Organic Chemistry The study of carboncontaining compounds and

Organic Chemistry The study of carbon-containing compounds and their properties. The vast majority of organic compounds contain chains or rings of carbon atoms.

Hydrocarbons. . . compounds composed of carbon and hydrogen. Saturated: carbon-carbon bonds are all single alkanes [Cn. H 2 n+2]

Hydrocarbons (continued) Unsaturated: contains carbon-carbon multiple bonds.

Rules for Naming Alkanes 1. For alkanes beyond butane, add -ane to the Greek root for the number of carbons. C-C-C-C = hexane 2. Alkyl substituents: drop the -ane and add -yl. -C 2 H 5 is ethyl

Rules for Naming Alkanes 3. Positions of substituent groups are specified by numbering the longest chain sequentially. C C-C-C-C 3 -methylhexane 4. Location and name are followed by root alkane name. Substituents in alphabetical order and use di-, tri-, etc.

Examples of Alkane Naming

Examples of Alkane Naming
Structural Isomers
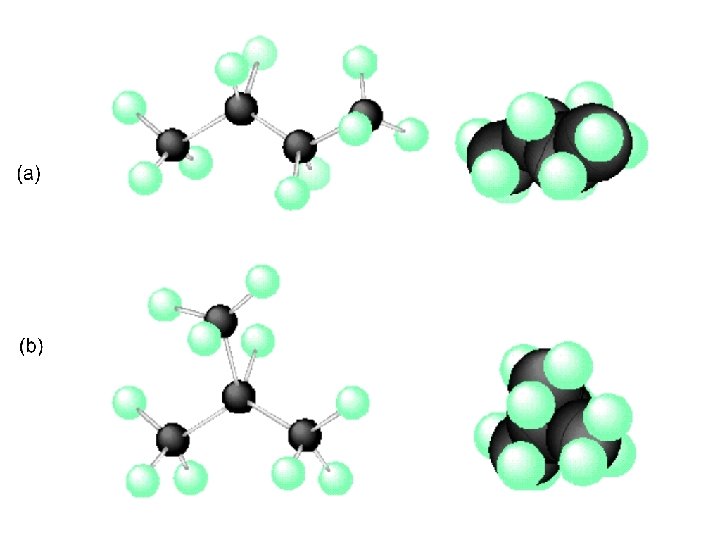

Examples of Isomers
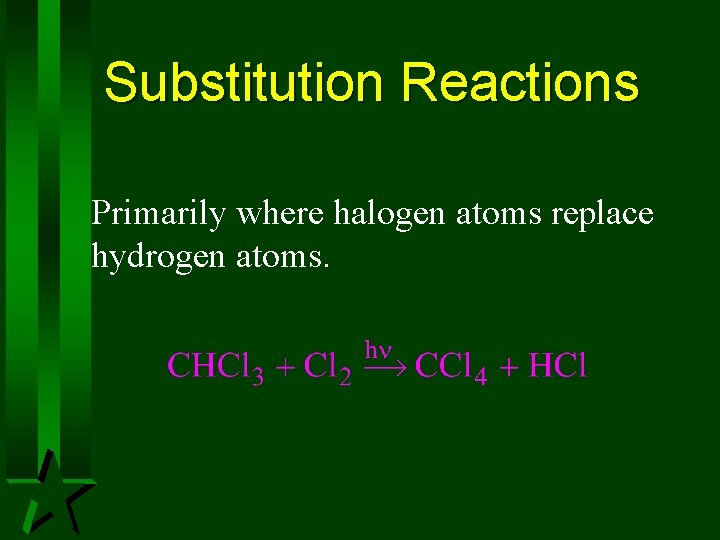
Substitution Reactions Primarily where halogen atoms replace hydrogen atoms.

Naming Alkyl Halides Named the same way as alkanes, but the halogen gets priority numbering.

