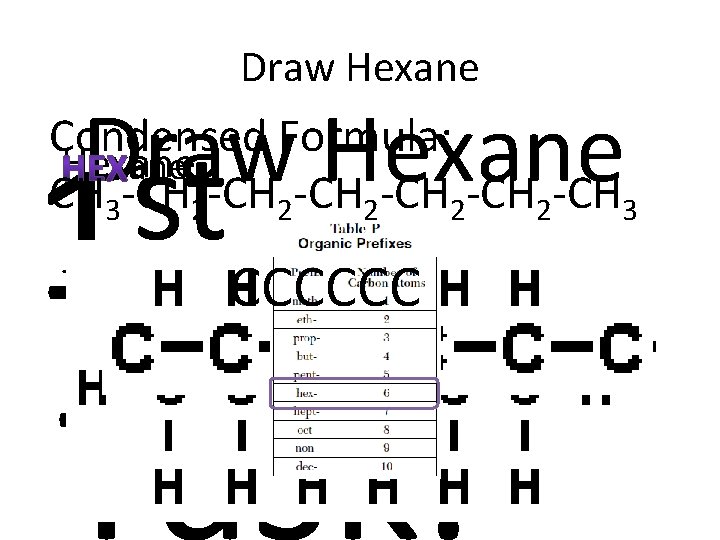
Draw Hexane Condensed Formula: Hexane CH 3 -CH 2 -CH 2 -CH 3 st 1 -ane = only single bonds CCCCCC Task:

Draw 1 -Pentene 5 4 3 -ane, Alkane: -ene, Alkene: draw all single bonds 2 1 nd 2 Task: except draw a double on the number carbon that the -ene, Alkene: prefix shows draw all single bonds except draw a double on the number carbon that the prefix shows 1 2 3 4 5 -yne, Alkyne: draw all single bonds except draw a triple bond on the number carbon that the prefix shows

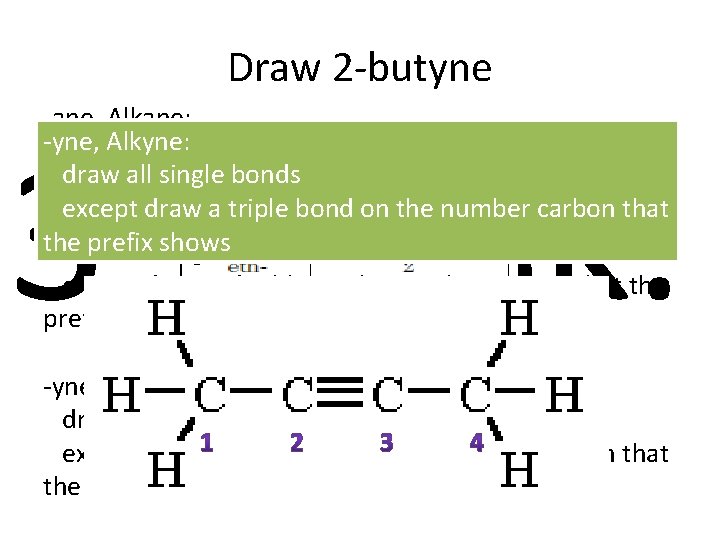
Draw 2 -butyne -ane, Alkane: -yne, Alkyne: draw all single bonds except draw a triple bond on the number carbon that -ene, Alkene: the prefix shows draw all single bonds rd 3 Task: except draw a double on the number carbon that the prefix shows -yne, Alkyne: draw all single bonds except draw a triple bond on the number carbon that the prefix shows

Draw: 2, 3 -dimethylbutane

Practice Problems • Using Table Q, what type of homologous structure is this and why? Why? • General formula • Structure • Name

May 31 • Objective : Functional groups. • How to distinguish them and what do they do to an organic compound? • Table R • HW P 204 Q 34 TO 48

