Organic Chemistry The chemistry of life Organic Chemistry




























- Slides: 42

Organic Chemistry The chemistry of life!



Organic Chemistry The study of carbon and most carbon compounds. Originally thought to only be made by living things, to get the name organic. It is now known that organic chemistry has more compounds than that made by living things.

Why so many compounds? Carbon can bond with carbon atoms to form rings, chains and networks They then form with groups of other atoms to form many compounds

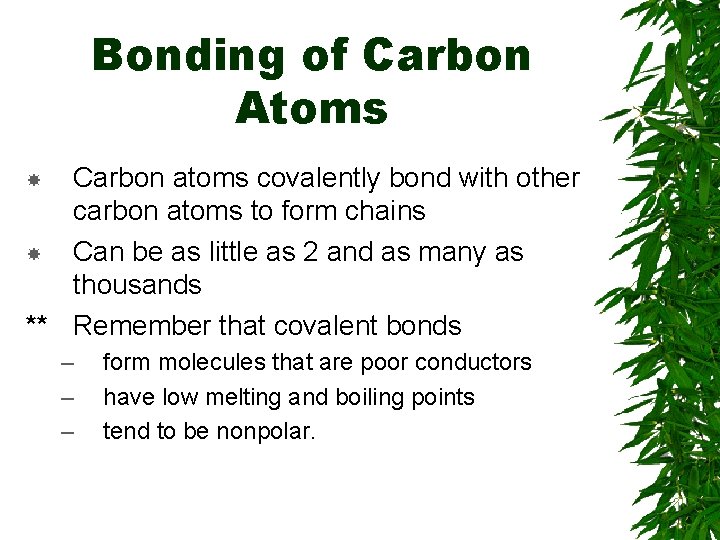
Bonding of Carbon Atoms Carbon atoms covalently bond with other carbon atoms to form chains Can be as little as 2 and as many as thousands ** Remember that covalent bonds – – – form molecules that are poor conductors have low melting and boiling points tend to be nonpolar.

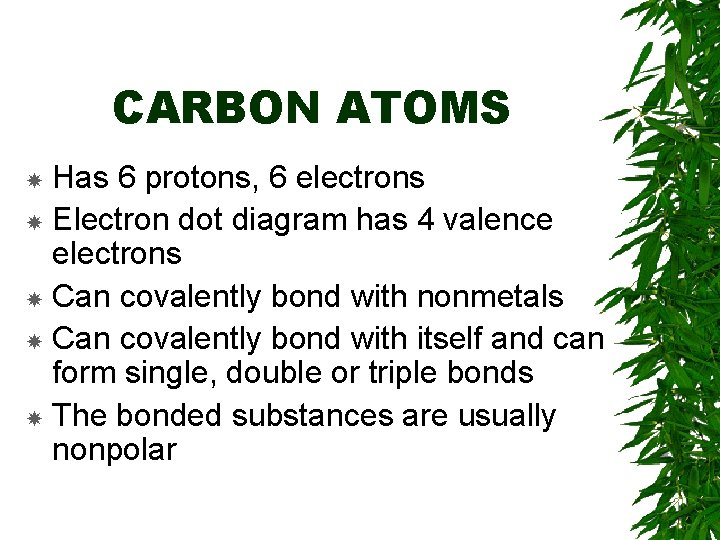
CARBON ATOMS Has 6 protons, 6 electrons Electron dot diagram has 4 valence electrons Can covalently bond with nonmetals Can covalently bond with itself and can form single, double or triple bonds The bonded substances are usually nonpolar

