Organic Chemistry Sh javanshir Faculty of Chemistry Iran











- Slides: 93

Organic Chemistry Sh. javanshir Faculty of Chemistry Iran University of Science & Technology

Chapter 4 -2. Alkenes: continue

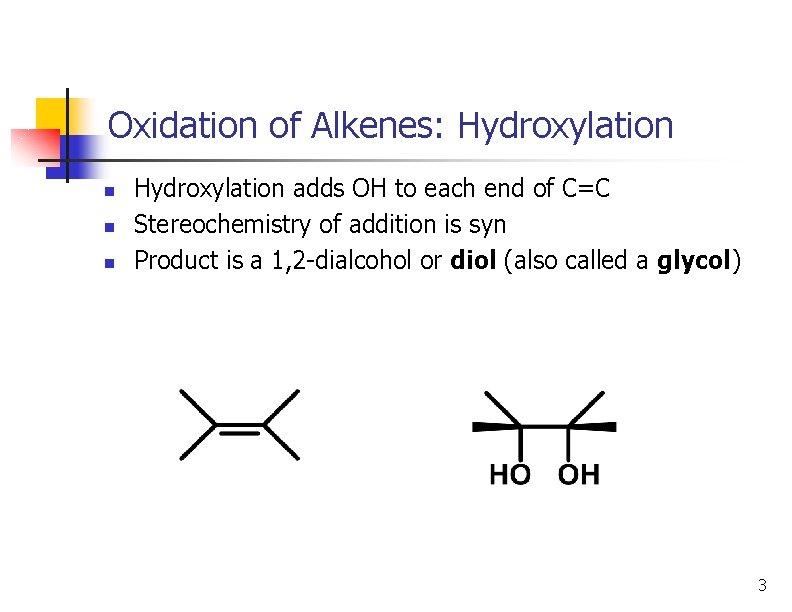
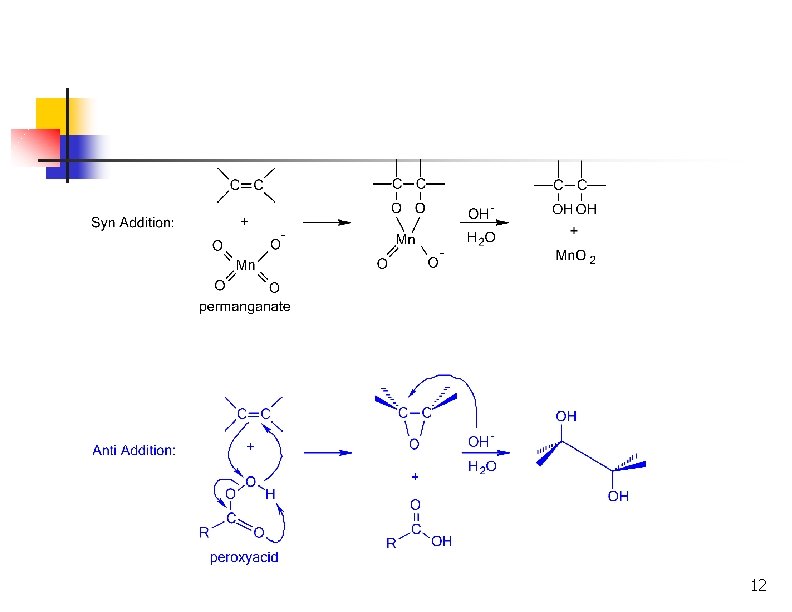
Oxidation of Alkenes: Hydroxylation n Hydroxylation adds OH to each end of C=C Stereochemistry of addition is syn Product is a 1, 2 -dialcohol or diol (also called a glycol) 3

Syn Hydroxylation of Alkenes n Two reagents: n n Osmium tetroxide (expensive!), followed by hydrogen peroxide or sodium bisulfate Cold, dilute aqueous potassium permanganate, followed by hydrolysis with base 4

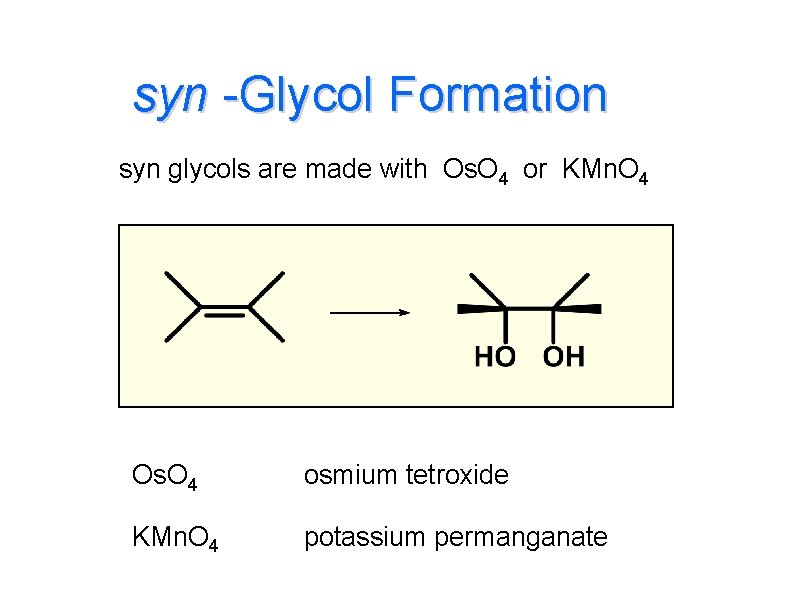
syn -Glycol Formation syn glycols are made with Os. O 4 or KMn. O 4 Os. O 4 osmium tetroxide KMn. O 4 potassium permanganate

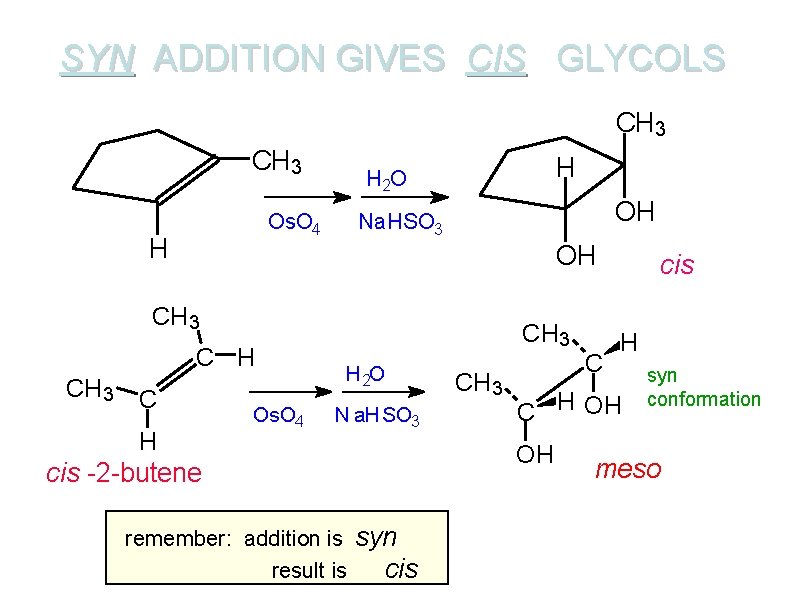
SYN ADDITION GIVES CIS GLYCOLS CH 3 Os. O 4 H H H 2 O OH Na. HSO 3 OH CH 3 C H Os. O 4 CH 3 H 2 O N a. HSO 3 syn cis H C H OH OH cis -2 -butene remember: addition is result is CH 3 C cis syn conformation meso