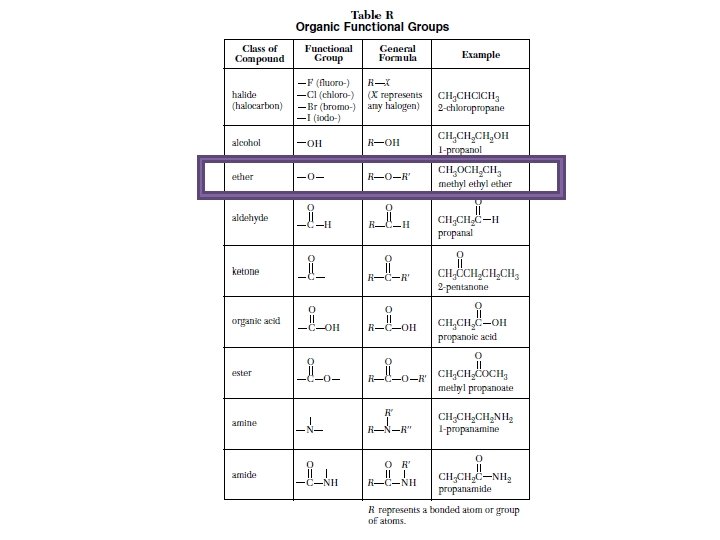
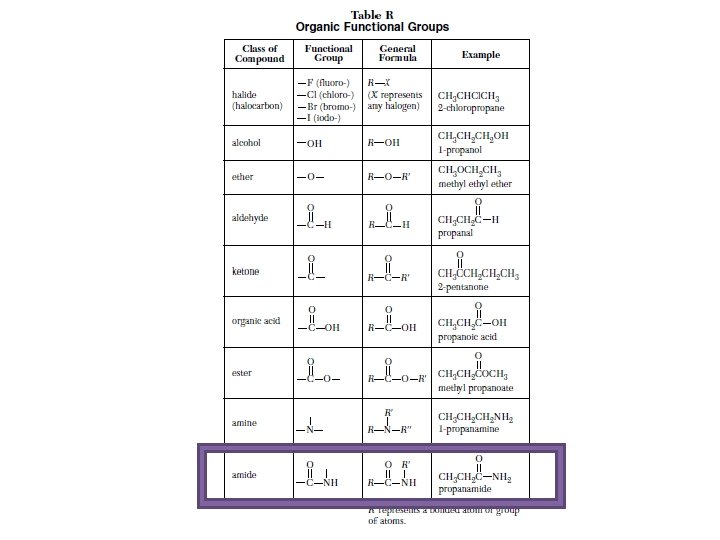
Table R Organic compounds and their functional groups


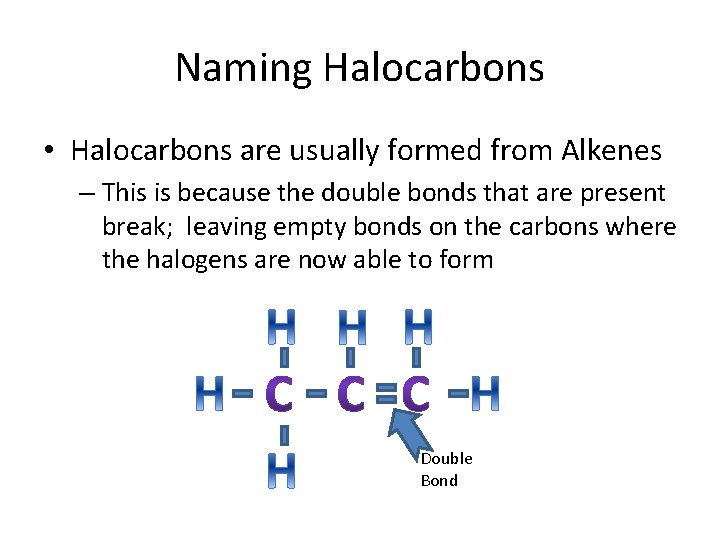
Halides (Halocarbons) • A halide is formed when one or more halogen elements attach themselves to a chain of carbons atoms • Halogen include all the elements in group 17

Naming Halocarbons • Halocarbons are usually formed from Alkenes – This is because the double bonds that are present break; leaving empty bonds on the carbons where the halogens are now able to form Double Bond

Naming Halocarbons • Every halogen has its own prefix to put at the beginning of its name 1, 2 -Di Fluoro propane – It is listed in Table R • When the bonds brake; the halogens fill the empty space F F


Alcohols • Contain 1 less Hydrogen and in its place there is an –OH group instead. • Even though alcohols have an –OH group, they are not a considered a base. – This is because there are covalent bonds holding the –OH to the carbons and bases don’t have covalent bonds present on the –OH. – When in solution, acids only release and H+ and bases release OH- O

Naming Alcohols • • You start with Alkane. (In this case, Methane) Take away one of the Hydrogen atoms. Add an –OH group to the empty space For the name; drop the –e at the end of the prefix (Methane) and add –ol to name the Alcohol! O Ethanol


