Organic Chemistry The study of carbon compounds Hydrocarbons
Organic Chemistry The study of carbon compounds Hydrocarbons and functional groups
Hydrocarbons • Organic compound with only hydrogen and carbon (think combustion reaction)
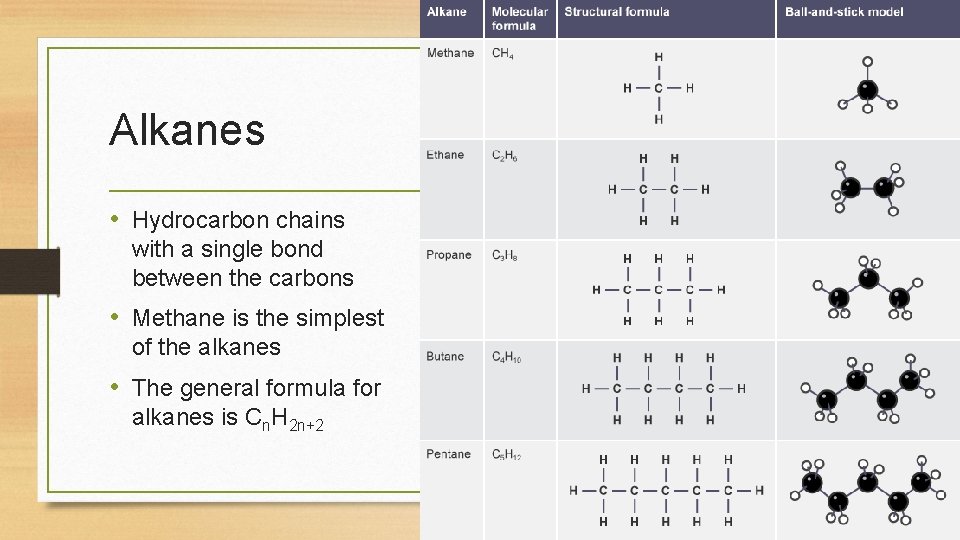
Alkanes • Hydrocarbon chains with a single bond between the carbons • Methane is the simplest of the alkanes • The general formula for alkanes is Cn. H 2 n+2
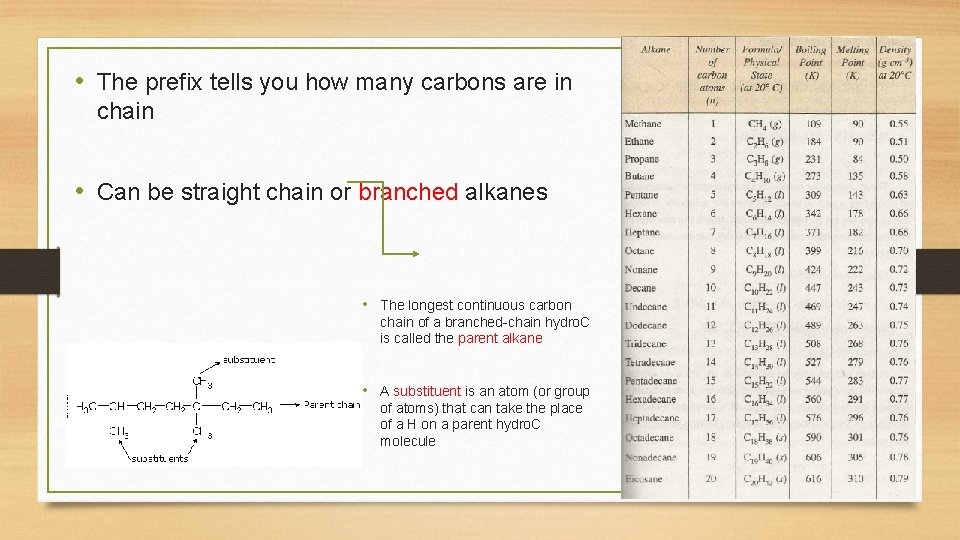
• The prefix tells you how many carbons are in chain • Can be straight chain or branched alkanes • The longest continuous carbon chain of a branched-chain hydro. C