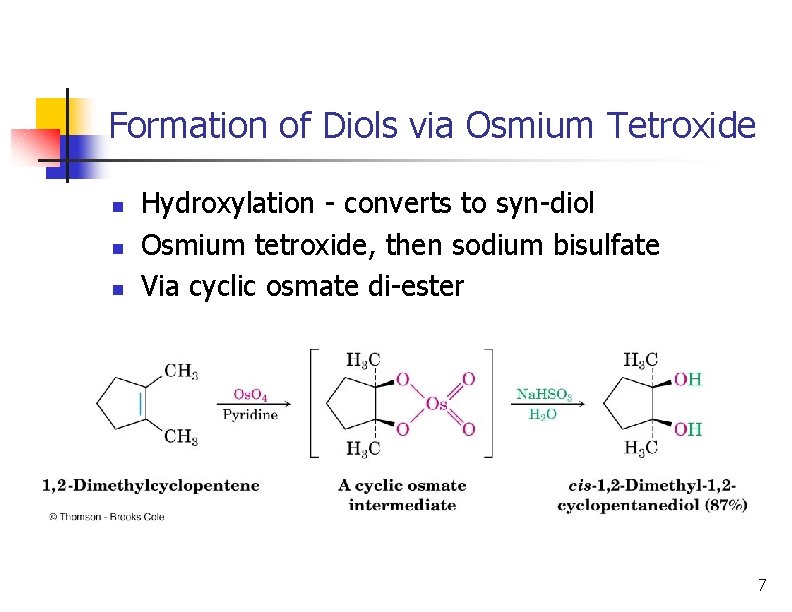
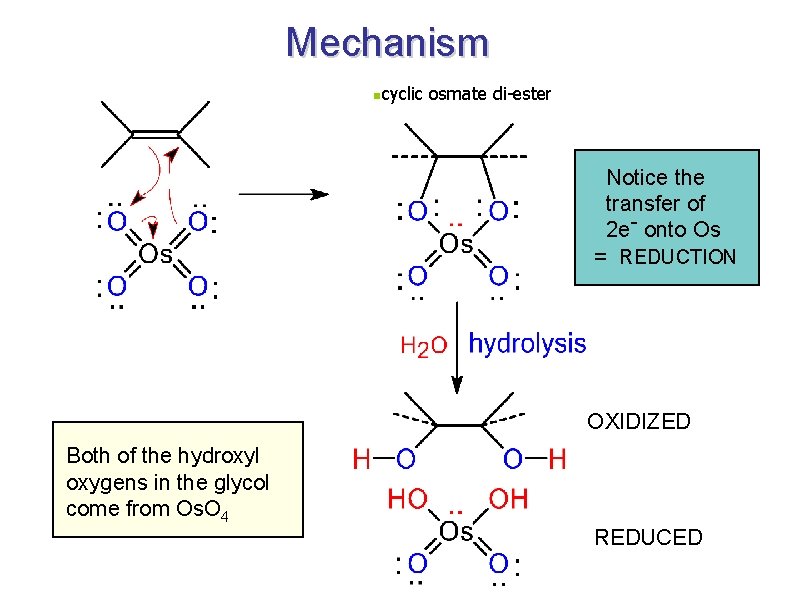
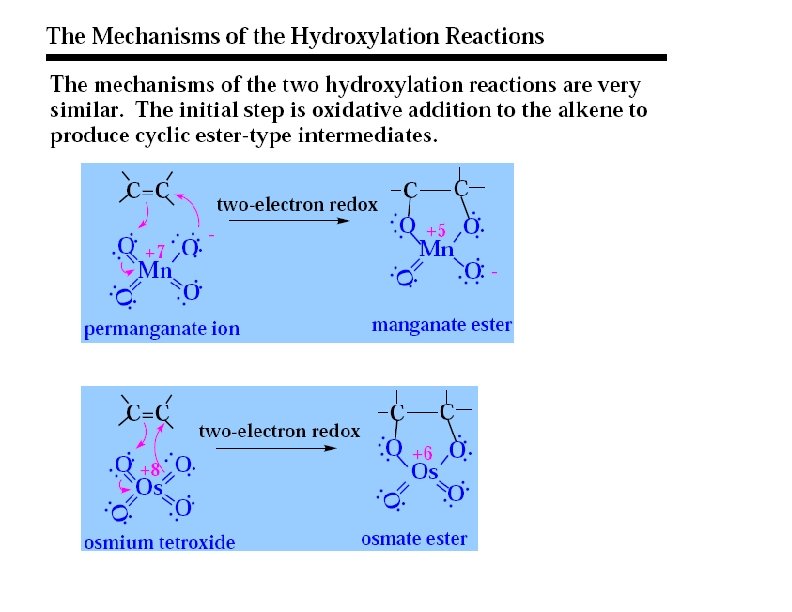
Formation of Diols via Osmium Tetroxide n n n Hydroxylation - converts to syn-diol Osmium tetroxide, then sodium bisulfate Via cyclic osmate di-ester 7

Mechanism ncyclic osmate di-ester Notice the transfer of 2 e- onto Os = REDUCTION OXIDIZED Both of the hydroxyl oxygens in the glycol come from Os. O 4 REDUCED

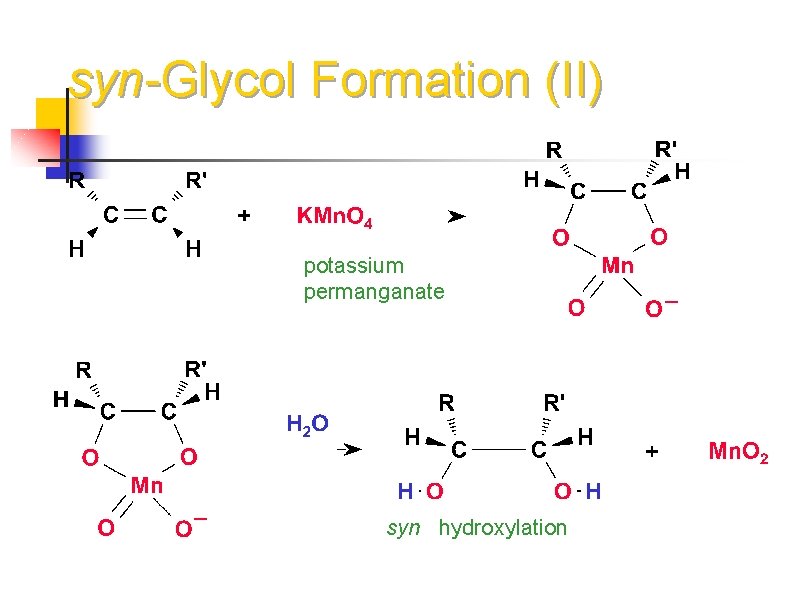
syn-Glycol Formation (II) potassium permanganate syn hydroxylation


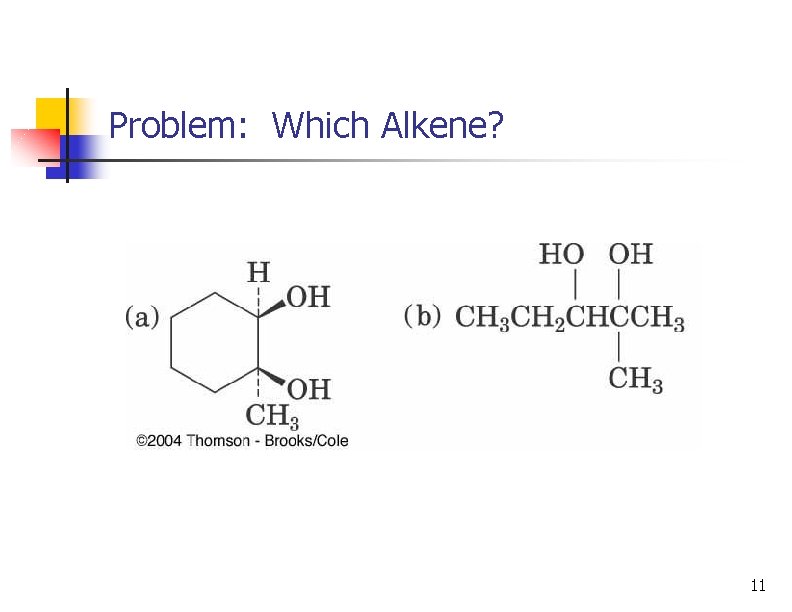
Problem: Which Alkene? 11

12

Cleavage Reactions of Alkenes Ozonolysis Hot Potassium Permanganate 13

Oxidative Cleavage n n n Both the pi and sigma bonds break. C=C becomes C=O. Two methods: n n n Warm or concentrated or acidic KMn. O 4. Ozonolysis Used to determine the position of a double bond in an unknown. 14

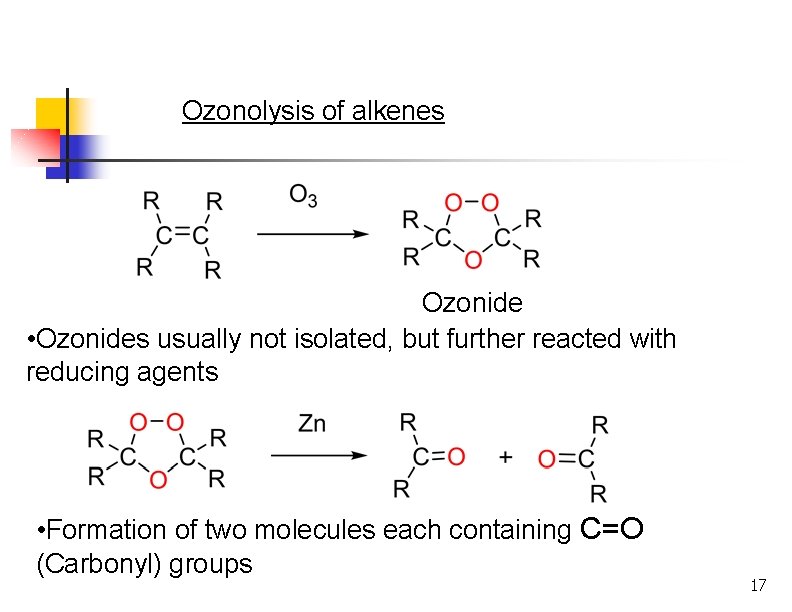
Ozonolysis n n Reaction with ozone forms an ozonide. Ozonides are not isolated, but are treated with a mild reducing agent like Zn or dimethyl sulfide. Milder oxidation than permanganate. Products formed are ketones or aldehydes. 15