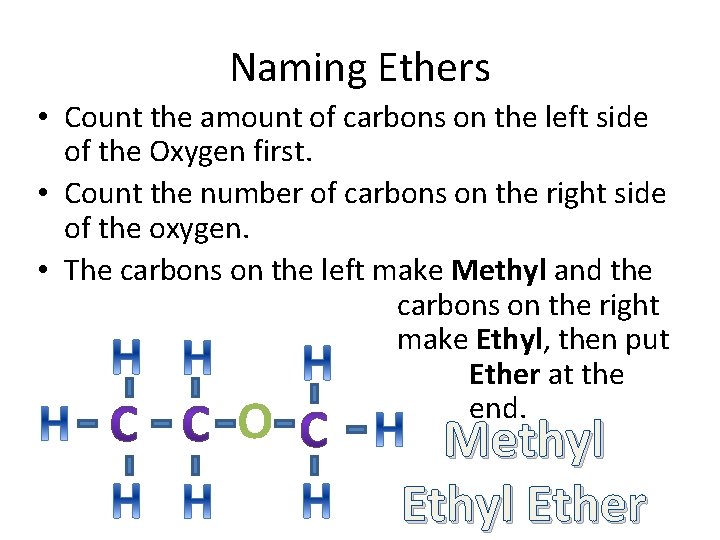
Ether • In an ether, there is always an oxygen atom in between two carbons. • And there can be any number of carbons on each side of the oxygen. O

Naming Ethers • Count the amount of carbons on the left side of the Oxygen first. • Count the number of carbons on the right side of the oxygen. • The carbons on the left make Methyl and the carbons on the right make Ethyl, then put Ether at the end. O Methyl Ether


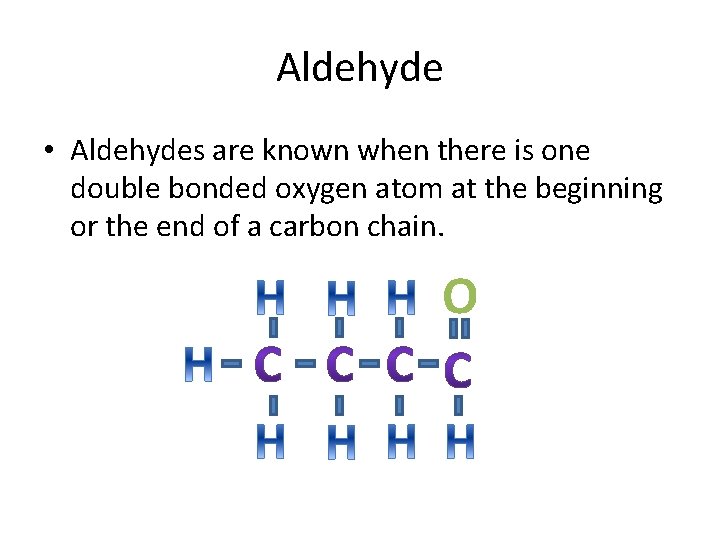
Aldehyde • Aldehydes are known when there is one double bonded oxygen atom at the beginning or the end of a carbon chain. O

Forming Aldehydes • Start with a carbon chain (butane). • Drop off two Hydrogen atoms. • Add a double bonded oxygen to the open carbon. O

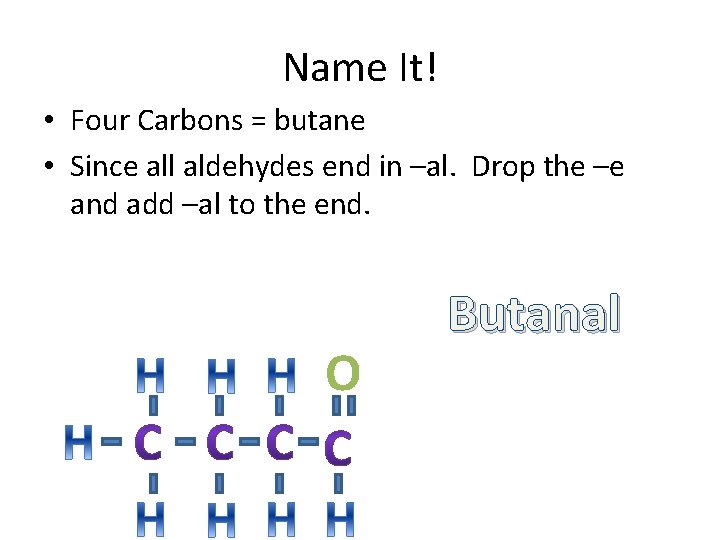
Name It! • Four Carbons = butane • Since all aldehydes end in –al. Drop the –e and add –al to the end. O Butanal