is called the parent alkane • A substituent is an atom (or group of atoms) that can take the place of a H on a parent hydro. C molecule
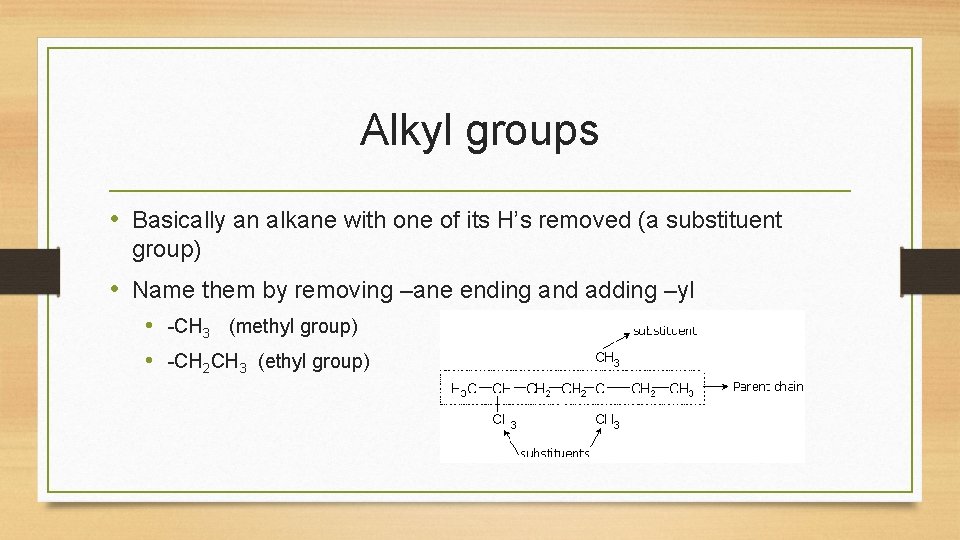
Alkyl groups • Basically an alkane with one of its H’s removed (a substituent group) • Name them by removing –ane ending and adding –yl • -CH 3 (methyl group) • -CH 2 CH 3 (ethyl group)
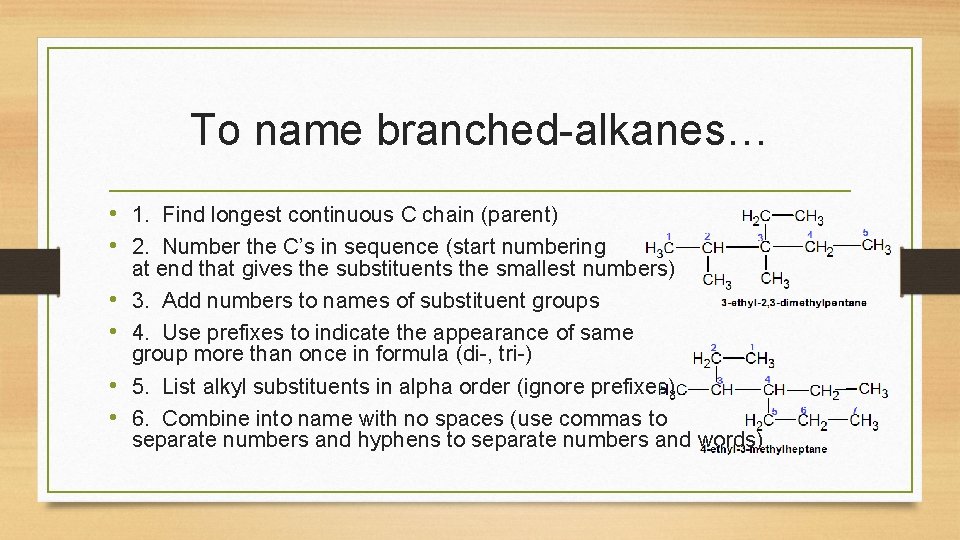
To name branched-alkanes… • 1. Find longest continuous C chain (parent) • 2. Number the C’s in sequence (start numbering • • at end that gives the substituents the smallest numbers) 3. Add numbers to names of substituent groups 4. Use