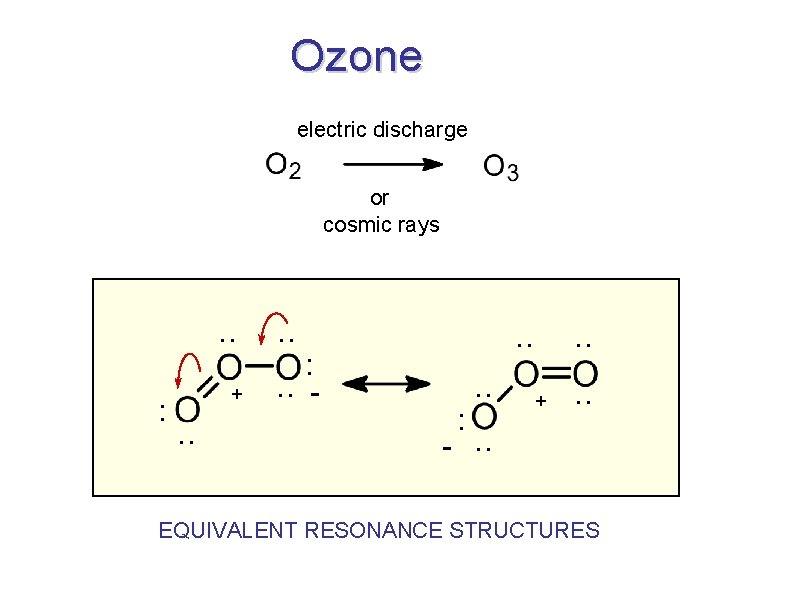
Ozone electric discharge or cosmic rays . . : + . . : . . - . . : . . + . . -. . EQUIVALENT RESONANCE STRUCTURES

Ozonolysis of alkenes Ozonide • Ozonides usually not isolated, but further reacted with reducing agents • Formation of two molecules each containing C=O (Carbonyl) groups 17

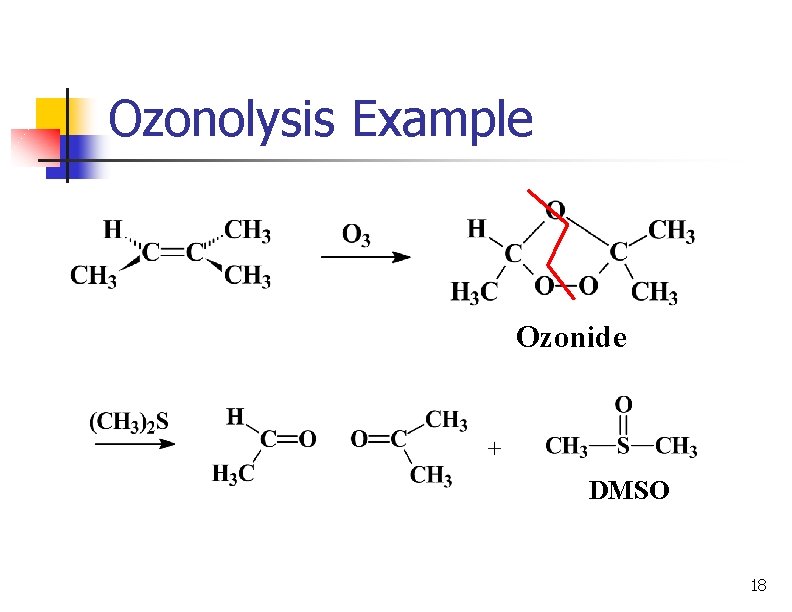
Ozonolysis Example Ozonide DMSO 18

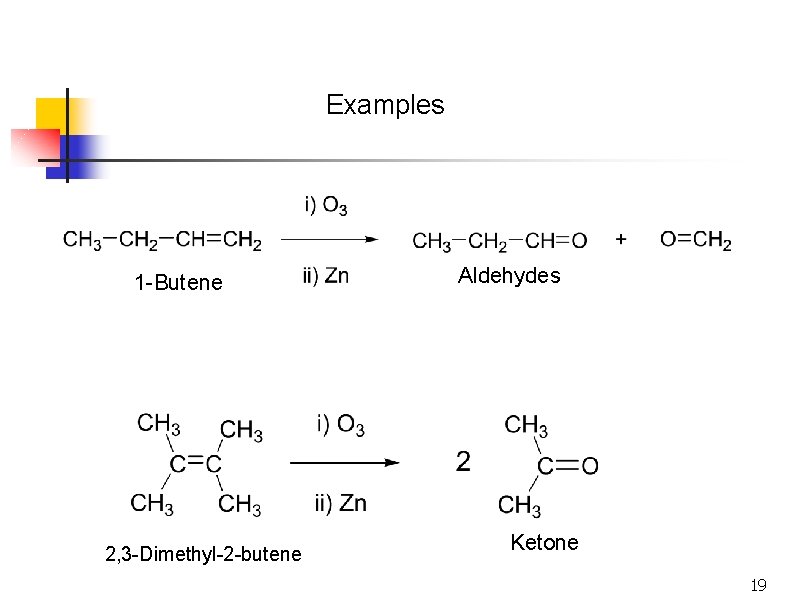
Examples 1 -Butene 2, 3 -Dimethyl-2 -butene Aldehydes Ketone 19

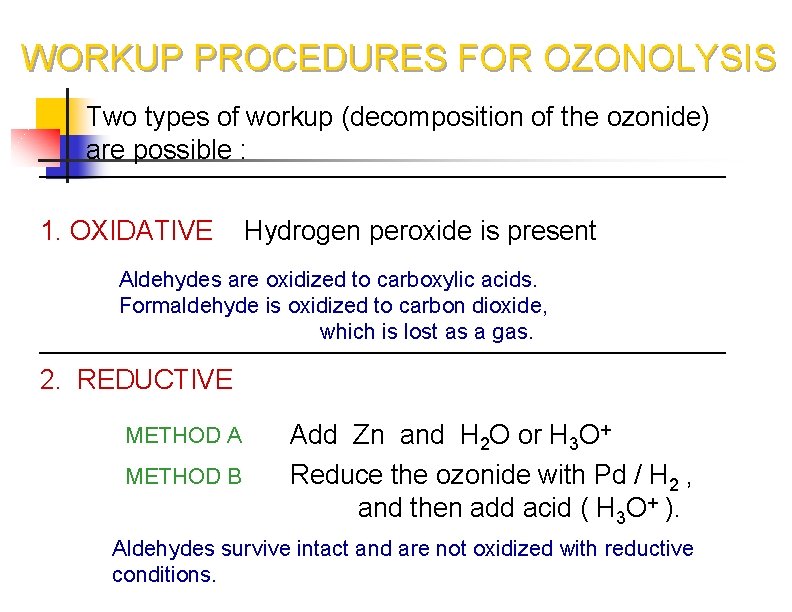
WORKUP PROCEDURES FOR OZONOLYSIS Two types of workup (decomposition of the ozonide) are possible : 1. OXIDATIVE Hydrogen peroxide is present Aldehydes are oxidized to carboxylic acids. Formaldehyde is oxidized to carbon dioxide, which is lost as a gas. 2. REDUCTIVE METHOD A Add Zn and H 2 O or H 3 O+ METHOD B Reduce the ozonide with Pd / H 2 , and then add acid ( H 3 O+ ). Aldehydes survive intact and are not oxidized with reductive conditions.

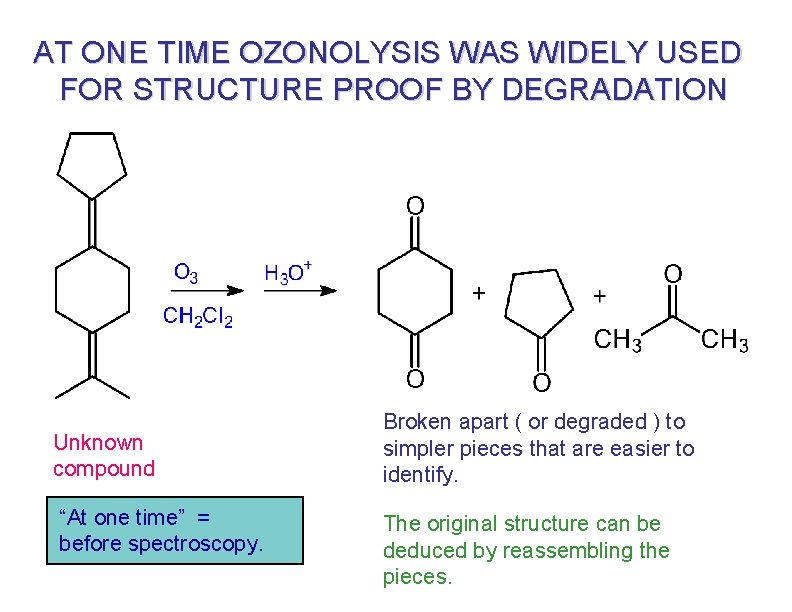
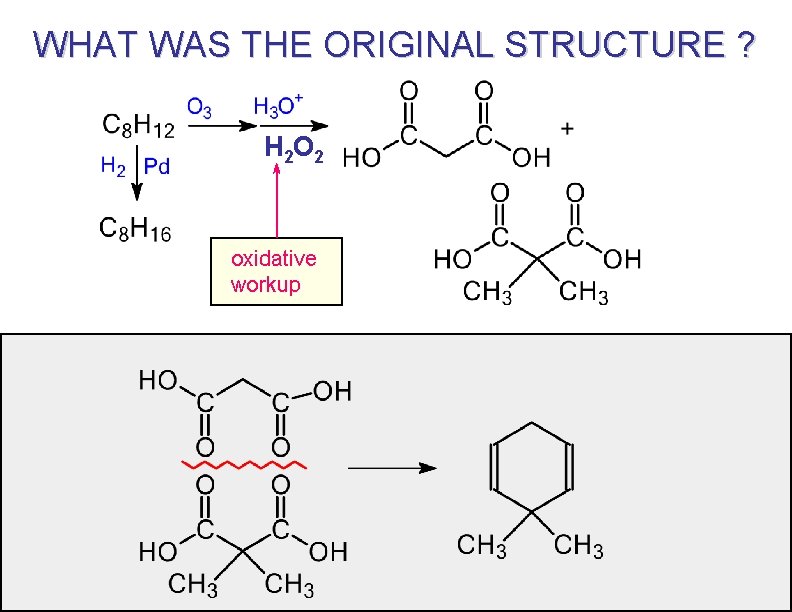
AT ONE TIME OZONOLYSIS WAS WIDELY USED FOR STRUCTURE PROOF BY DEGRADATION Unknown compound “At one time” = before spectroscopy. Broken apart ( or degraded ) to simpler pieces that are easier to identify. The original structure can be deduced by reassembling the pieces.