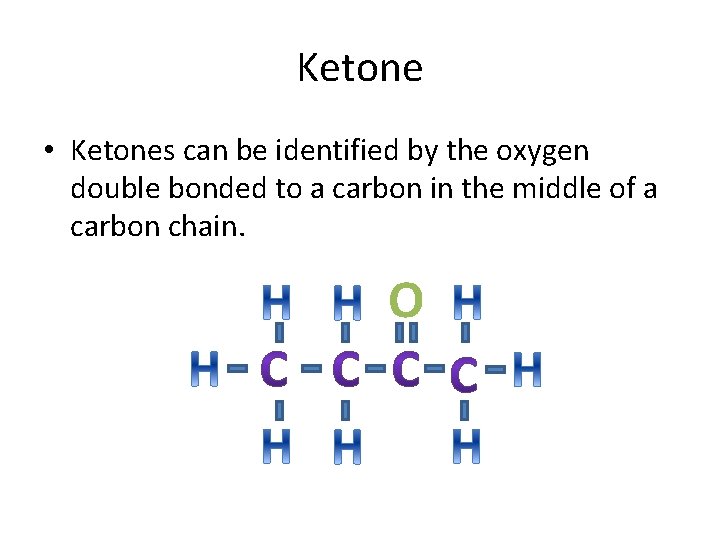
Ketone • Ketones can be identified by the oxygen double bonded to a carbon in the middle of a carbon chain. O

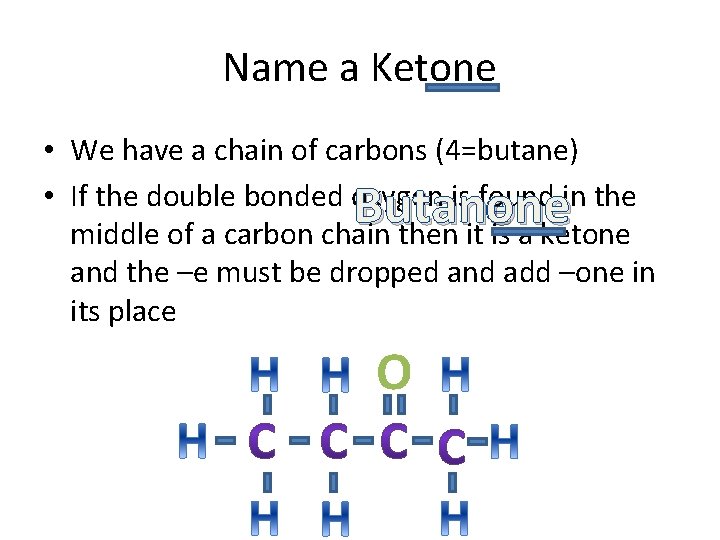
Name a Ketone • We have a chain of carbons (4=butane) • If the double bonded oxygen is found in the middle of a carbon chain then it is a ketone and the –e must be dropped and add –one in its place Butaneone O


Organic Acids!!!!!! • Contains a double bonded oxygen and an –OH to the last carbon in the chain • Called acids because H+ ions are released when dissolved in water • Since ions are present when dissolved, an electric current can be conducted through the water • Organic acids are electrolytes! O O

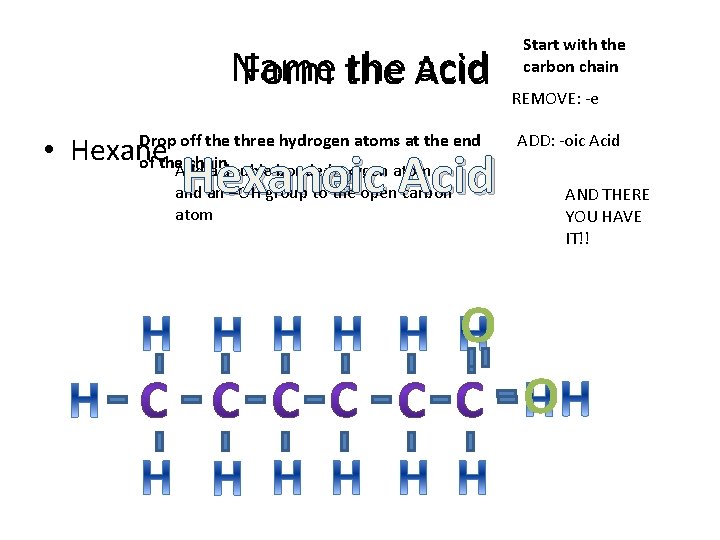
Name the acid Form the Acid Drop off the three hydrogen atoms at the end • Hexane of the chain Hexanoic e Acid Start with the carbon chain REMOVE: -e ADD: -oic Acid Add a double bonded oxygen atom and an –OH group to the open carbon atom AND THERE YOU HAVE IT!! O O