Cyclic Alkanes Carbon atoms can form rings containing only carbon-carbon single bonds. C 3 H 6, C 4 H 8, C 6 H 12
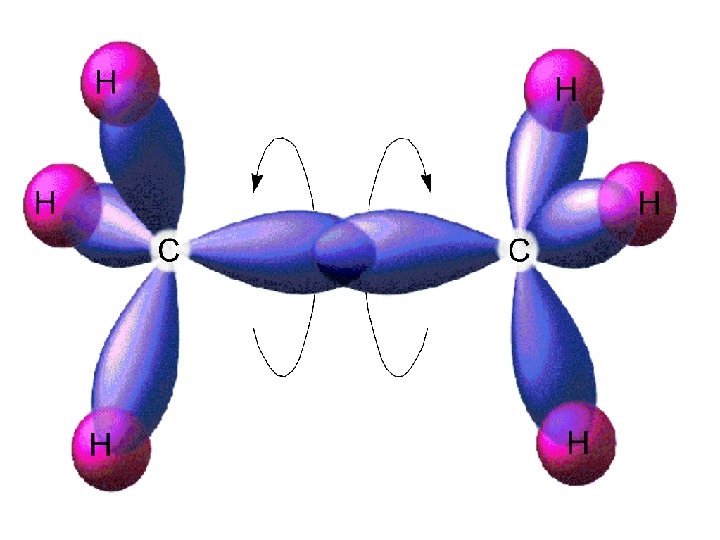
22_499 C No "head-on" overlap of atomic orbitals 109. 5° 60° C (a) (b) C

Naming Cyclic Alkanes

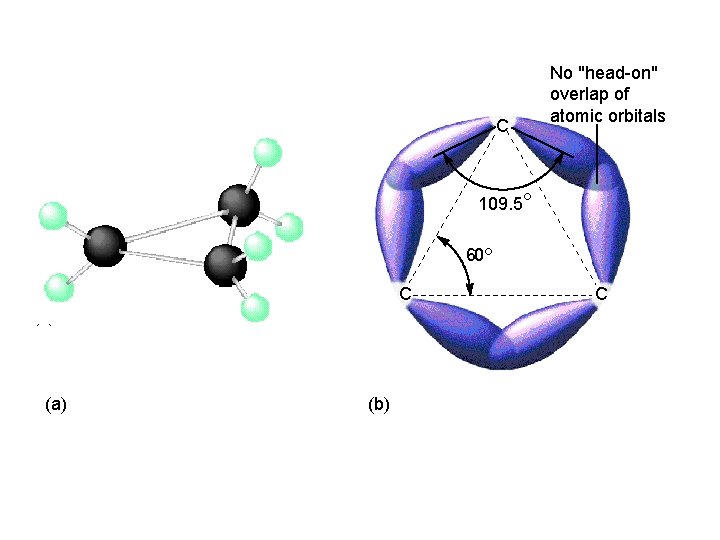
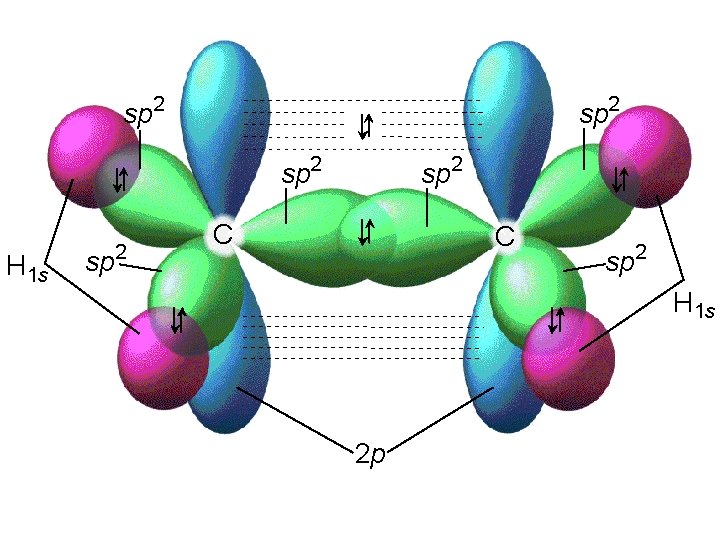
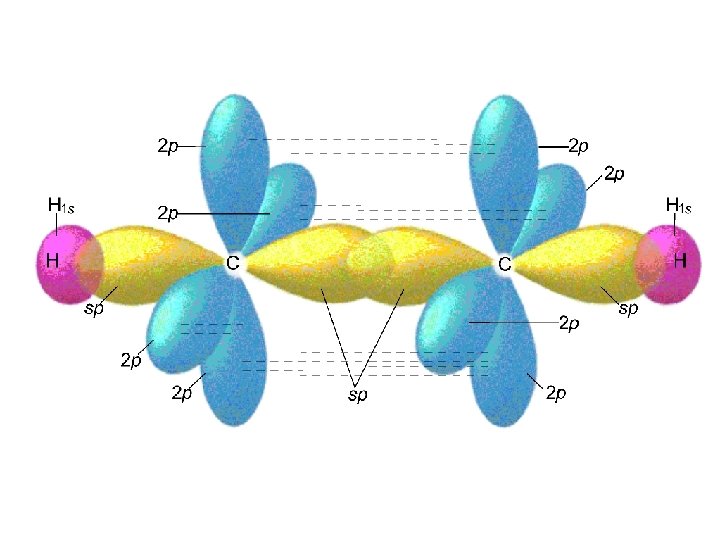
Alkenes and Alkynes Alkenes: hydrocarbons that contain a carbon double bond. [Cn. H 2 n] C C=C propene Alkynes: hydrocarbons containing a carbon triple bond. C C C 2 -pentyne
22_501 sp 2 H 1 s sp 2 C C sp 2 H 1 s 2 p