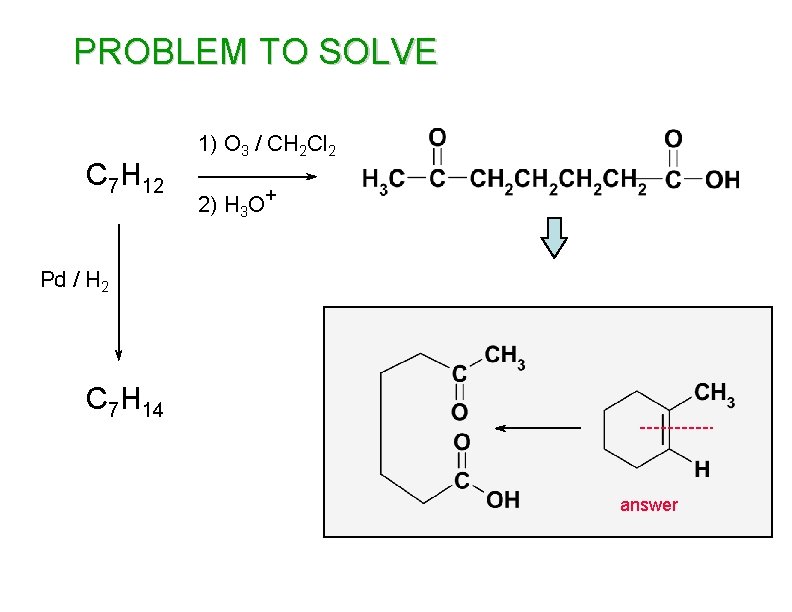
PROBLEM TO SOLVE C 7 H 12 1) O 3 / CH 2 Cl 2 2) H 3 O+ Pd / H 2 C 7 H 14 answer

WHAT WAS THE ORIGINAL STRUCTURE ? H 2 O 2 oxidative workup

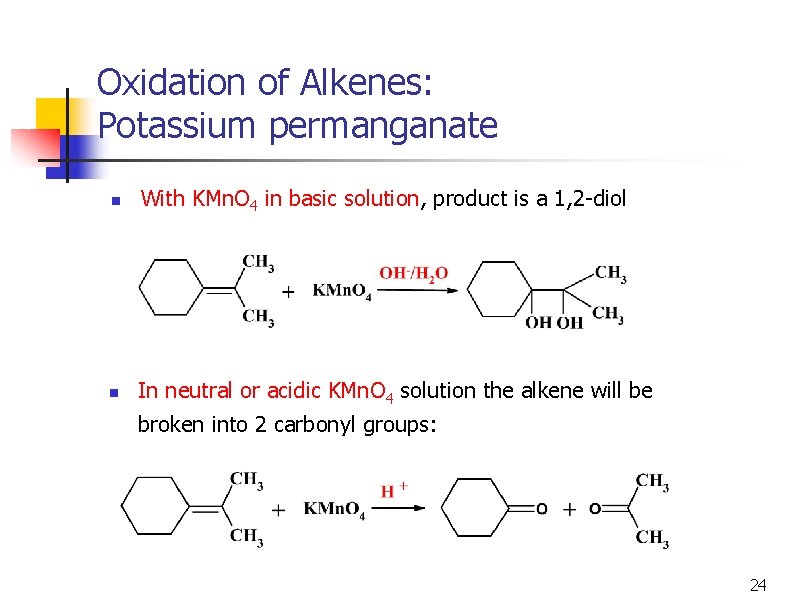
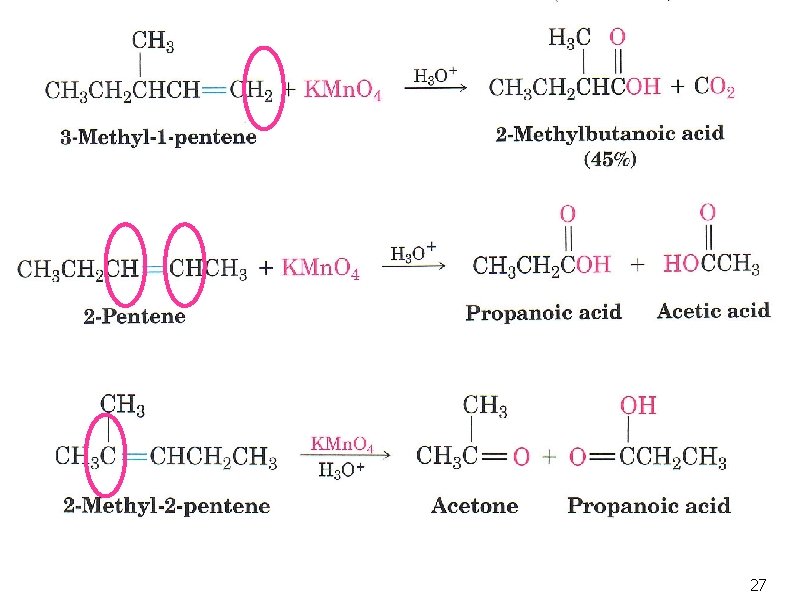
Oxidation of Alkenes: Potassium permanganate n With KMn. O 4 in basic solution, product is a 1, 2 -diol n In neutral or acidic KMn. O 4 solution the alkene will be broken into 2 carbonyl groups: 24

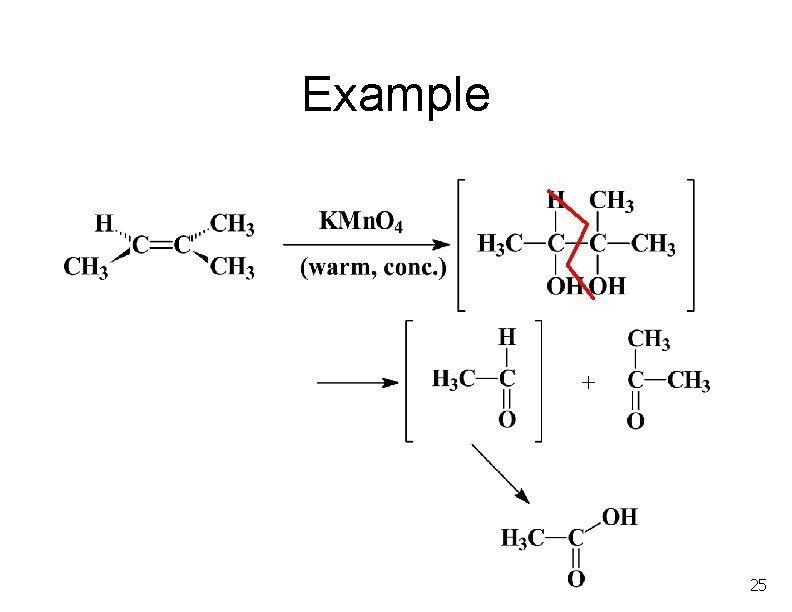
Example 25

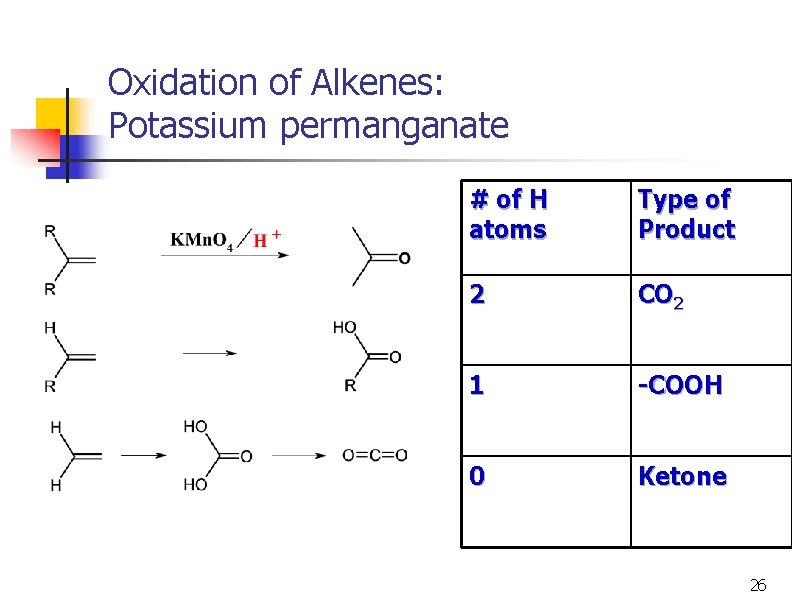
Oxidation of Alkenes: Potassium permanganate # of H atoms Type of Product 2 CO 2 1 -COOH 0 Ketone 26