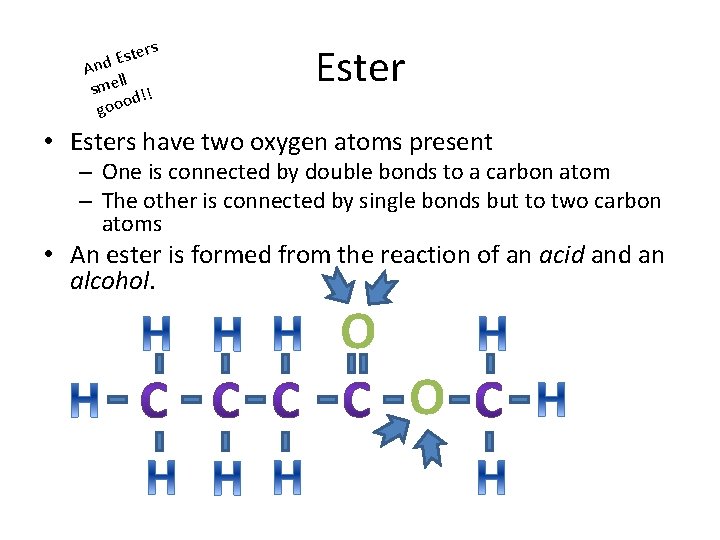
ters s E And ll sme !! d gooo Ester • Esters have two oxygen atoms present – One is connected by double bonds to a carbon atom – The other is connected by single bonds but to two carbon atoms • An ester is formed from the reaction of an acid an alcohol. O O

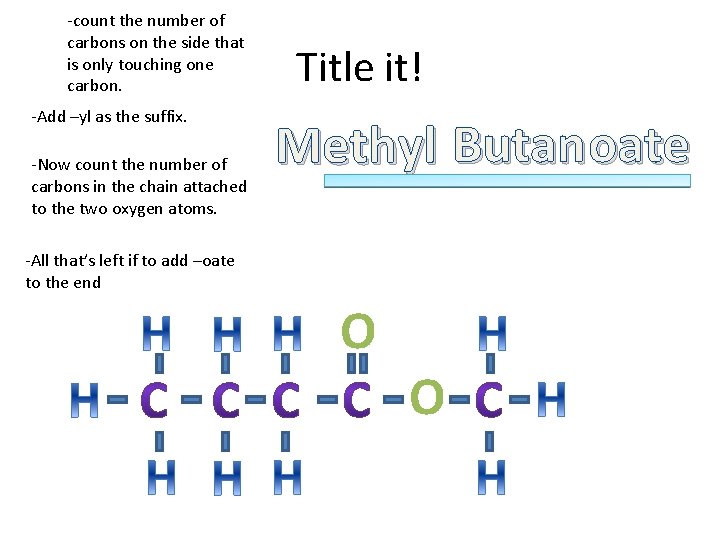
-count the number of carbons on the side that is only touching one carbon. -Add –yl as the suffix. -Now count the number of carbons in the chain attached to the two oxygen atoms. Title it! Methyl Butan oate -All that’s left if to add –oate to the end O O


Amine • Amines contain a nitrogen atom N – the nitrogen atom is found at the end of a carbon chain; attached to one carbon as well as two hydrogen. N

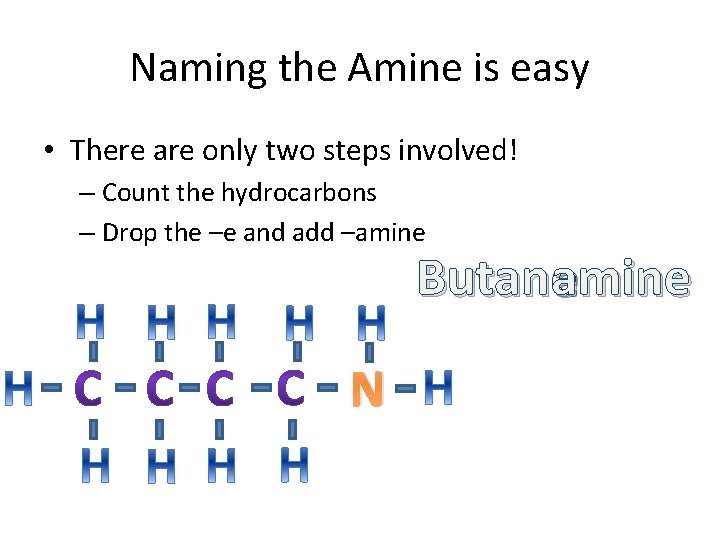
Naming the Amine is easy • There are only two steps involved! – Count the hydrocarbons – Drop the –e and add –amine Butaneamine N