Nomenclature for Alkenes 1. Root hydrocarbon name ends in -ene C 2 H 4 is ethene 2. With more than 3 carbons, double bond is indicated by the lowest numbered carbon atom in the bond. C=C C C is 1 -butene

Naming Unsaturated HC’s
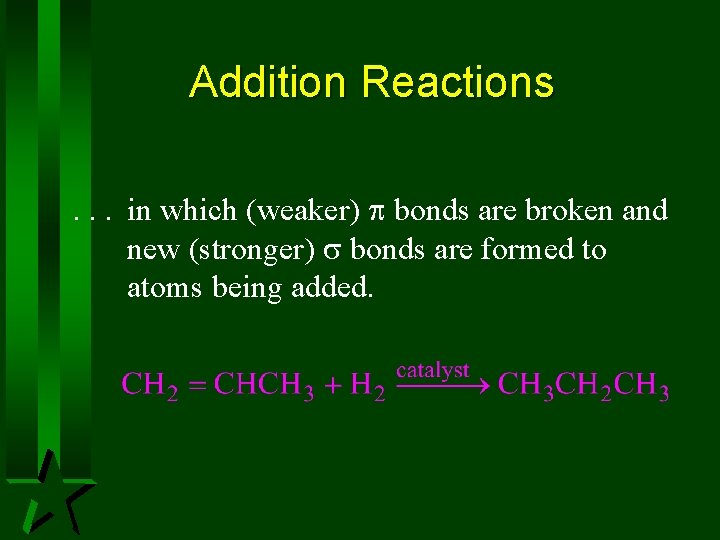
Addition Reactions. . . in which (weaker) bonds are broken and new (stronger) bonds are formed to atoms being added.
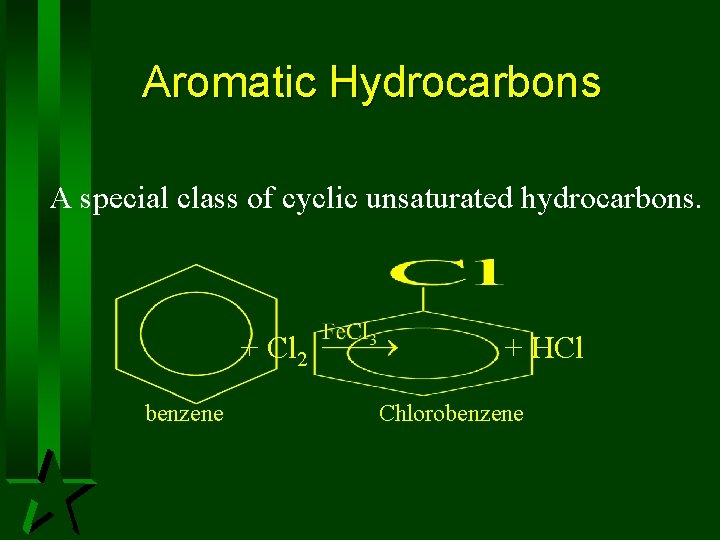
Aromatic Hydrocarbons A special class of cyclic unsaturated hydrocarbons. + Cl 2 benzene + HCl Chlorobenzene