27

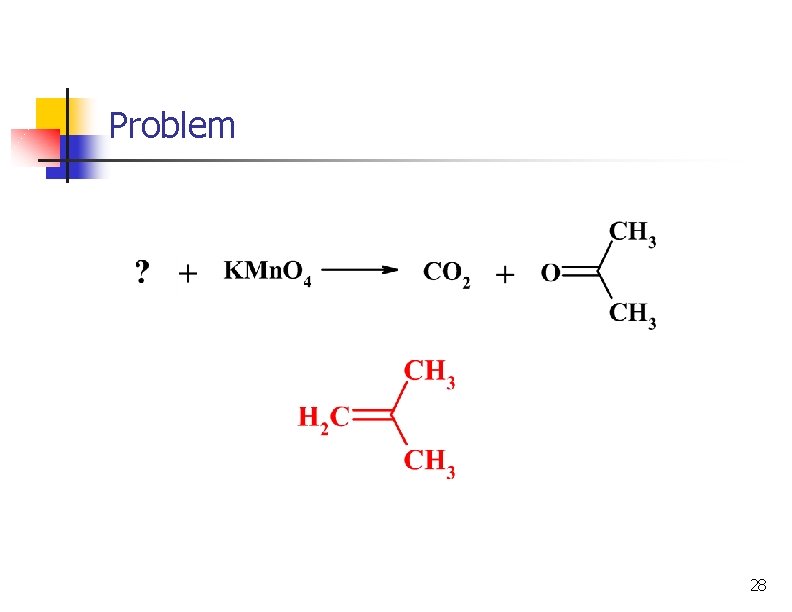
Problem 28

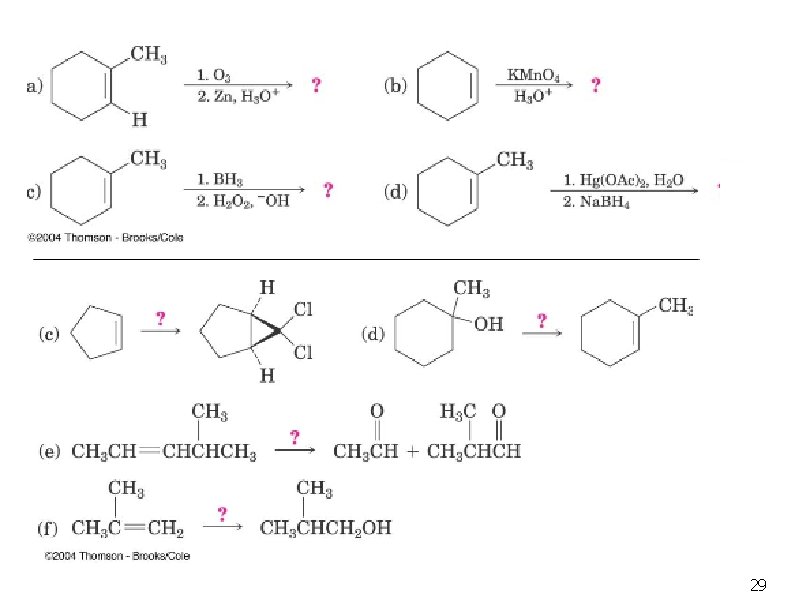
29

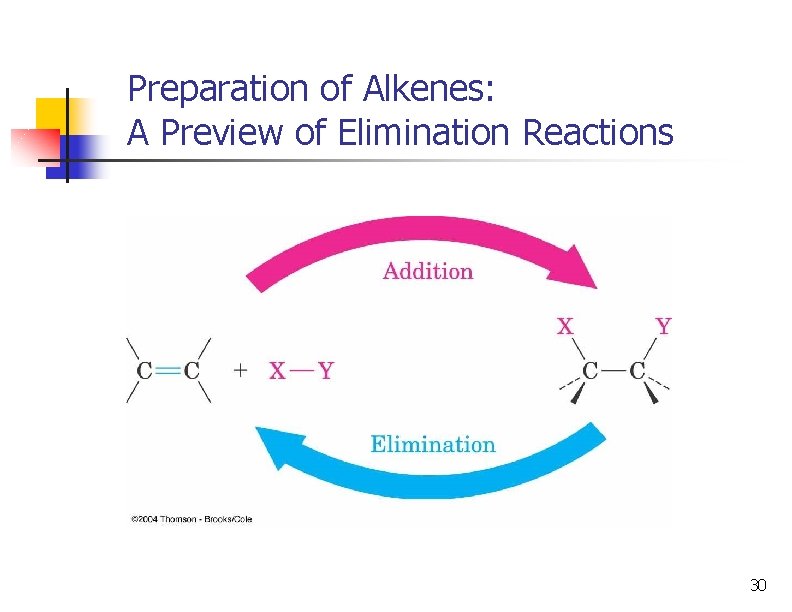
Preparation of Alkenes: A Preview of Elimination Reactions 30

Alkene Synthesis Overview n n E 2 dehydrohalogenation (-HX) E 1 dehydrohalogenation (-HX) Dehalogenation of vicinal dibromides (-X 2) Dehydration of alcohols (-H 2 O)

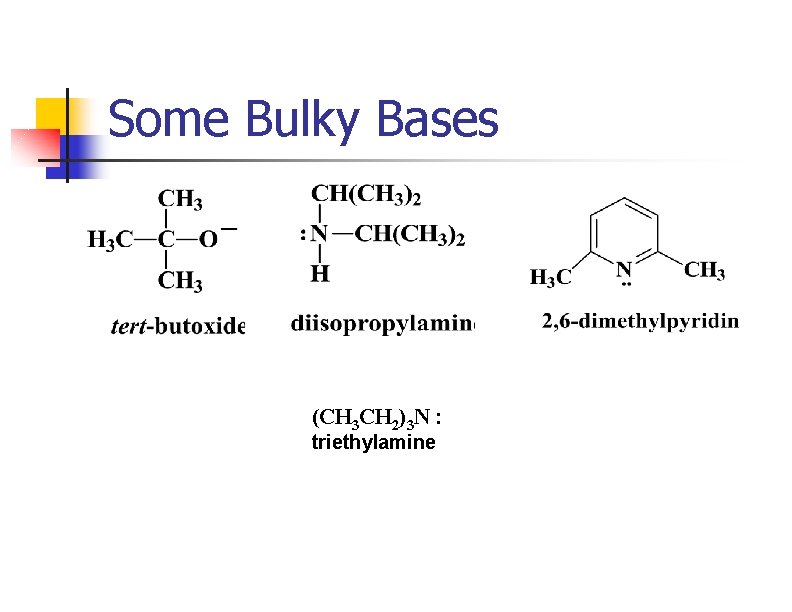
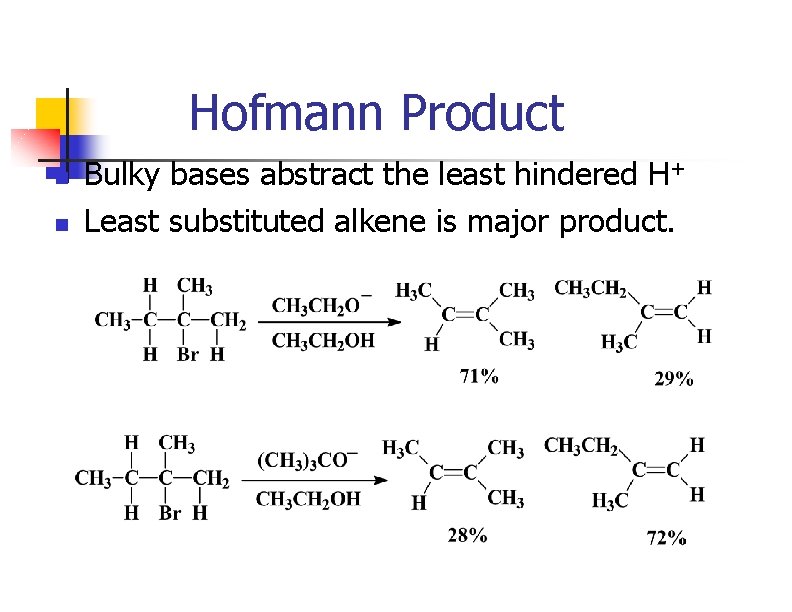
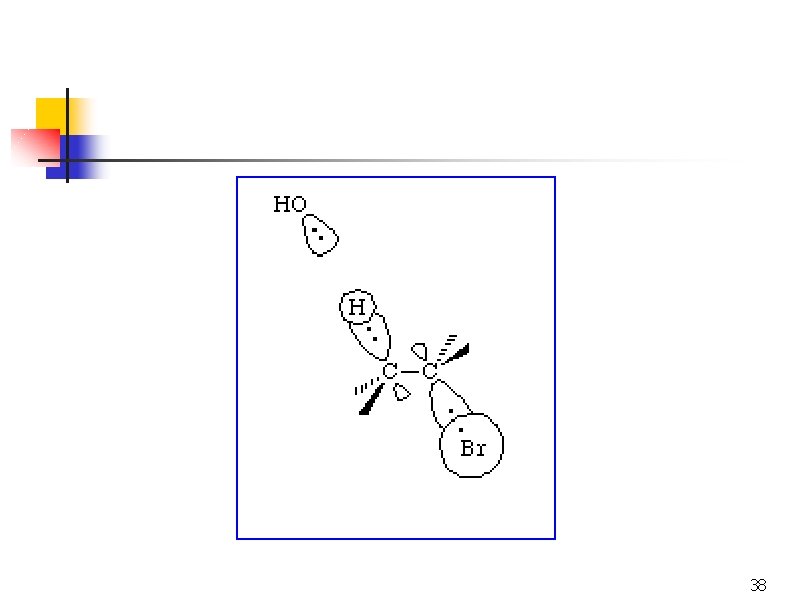
Removing HX via E 2 n n n Strong base abstracts H+ as X- leaves from the adjacent carbon. Tertiary and hindered secondary alkyl halides give good yields. Use a bulky base if the alkyl halide usually forms substitution products.