prefixes to indicate the appearance of same group more than once in formula (di-, tri-) 5. List alkyl substituents in alpha order (ignore prefixes) 6. Combine into name with no spaces (use commas to separate numbers and hyphens to separate numbers and words)
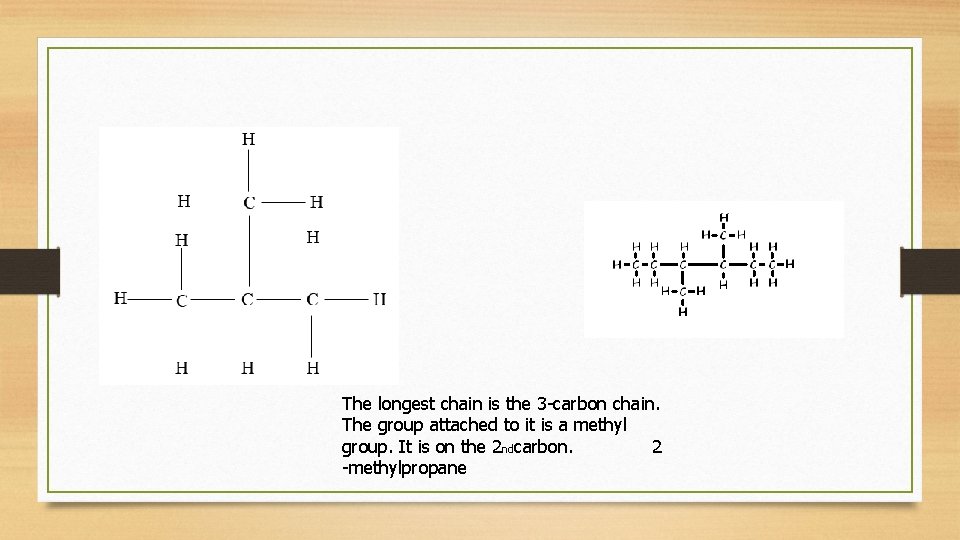
The longest chain is the 3 -carbon chain. The group attached to it is a methyl group. It is on the 2 ndcarbon. 2 -methylpropane
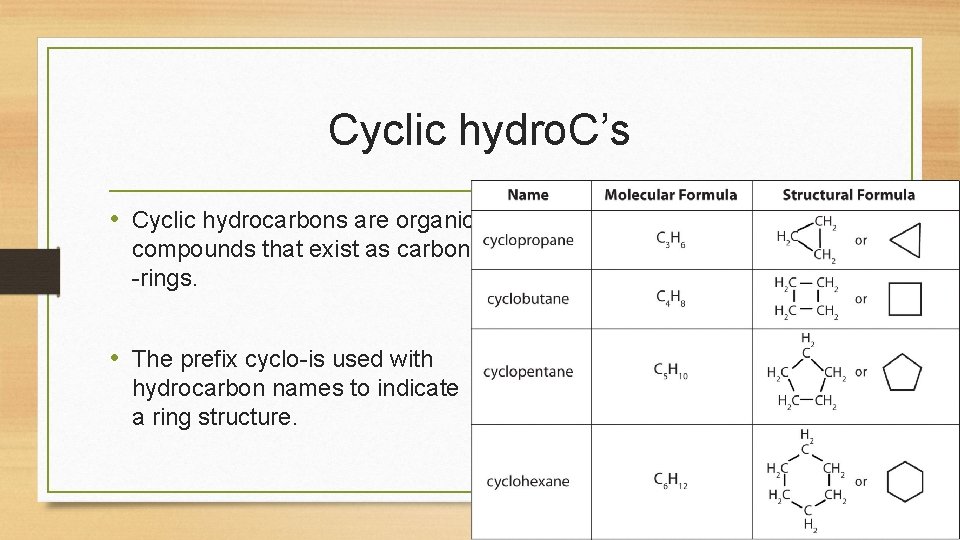
Cyclic hydro. C’s • Cyclic hydrocarbons are organic compounds that exist as carbon -rings. • The prefix cyclo-is used with hydrocarbon names to indicate a ring structure.