Naming Aromatic HC’s
Refinery Processes Cracking: large molecules broken down to smaller ones by breaking carbon-carbon bonds. Pyrolysis (thermal cracking): The process that produces cracking at high temperatures. Catalytic Cracking: Cracking at lower temperatures. Catalytic reforming: Alkanes and cycloalkanes converted to aromatic compounds.
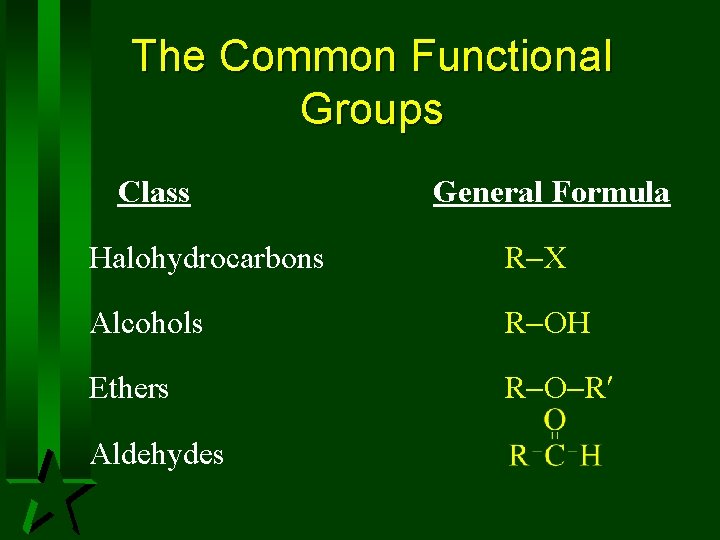
The Common Functional Groups Class General Formula Halohydrocarbons R X Alcohols R OH Ethers R O R Aldehydes

The Common Functional Groups Class General Formula Ketones Carboxylic Acids Esters Amines R NH 2

Naming Hydrocarbons with Functional Groups

Naming Hydrocarbons with Functional Groups
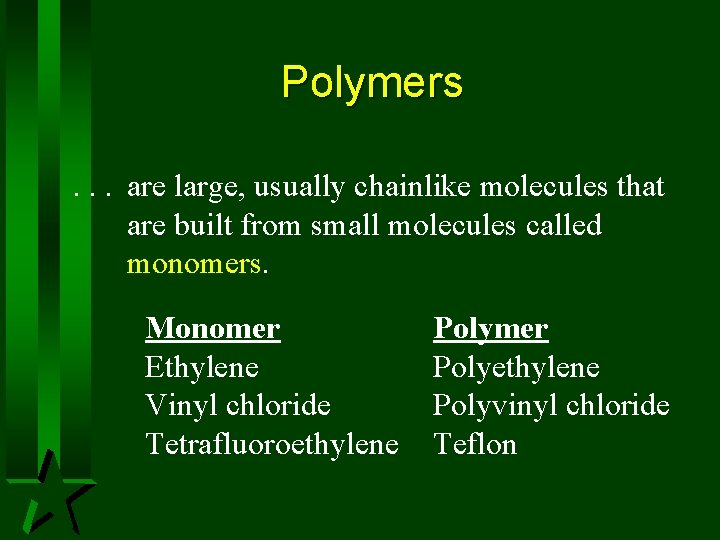
Polymers. . . are large, usually chainlike molecules that are built from small molecules called monomers. Monomer Ethylene Vinyl chloride Tetrafluoroethylene Polymer Polyethylene Polyvinyl chloride Teflon
Types of Polymerization Addition Polymerization: monomers “add together” to form the polymer, with no other products. (Teflon) Condensation Polymerization: A small molecule, such as water, is formed for each extension of the polymer chain. (Nylon)
Biochemistry The study of the chemistry of living things.
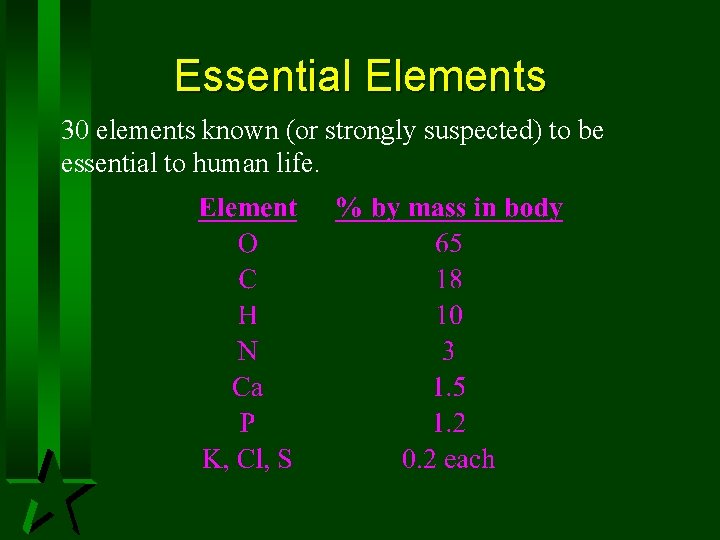
Essential Elements 30 elements known (or strongly suspected) to be essential to human life.
Proteins Natural polymers made up of -amino acids (molecular weight from 6000 to >1, 000 g/mol). Fibrous Proteins: provide structural integrity and strength to muscle, hair and cartilage.