Some Bulky Bases (CH 3 CH 2)3 N : triethylamine

Hofmann Product n n Bulky bases abstract the least hindered H+ Least substituted alkene is major product.

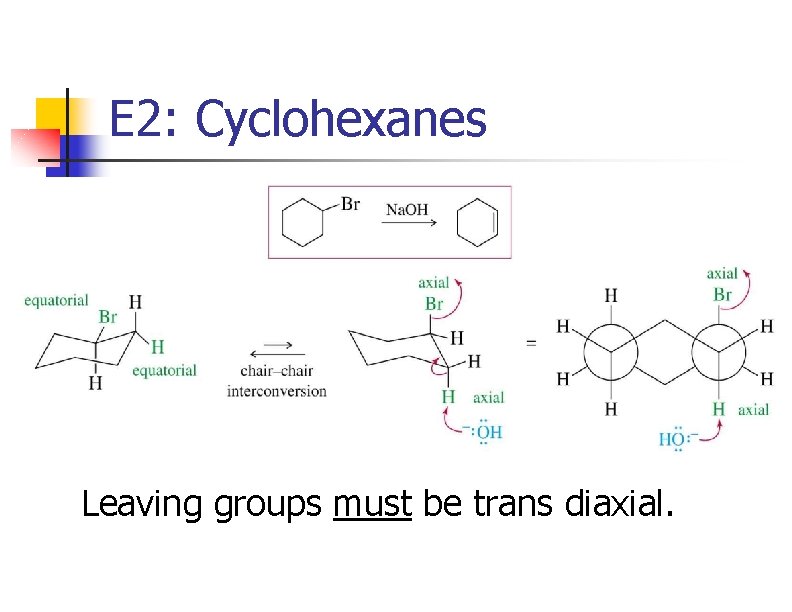
E 2: Cyclohexanes Leaving groups must be trans diaxial.

Removing HX via E 1 n n Secondary or tertiary halides Formation of carbocation intermediate Weak nucleophile Usually have substitution products too

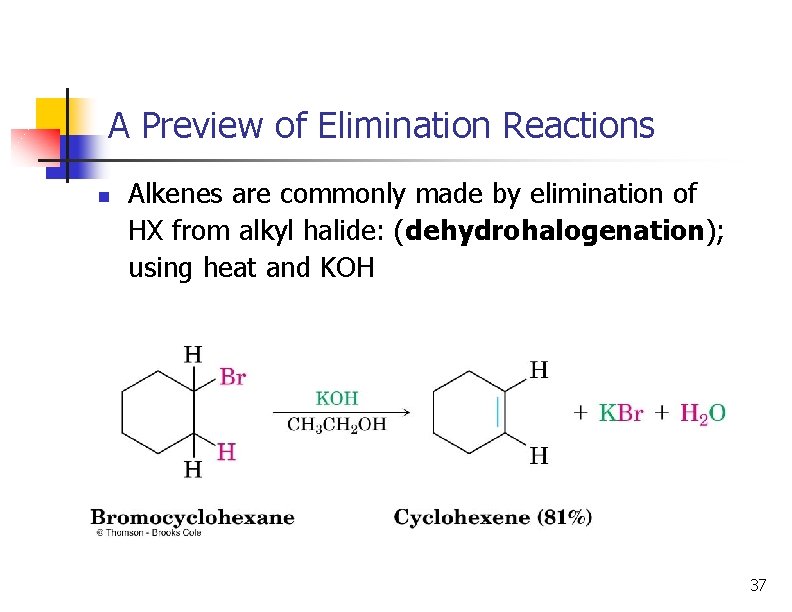
A Preview of Elimination Reactions n Alkenes are commonly made by elimination of HX from alkyl halide: (dehydrohalogenation); using heat and KOH 37

38

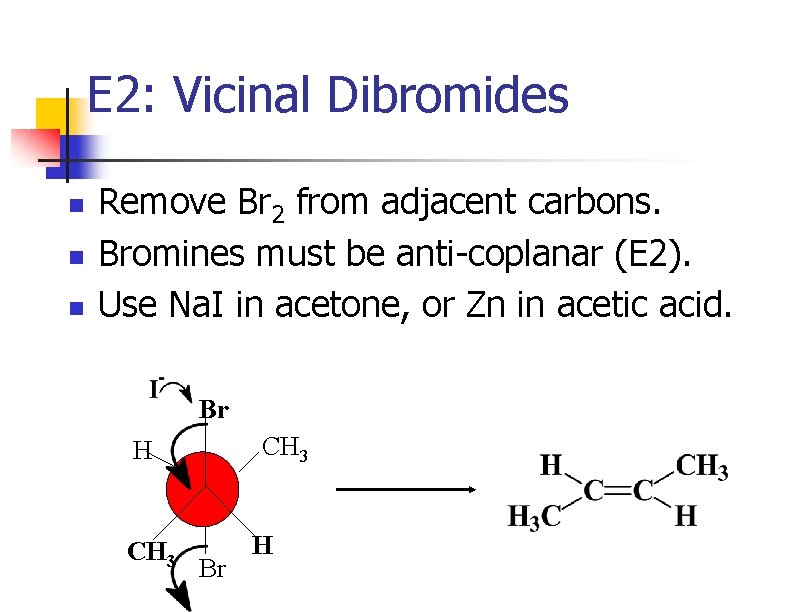
E 2: Vicinal Dibromides n n n Remove Br 2 from adjacent carbons. Bromines must be anti-coplanar (E 2). Use Na. I in acetone, or Zn in acetic acid. Br CH 3 H CH 3 Br H

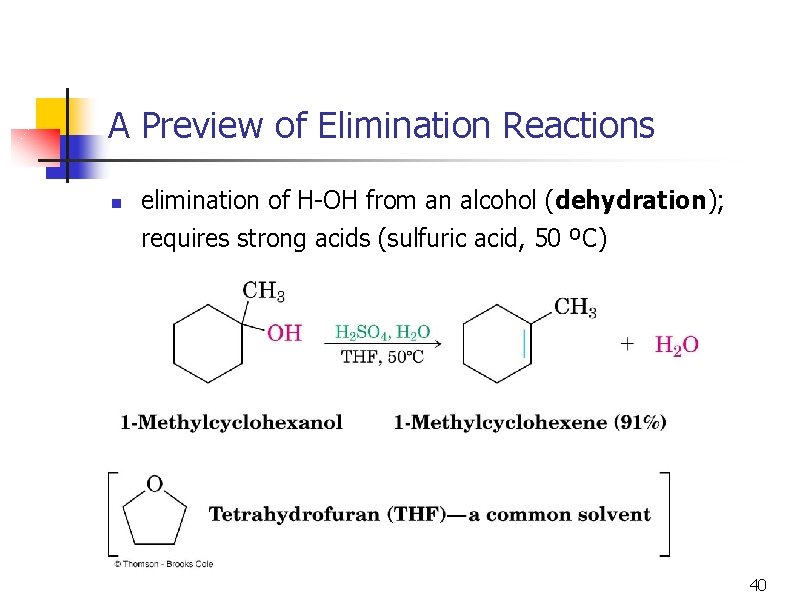
A Preview of Elimination Reactions n elimination of H-OH from an alcohol (dehydration); requires strong acids (sulfuric acid, 50 ºC) 40

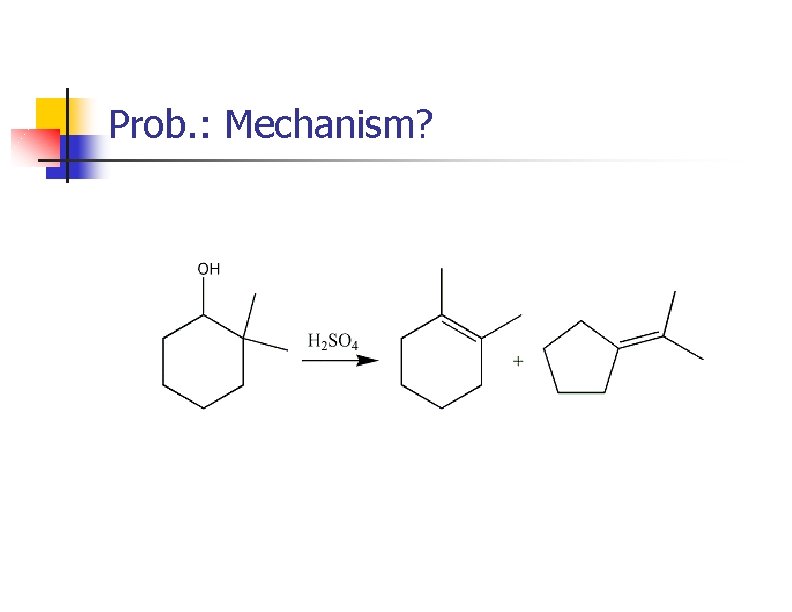
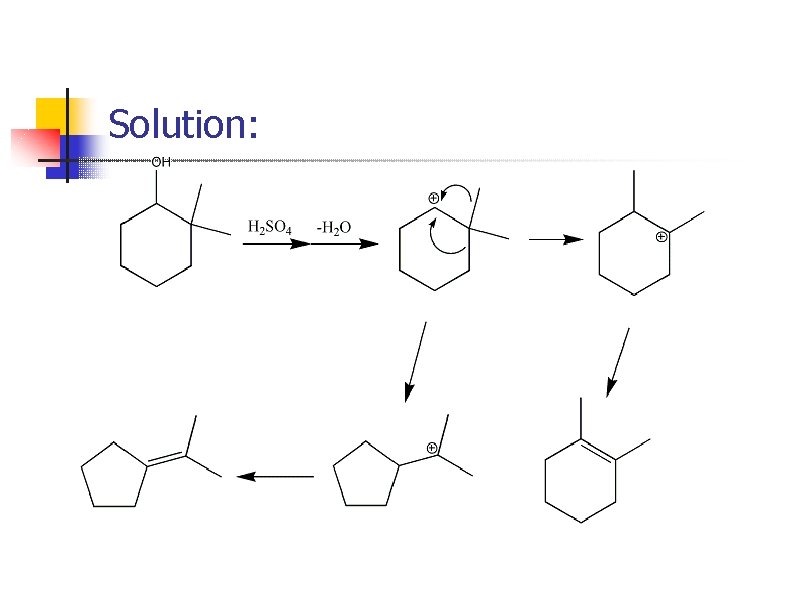
Prob. : Mechanism?