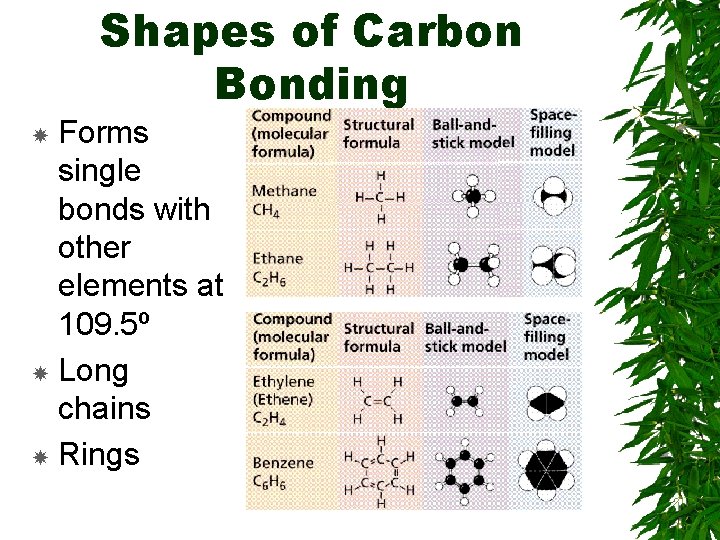
Shapes of Carbon Bonding Forms single bonds with other elements at 109. 5º Long chains Rings

Carbon Bonds Single Bonds where only one pair of electrons are shared is saturated Double and triple bonds where 2 and 3 pairs of electrons are shared are unsaturated Can be open (chains), closed (rings) or networked (lattice) ex: Diamonds

Hydrocarbons – organic compounds that contain only hydrogen and carbon Homologous series – a group of related compounds in which each member differs from the one before by the number of carbons Alkanes, Alkynes and Alkenes are 3 important homologous series of hydrocarbons ***listed in Table Q

Alkanes A homologous series of saturated hydrocarbons that release energy when burned – Made only of hydrogen and carbon – Homologous series – each Alkane differs from another by the number of carbons – Saturated means all carbons in Alkanes have single bonds

Examples of Alkanes General Formula Cn. H 2 n+2 Methane – 90% of natural gas CH 4 Ethane – 10% of natural gas C 2 H 6 Propane – used for outdoor grills C 3 H 8 Butane – used as lighter fluid C 4 H 10 As the number of carbons increase the boiling point increases and bond strength increases ** Table P lists all organic prefixes

Alkenes A homologous series of unsaturated hydrocarbons that are used to make other items like plastics – Made only of hydrogen and carbon – Homologous series – each Alkene differs from another by the number of carbons – Unsaturated means all carbons in Alkenes have double bonds

Examples of Alkenes General Formula Cn. H 2 n Ethene – C 2 H 4 Propene – C 3 H 6 Butene – C 4 H 8 ** Table P lists all organic prefixes

Alkynes A homologous series of unsaturated hydrocarbons that are used to make other items like fuels – Made only of hydrogen and carbon – Homologous series – each Alkyne differs from another by the number of carbons – Unsaturated means all carbons in Alkynes have triple bonds

Examples of Alkynes General Formula Cn. H 2 n-2 Ethyne – C 2 H 2 Propyne – C 3 H 4 Butyne – C 4 H 6 ** Table P lists all organic prefixes

Isomers When hydrocarbons are formed there are more than one way to arrange the atoms

Facts on Isomers: Have the same molecular formula Different chemical and physical properties As number of carbons increases so does the number of isomers for that molecule

Naming Hydrocarbon Compounds Naming the Normal Form (straight line) 1. Must have continuous straight chain of carbons 2. Count number of carbons 3. Choose prefix from table P 4. Check for # of bonds between carbons 5. Use n and then the name Ex: n- butane IUPAC (international union of pure and applied chemistry)

Naming Branched Chains of Hydrocarbons Name the longest continuous hydrocarbon chain first Check for each branch attached. – Branch names use the same prefix – Uses –yl ending – Has one less hydrogen Carbons are numbered to give the lowest number in the name for the location More than 1 of the same group use di-, triand tetra- prefixes before the branch name

Functional Groups Atoms or groups of atoms that replace one or more hydrogen atoms in a hydrocarbon. ***Listed on Table R Halides Alcohols Aldehydes Ketones Ethers Amides Amino Acids Amines Esters Organic Acids

Halides A. k. a organic halides or halocarbon) When group 17 atoms replace a hydrogen Used as solvents and pesticides Named by citing the location where it is attached to the hydrocarbon

Aldehydes When an O atom is attached to a carbon chain by a double covalent bond Called a carbonyl group Carbonyl group formula is – C = O The carbonyl group is on the end of a hydrocarbon Named by substituting the e ending with -al Often used as preservatives