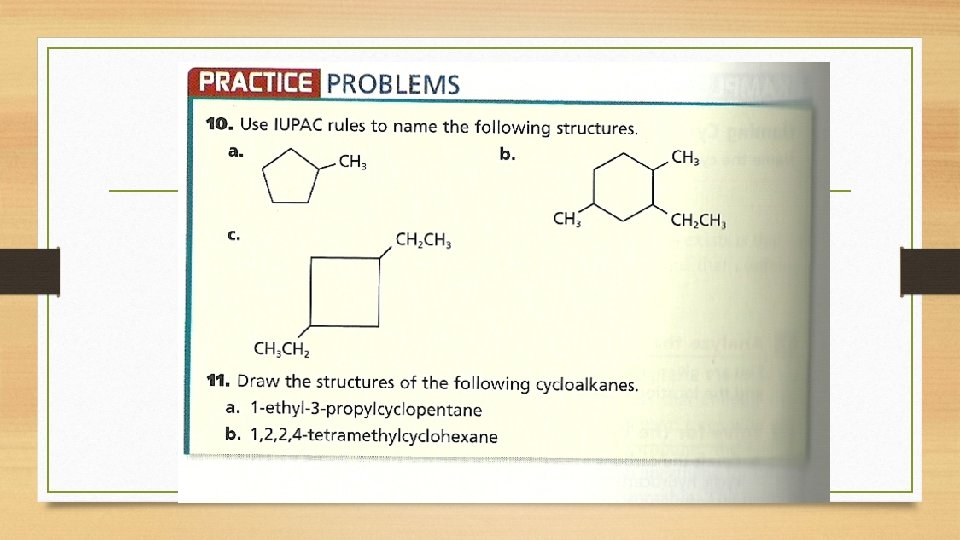
Naming branched cycloalkanes • The ring is always considered the parent chain. • Number the carbons beginning with one attached to a substituent group and in such a way to give the lowest possible numbers in the name.
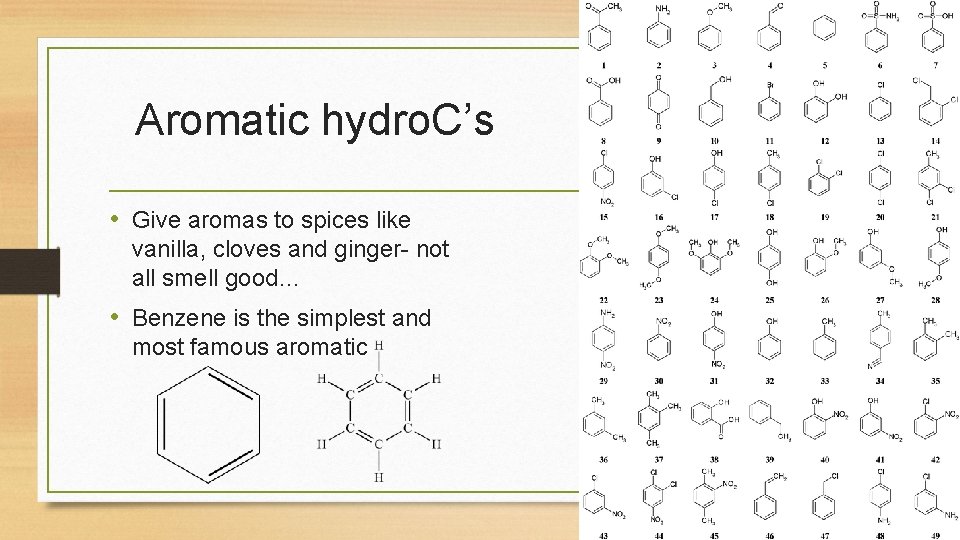
Aromatic hydro. C’s • Give aromas to spices like vanilla, cloves and ginger- not all smell good… • Benzene is the simplest and most famous aromatic
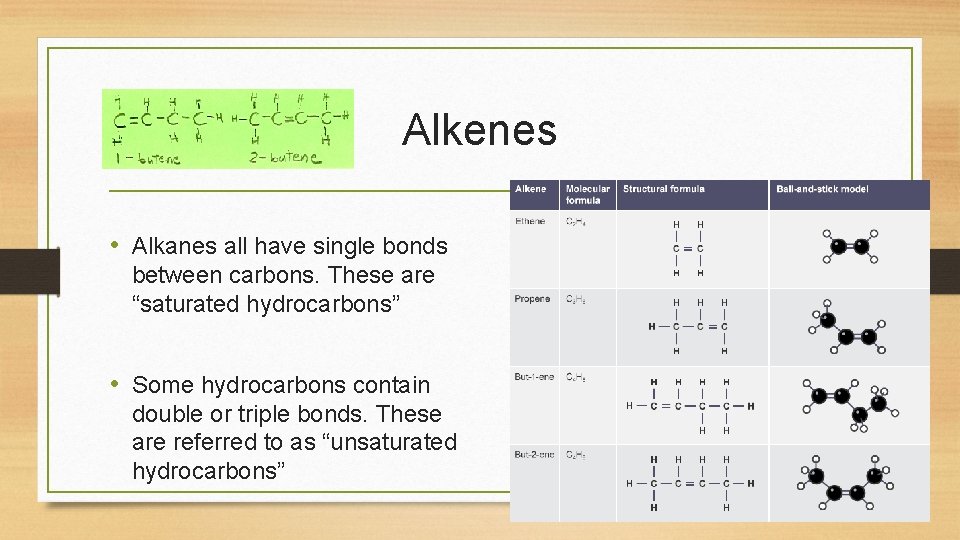