Solution:

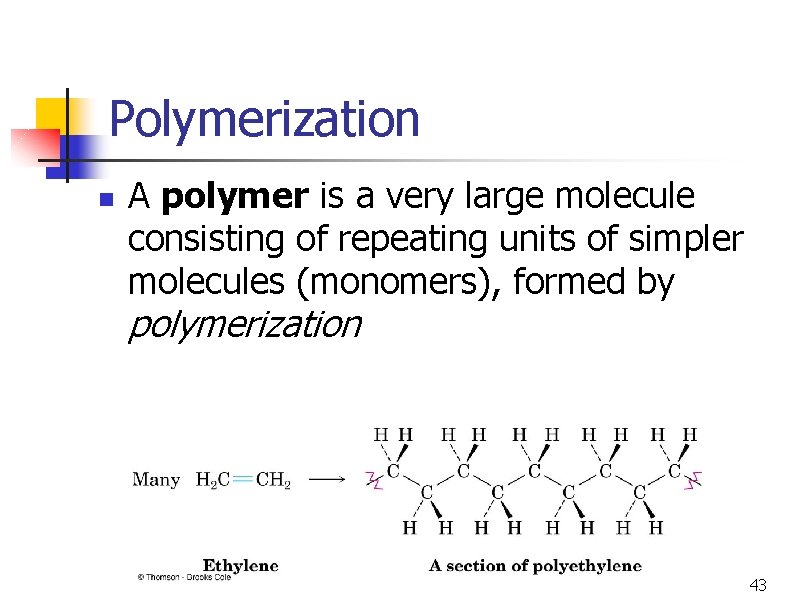
Polymerization n A polymer is a very large molecule consisting of repeating units of simpler molecules (monomers), formed by polymerization 43

Polymerization • An alkene (monomer) can add to another molecule like itself to form a chain (polymer). • Three methods: ØCationic, a carbocation intermediate ØFree radical ØAnionic, a carbanion intermediate (rare) 44

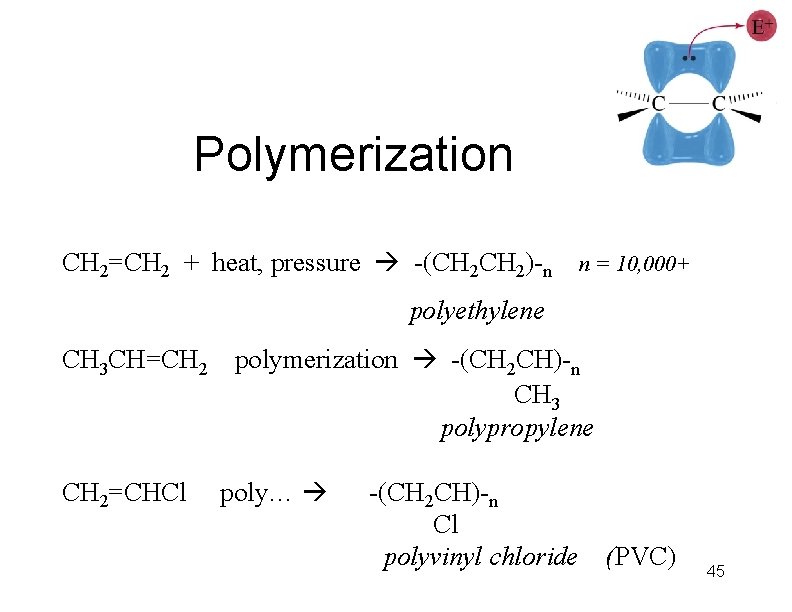
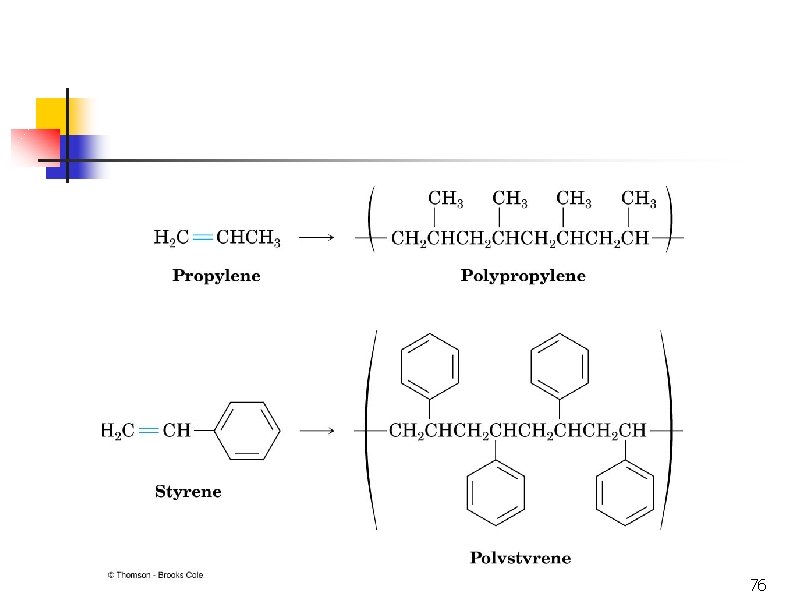
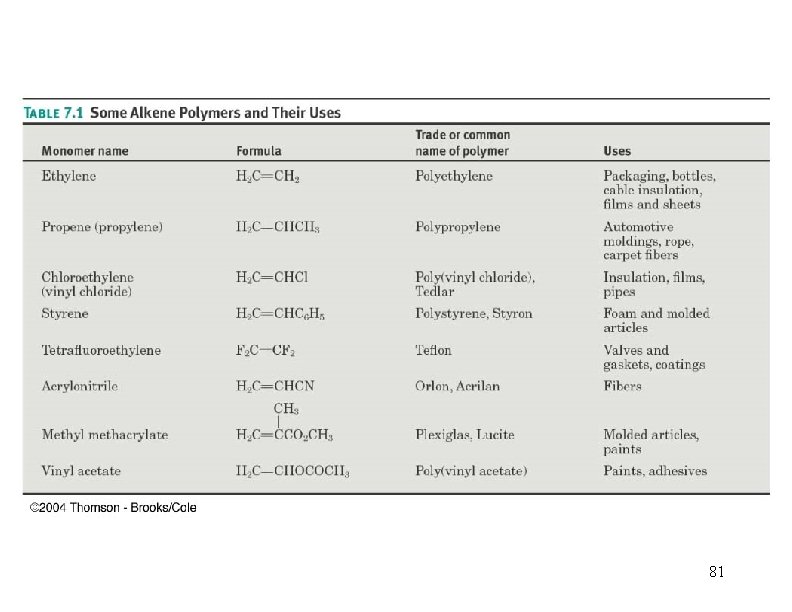
Polymerization CH 2=CH 2 + heat, pressure -(CH 2)-n n = 10, 000+ polyethylene CH 3 CH=CH 2=CHCl polymerization -(CH 2 CH)-n CH 3 polypropylene poly… -(CH 2 CH)-n Cl polyvinyl chloride (PVC) 45