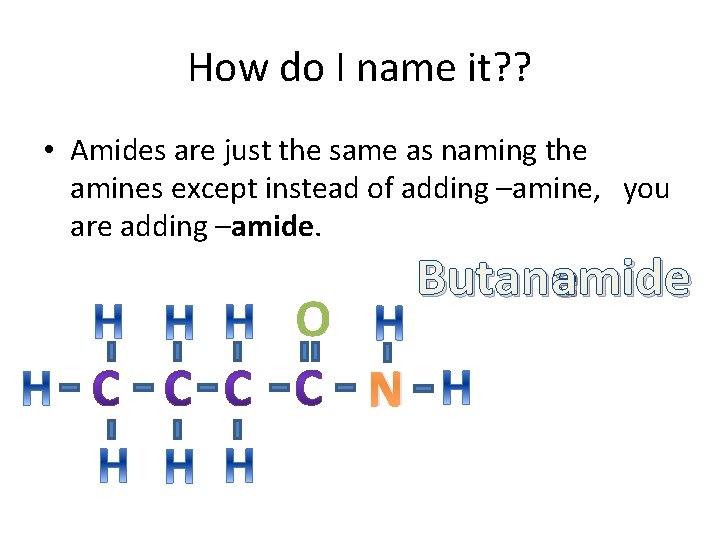
Amide • Amides also contains a Nitrogen atom but attached to the same carbon is a double bonded oxygen. O N

How do I name it? ? • Amides are just the same as naming the amines except instead of adding –amine, you are adding –amide. Butaneamide O N

Amino Acids

Amino Acids… • Contains both an amine and an organic acid Organic Acid O O N Amine

Hmmmm……. Where are the Amino Acids? ?

NOTE: All the example on how the groups are named are shown on table R in the far right column. Note: The formulas on how each group is drawn is shown in the formula column.

June 1 • • OBJECTIVE: ORGANIC REACTIONS P 208 q 49 to 61 TAKE HOME TEST P 210 ANSWERS IN SCANTRON Q 1 TO 30

Organic Reactions

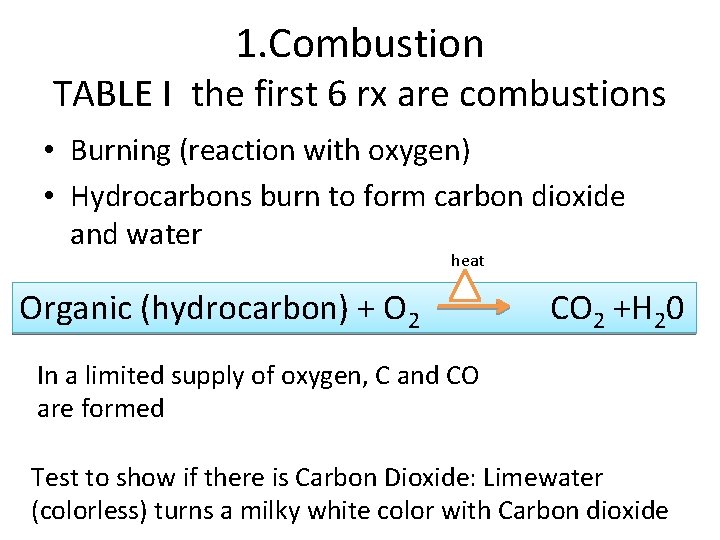
1. Combustion TABLE I the first 6 rx are combustions • Burning (reaction with oxygen) • Hydrocarbons burn to form carbon dioxide and water heat Organic (hydrocarbon) + O 2 CO 2 +H 20 In a limited supply of oxygen, C and CO are formed Test to show if there is Carbon Dioxide: Limewater (colorless) turns a milky white color with Carbon dioxide

2. Substitution • Replacement of one or more hydrogens in a saturated hydrocarbon by an halogen. Alkane + halogen(X 2) halocarbon + HX(g) + F 2 F + HF

3. Addition • Adding one or more atoms at a double/ triple bond. Could be Hydrogenation (add H) Or Halogenation (add halogens) For alkenes and alkynes! + F 2 H H F F

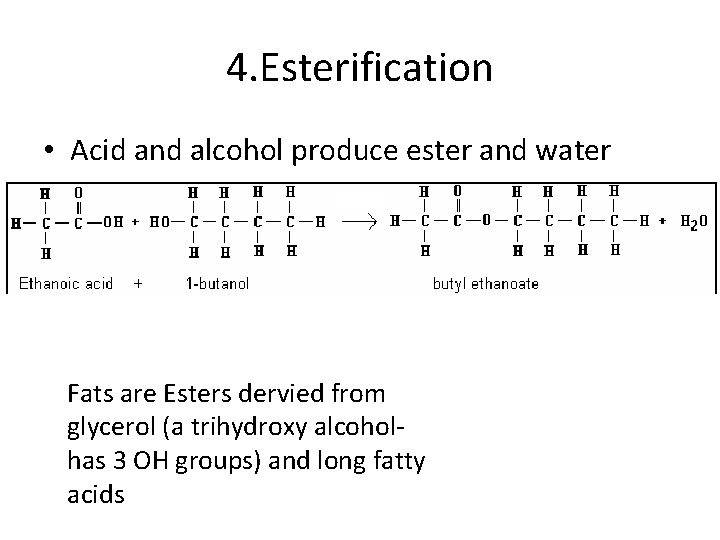
4. Esterification • Acid and alcohol produce ester and water Fats are Esters dervied from glycerol (a trihydroxy alcohol- has 3 OH groups) and long fatty acids