Proteins (continued) Globular Proteins: - roughly spherical shape - transport and store oxygen and nutrients - act as catalysts - fight invasion by foreign objects - participate in the body’s regulatory system - transport electrons in metabolism
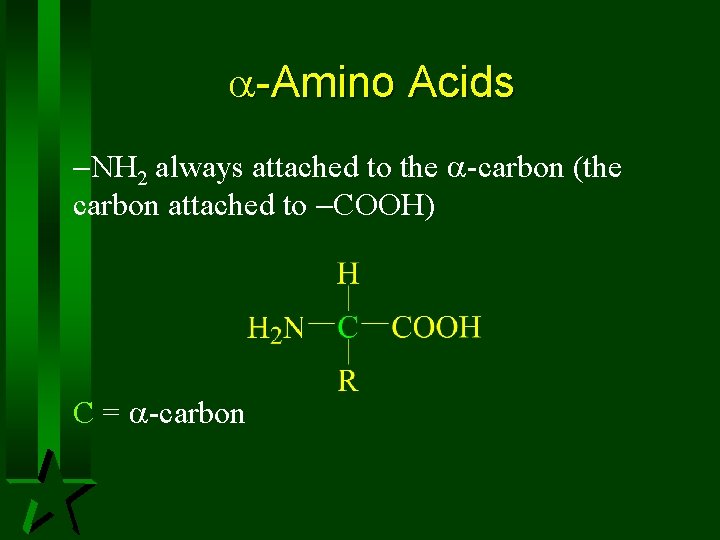
-Amino Acids NH 2 always attached to the -carbon (the carbon attached to COOH) C = -carbon
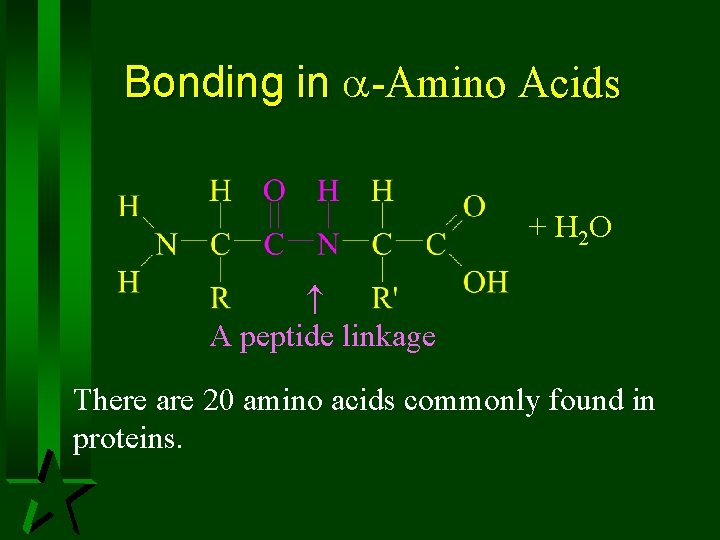
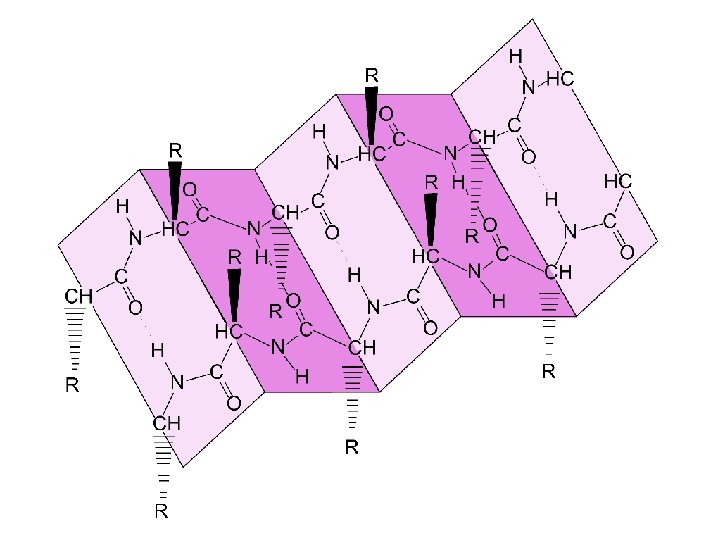
Bonding in -Amino Acids + H 2 O A peptide linkage There are 20 amino acids commonly found in proteins.
Levels of Structure Primary: Sequence of amino acids in the protein chain. Secondary: The arrangement of the protein chain in the long molecule (hydrogen bonding determines this). Tertiary: The overall shape of the protein (determined by hydrogen-bonding, dipole-dipole interactions, ionic bonds, covalent bonds and London forces).
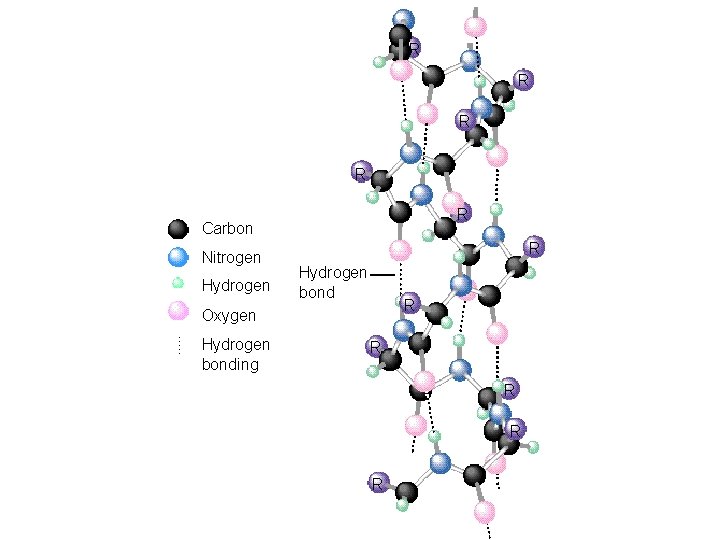
23_519 R R R Carbon Nitrogen Hydrogen R Hydrogen bond R Oxygen Hydrogen bonding R R