Alkenes • Alkanes all have single bonds between carbons. These are “saturated hydrocarbons” • Some hydrocarbons contain double or triple bonds. These are referred to as “unsaturated hydrocarbons”
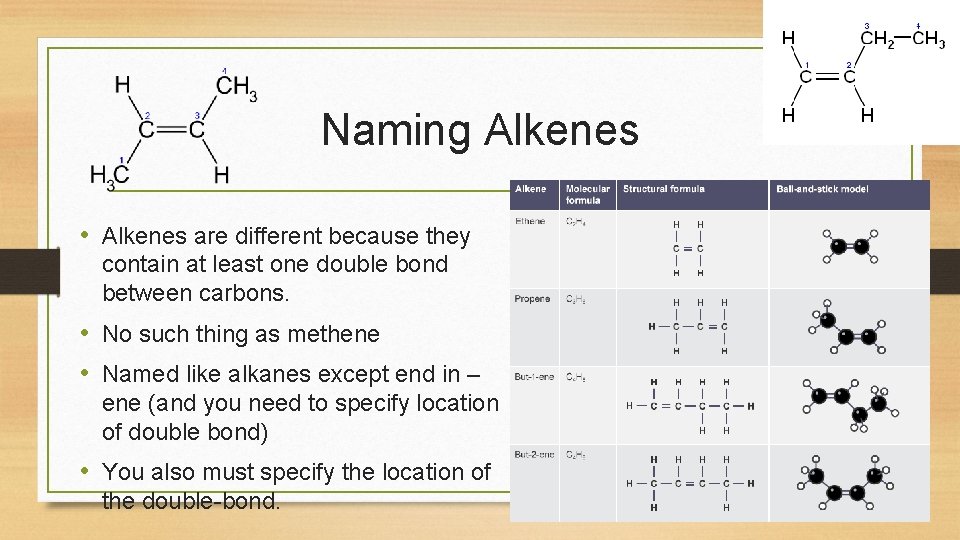
Naming Alkenes • Alkenes are different because they contain at least one double bond between carbons. • No such thing as methene • Named like alkanes except end in – ene (and you need to specify location of double bond) • You also must specify the location of the double-bond.