Ketones When the carbonyl group is found on an inside carbon Named by replacing the e ending with –one Often used as solvents

Ethers A series of organic compounds where two carbon chains are joined together by an oxygen atom Named by using alkyl groups and ending with ether

Organic Acids Homologous series of organic compounds with a carboxyl group Carboxyl group formula is – COOH Named by replacing the e ending with –oic acid Creates a weak electrolyte

Esters Compounds whose formula is CO – O Is part of an organic acid Has strong fragrant aromas and is responsible for many smells in fruits

Amines Derivative of ammonia Formed when 1 or more hydrogen atoms of ammonia is replaced with an alkyl group The Alkane chain is numbered to show the location Named by replacing the e ending with – amine Important for B vitamins, hormones and anesthetics

Amino Acids Contains carboxyl groups and amine groups The amine group is attached to the carbon atom that is adjacent to the acid group The rest of the molecule is a hydrocarbon chain Necessary for the building blocks of protein

Amides A compound formed by the combination of two amino acids When on of the hydrogen atoms of an amino group reacts with an –OH of an organic acid condensation occurs Water and an amide is formed Known as a peptide link Eventually a polypeptide will form a protein

Alcohols When one or more of the hydrogen atoms are replaced by an –OH group The -OH group is called a hydroxyl Does not form electrolytes Does not form hydroxide ions in water Does not react with indicators Forms polar molecules

Classification of Alcohols Classified as primary, secondary or tertiary depending on where the hydroxyl group is located Primary, attached to the carbon at the end of the chain Secondary, attached to a carbon in the middle of a chain Tertiary, attached to a carbon with a branching alkyl group

Dihydroxy and Trihydroxy Alcohols Can also be classified by the number of hydroxyl groups Dihydroxy alcohols have 2 groups attached to a carbon chain Trihydroxy alcohols have 3 hydroxyl groups attached to a carbon chain

Types of Organic Reactions – Combustion – Substitution – Addition – Esterification – Saponification – Fermentation – Polymerization

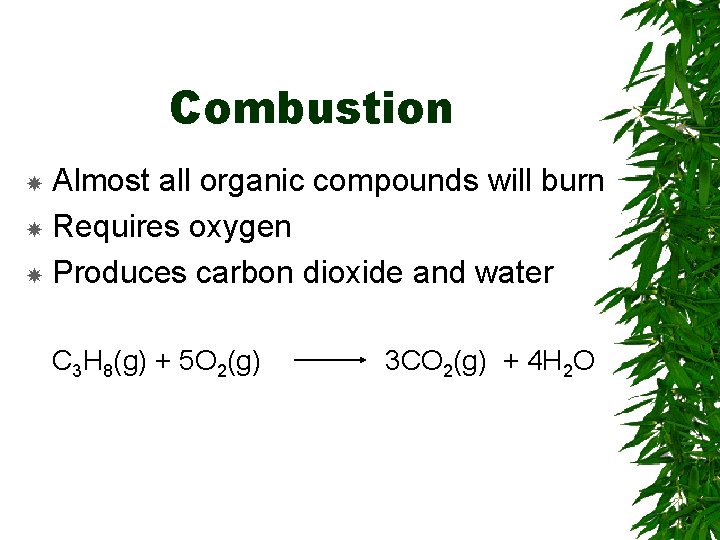
Combustion Almost all organic compounds will burn Requires oxygen Produces carbon dioxide and water C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O

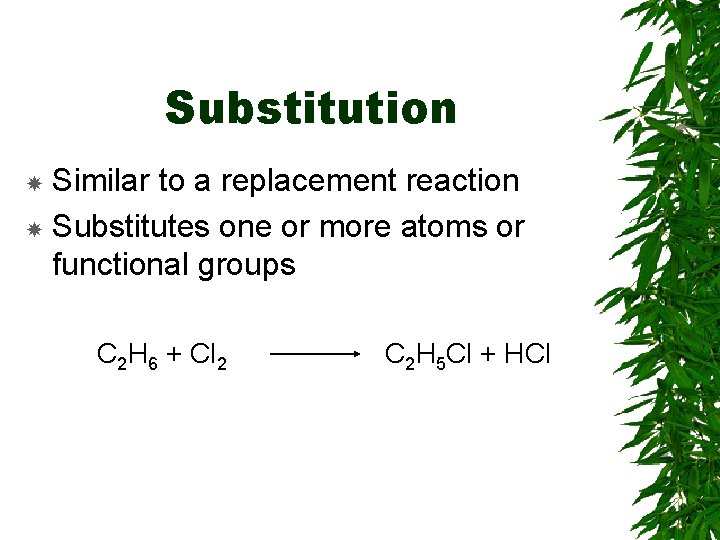
Substitution Similar to a replacement reaction Substitutes one or more atoms or functional groups C 2 H 6 + Cl 2 C 2 H 5 Cl + HCl