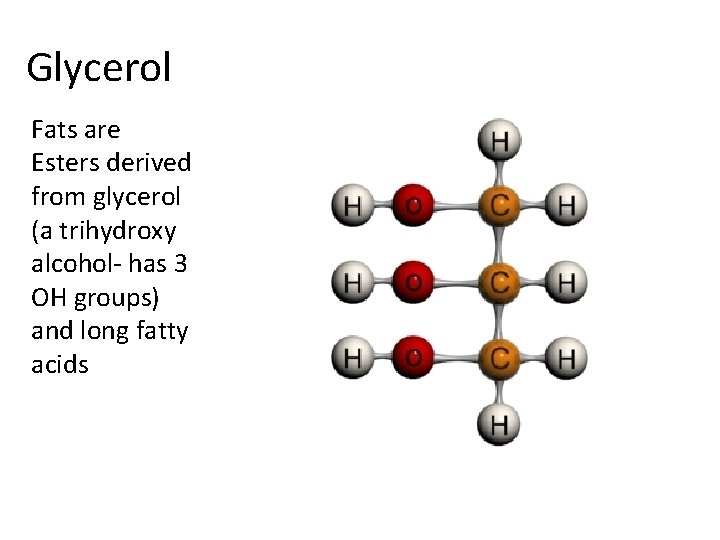
Glycerol Fats are Esters derived from glycerol (a trihydroxy alcohol- has 3 OH groups) and long fatty acids

5. Saponification • (hydrolysis) Ester breaks up into Acid and Alcohol (reverse of esterification) • Produces soap Fat + Strong Base Soap + Glycerol

6. Fermentation Zymase (enzyme) C 6 H 12 O 6 -------> 2 C 2 H 5 OH + 2 CO 2 Glucose Ethanol Carbon Dioxide

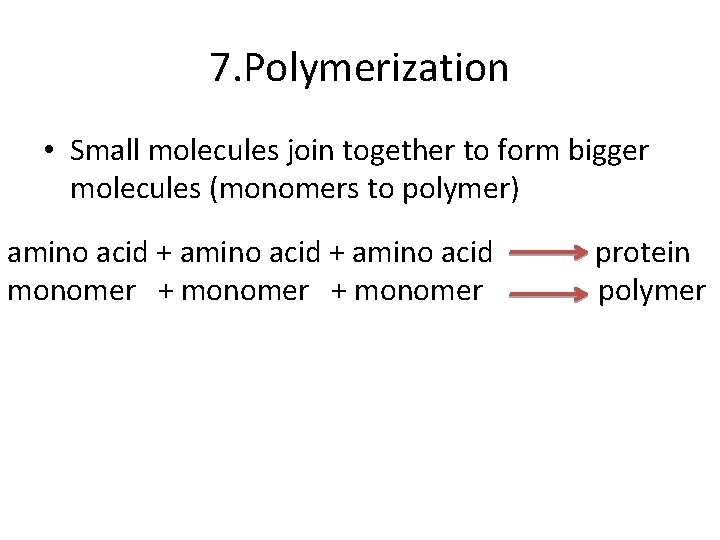
7 POLYMERIZATION • Polymers are made of chains of smaller units called MONOMERS • NATURAL POLYMERS • Protein, starches, cellulose • SYNTHETIC POLYMERS • Nylon, rayon, polyethylene

7. Polymerization • Small molecules join together to form bigger molecules (monomers to polymer) amino acid + amino acid protein monomer + monomer polymer

Polymerization • 2 Types: • Condensation Polymerization: – Dehydration synthesis, occur when water is remove from primary alcohols. Have ether or ether linkages. – Make water and polymer Natural • Protein (DNA) • Starch • cellulose Artificial • Nylon • Polyester • silicone