Naming branched alkenes • The longest carbon-chain must contain the double bond. Always start numbering closest to double-bond. • If there is more than one double bond, use a prefix (di, tri, tetra, etc) before the – ene to indicate the number. • Draw the structure for 5, 5 -dimethyl-1 -hexene
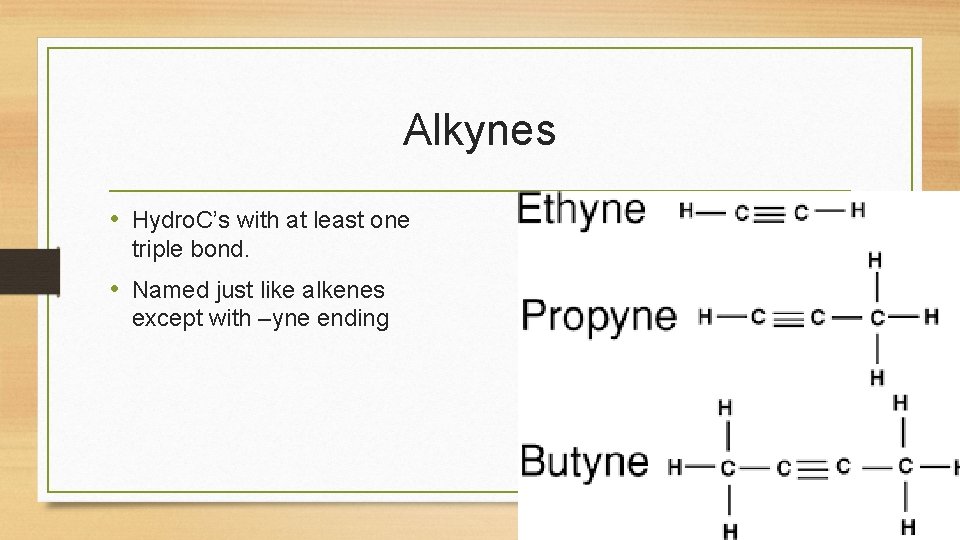
Alkynes • Hydro. C’s with at least one triple bond. • Named just like alkenes except with –yne ending
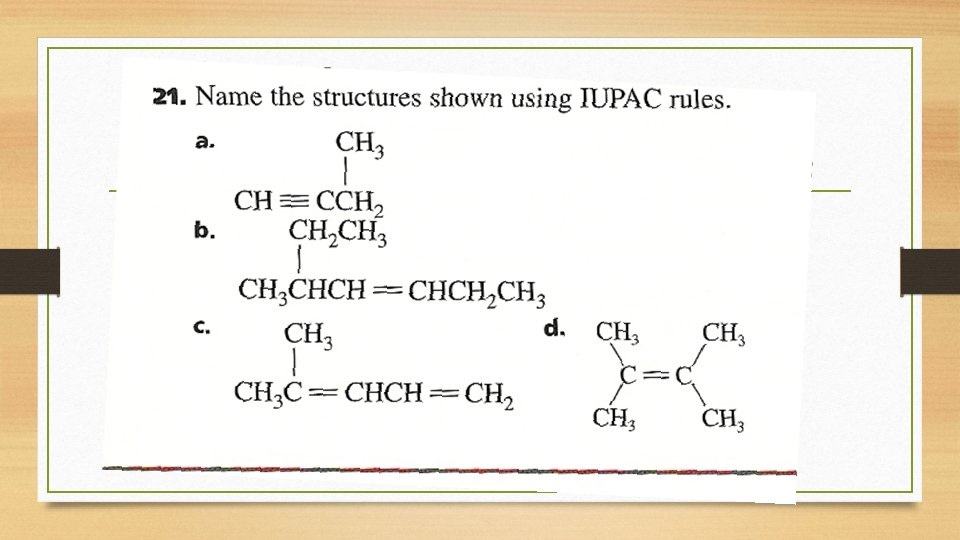
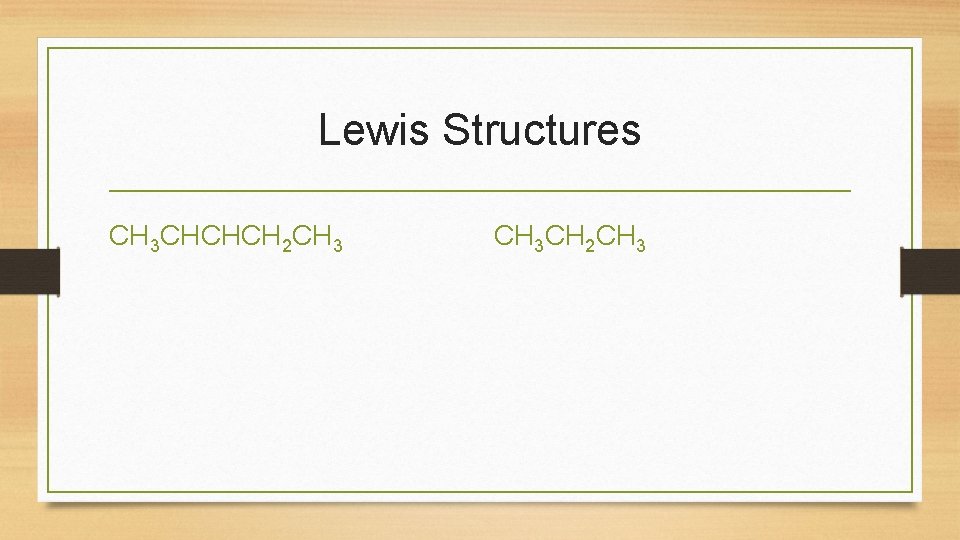
Lewis Structures CH 3 CHCHCH 2 CH 3 CH 2 CH 3
- Slides: 21