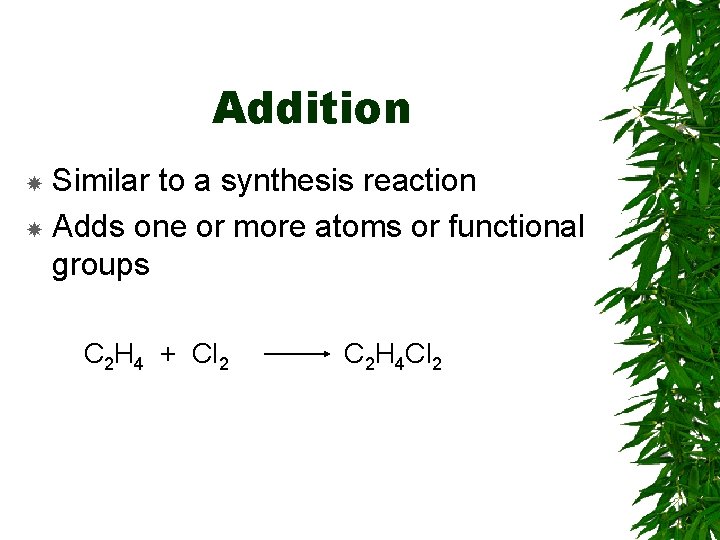
Addition Similar to a synthesis reaction Adds one or more atoms or functional groups C 2 H 4 + Cl 2 C 2 H 4 Cl 2

Esterification A reaction between an organic acid an alcohol Produces an ester and water CH 3 OOH + CH 3 CH 2 OH H 2 O + CH 3 COOCH 2 CH 3

Saponification A reaction between an inorganic base and an ester Produces an alcohol and a soap Fat + Base Glycerol + Soap

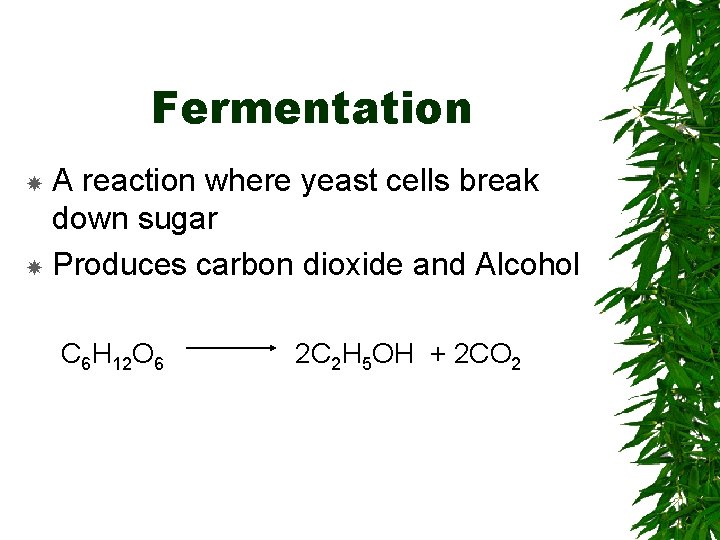
Fermentation A reaction where yeast cells break down sugar Produces carbon dioxide and Alcohol C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2

Polymerization Organic compound made up of log carbon chains bonded together Each unit of polymer is called a monomer Produces products like rayon, nylon, and polyethylene Organic polymers include proteins, starches and cellulose

Addition & Condensation Polymerization Addition is the joining of monomers of unsaturated compounds Condensation is the removing of water from hydroxyl groups and joining by ether or ester linkage