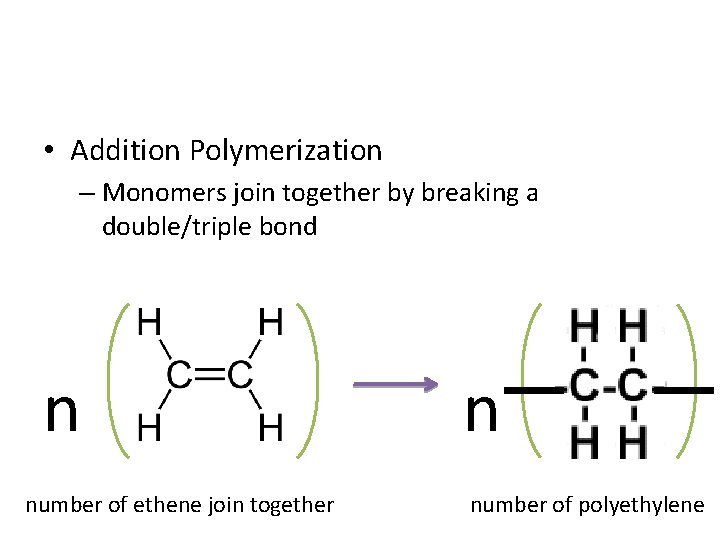
• Addition Polymerization – Monomers join together by breaking a double/triple bond n n number of ethene join together number of polyethylene

Finding missing reactants and products in Organic Reactions • # of atoms on the left side of the arrow must equal # on the right • After the elements/compounds are correctly written, change the coefficient • Ex: • C 2 H 6 + Cl 2 C 2 H 5 Cl + ______ HCl

Practice Regents Questions • Go online to regentsprep. org! • http: //regentsprep. org/Regents/core/questio ns/questions. cfm? Course=CHEM&Topic. Code= 06

• http: //www. chemguide. co. uk/basicorg/conventions/n ames. html#top/draw. html#top • http: //www. chemguide. co. uk/basicorg/conventions/n ames 2. html#top • http: //www. chemguide. co. uk/orgpropsmenu. html#top • http: //www. chalkbored. com/lessons/chemistry 11/unit-5 -answers-handout. pdf • http: //www. chalkbored. com/lessons/chemistry 11/hydrocarbon-nomenclature-handout. pdf

• http: //reviewgamezone. com/game. php? id=66 9 • http: //www. youtube. com/watch? v=1 q. GPWd m 2 Ml. I
 Topic 11 organic chemistry
Topic 11 organic chemistry Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Nonene
Nonene Meth eth
Meth eth Organic synthesis via enolates
Organic synthesis via enolates Hybridisation
Hybridisation Leveling effect organic chemistry
Leveling effect organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s What is organic chemistry like
What is organic chemistry like Organic chemistry
Organic chemistry Cracking organic chemistry
Cracking organic chemistry Numbering carbon chains
Numbering carbon chains Organic chemistry
Organic chemistry Objective lab report example
Objective lab report example Conformation of sugars ppt
Conformation of sugars ppt Radicals
Radicals Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry nomenclature
Organic chemistry nomenclature Functional groups table class 10
Functional groups table class 10 Separation scheme of caffeine from vivarin tablets
Separation scheme of caffeine from vivarin tablets Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? High resolution
High resolution Organic chemistry
Organic chemistry Chemistry mind map
Chemistry mind map Intro to organic chemistry
Intro to organic chemistry Analytical chemistry chapters
Analytical chemistry chapters Ferrox paper test for oxygen
Ferrox paper test for oxygen Organic chemistry
Organic chemistry Condensed structural formula
Condensed structural formula How to calculate percent yield in organic chemistry
How to calculate percent yield in organic chemistry Wiley
Wiley Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Neon organic or inorganic
Neon organic or inorganic Conjugation organic chemistry
Conjugation organic chemistry Eth meth prop but pent
Eth meth prop but pent Canola oil
Canola oil Organic chemistry conversion chart
Organic chemistry conversion chart Organic chemistry
Organic chemistry Functional group
Functional group Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Organic chemistry case studies
Organic chemistry case studies Chemistry organic
Chemistry organic A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Ee organic chemistry
Ee organic chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Halohydrin formation
Halohydrin formation Hono organic chemistry
Hono organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis John wiley & sons, inc.
John wiley & sons, inc. Ethos
Ethos Brooklyn college organic chemistry
Brooklyn college organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Organic chemistry william h brown
Organic chemistry william h brown Iodine test for starch
Iodine test for starch Polarimetry organic chemistry
Polarimetry organic chemistry David klein
David klein Nbs chemistry
Nbs chemistry Is alkane an organic compound
Is alkane an organic compound Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry
Organic chemistry Organic chemistry myanmar
Organic chemistry myanmar Ester organic chemistry
Ester organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Chapter 7 organic chemistry
Chapter 7 organic chemistry Organic chemistry class 11 notes
Organic chemistry class 11 notes Rancidity meaning
Rancidity meaning Father of organic chemistry
Father of organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Alkene alcohol naming
Alkene alcohol naming Structural isomers of hexane
Structural isomers of hexane Meth eth prop chart
Meth eth prop chart Organic chemistry cheat sheet
Organic chemistry cheat sheet Bu organic chemistry
Bu organic chemistry