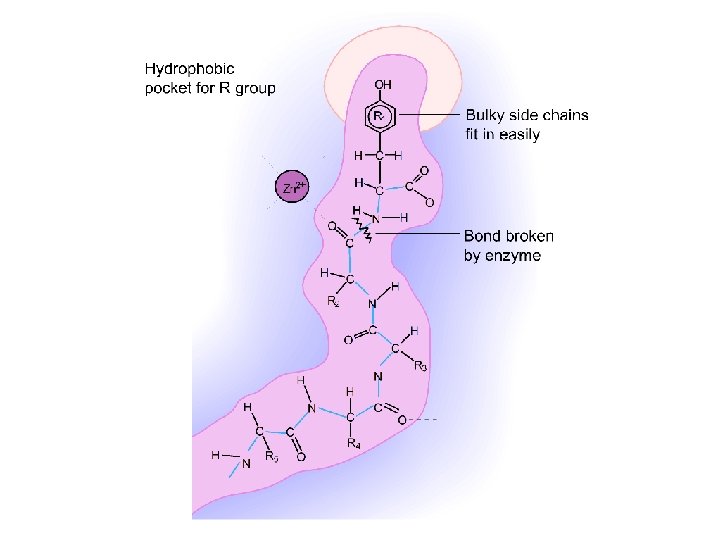
Enzymes Proteins tailored to catalyze specific biologic reactions.

Enzymes (continued) Many enzymes seem to use the lock-and-key model in which the substrate and enzyme connect via - H-bonding - ionic bonding and/or - metal ion-ligand bonding such that part of the substrate where the reaction is to occur occupies the active site of the enzyme.
Carbohydrates Food source for most organisms and structural material for plants. Empirical formula = CH 2 O Monosaccharides (simple sugars) - pentoses - ribose, arabinose - hexoses - fructose, glucose

Carbohydrates (continued) Disaccharides (formed from 2 monosaccharides joined by a glycoside linkage) - sucrose (glucose + fructose) Polysaccharides (many monosaccharide units) - starch, cellulose
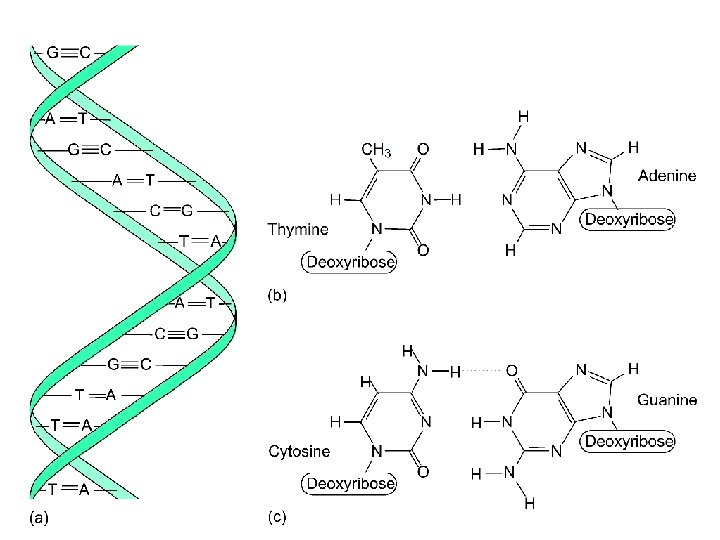
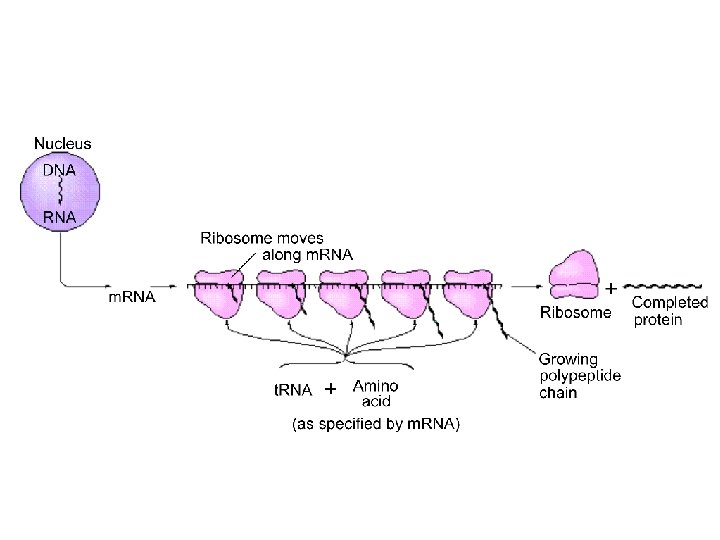
Nucleic Acids DNA (deoxyribonucleic acids): stores and transmits genetic information, responsible (with RNA) for protein synthesis. (Molar mass = several billion) RNA (ribonucleic acid): helps in protein synthesis. (Molecular weight = 20, 000 to 40, 000) - messenger RNA - transfer RNA
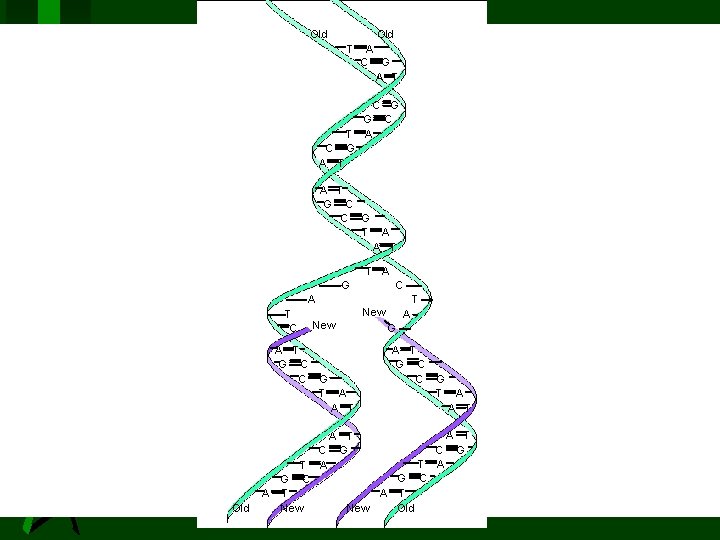
Old 23_539 Old T C A T A C G A T G T A G A T G C C G T C G C A A T T A G A T C Old C T New A G A T G C C G T A A T A T C G A T G C A T New Old

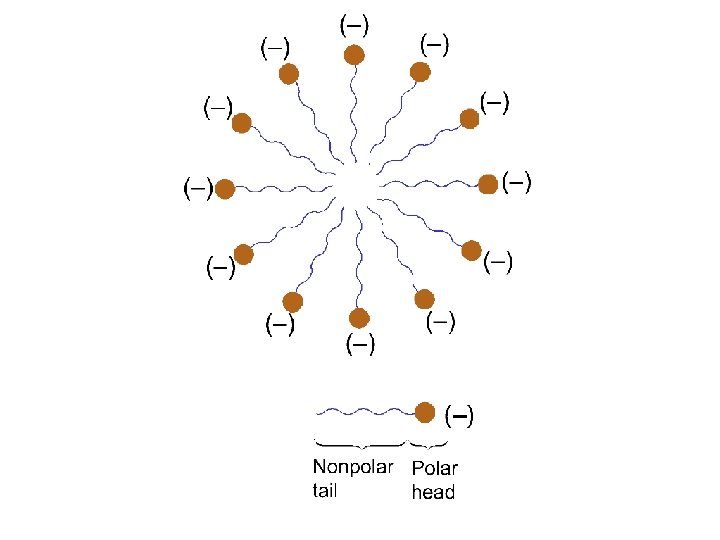
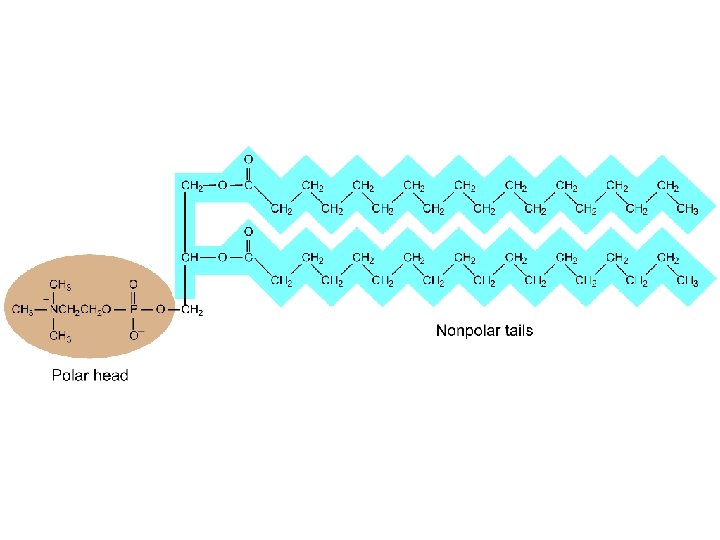
Lipids Water-insoluble substances that can be extracted from cells by nonpolar organic solvents. - fats - phospholipids - waxes - steroids
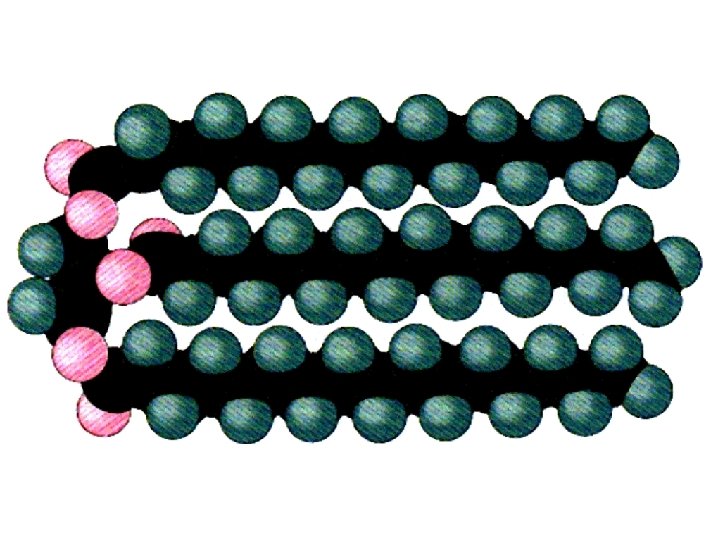

Soaps and Detergents Made from a reaction of a strong base with a lipid such as glyceryl tristearate The strong base breaks the link between glycerol and fatty acid to make the soap molecule Soap molecule have an ionic head which is water soluble and a nonpolar fatty acid tail which attracts and dissolves the grease which holds dirt to surfaces
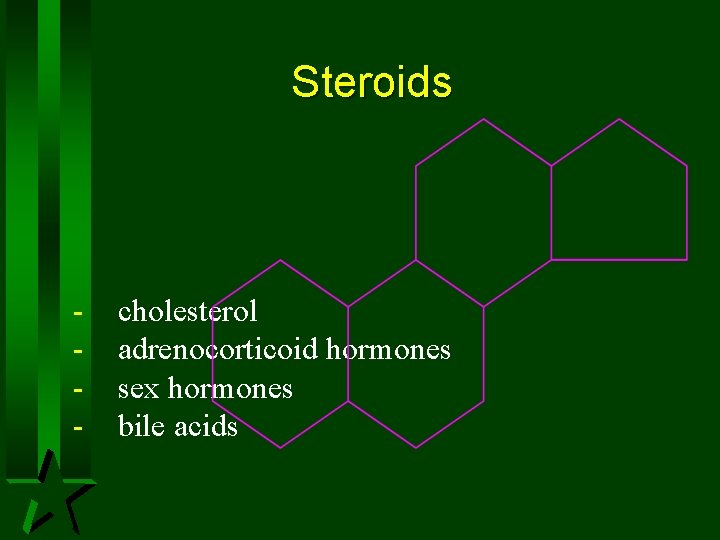
Steroids - cholesterol adrenocorticoid hormones sex hormones bile acids
- Slides: 56