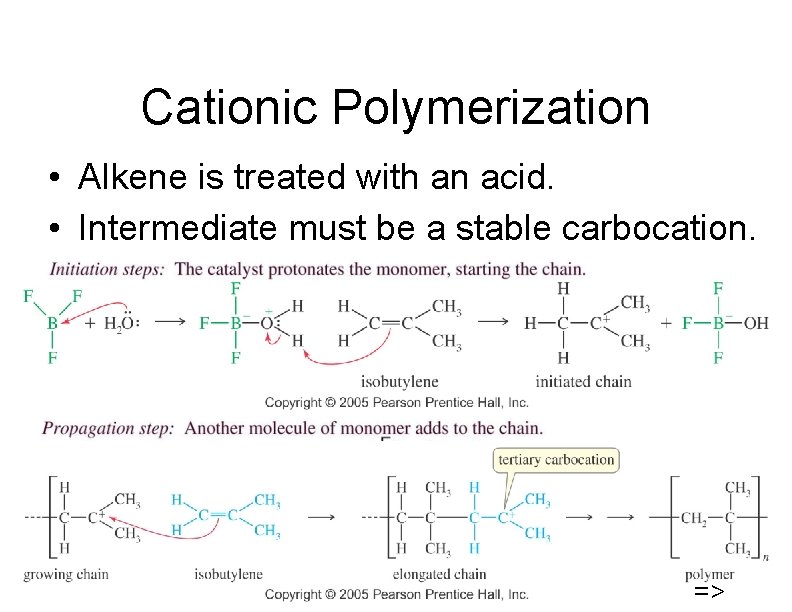
Cationic Polymerization • Alkene is treated with an acid. • Intermediate must be a stable carbocation. 46 =>

47

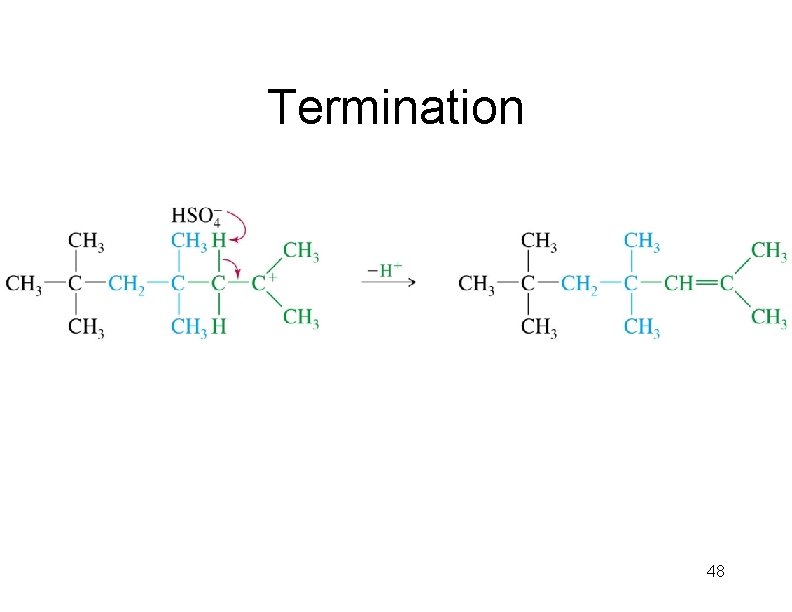
Termination 48

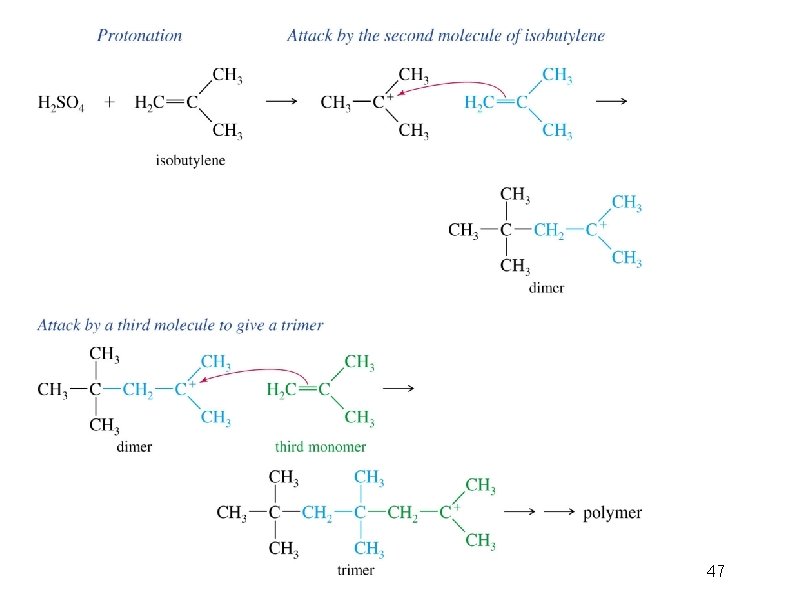
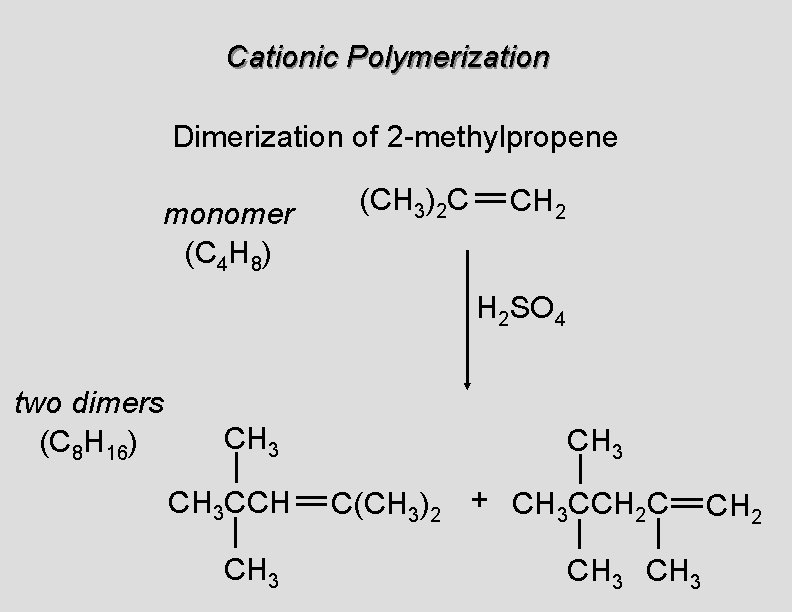
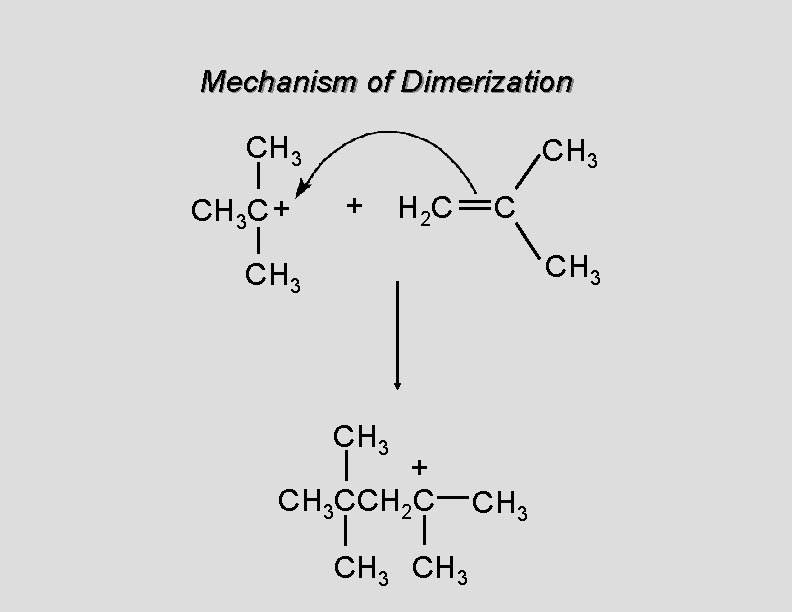
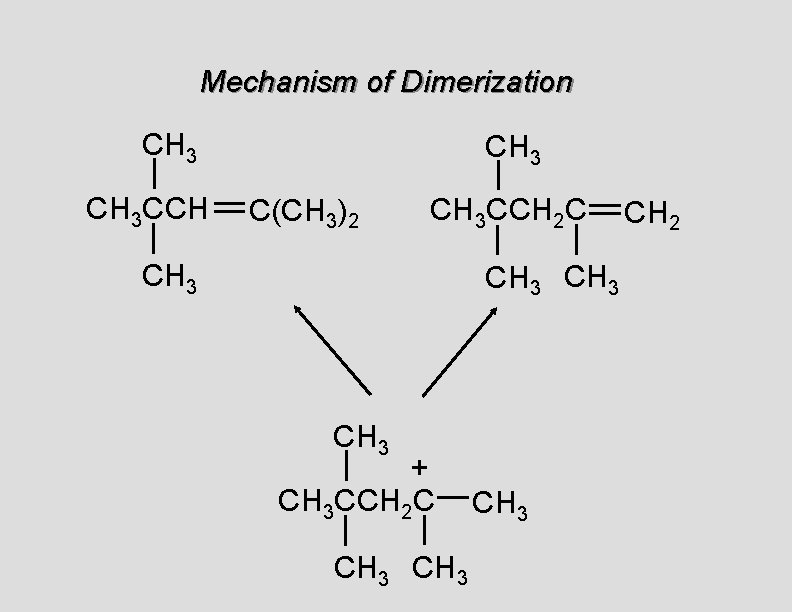
Cationic Polymerization Dimerization of 2 -methylpropene monomer (C 4 H 8) (CH 3)2 C CH 2 H 2 SO 4 two dimers (C 8 H 16) CH 3 CCH CH 3 C(CH 3)2 + CH 3 CCH 2 C CH 3 CH 2

Mechanism of Dimerization CH 3 C + CH 3 + H 2 C C CH 3 + CH 3 CCH 2 C CH 3

Mechanism of Dimerization CH 3 CCH CH 3 C(CH 3)2 CH 3 CCH 2 C CH 3 + CH 3 CCH 2 C CH 3 CH 2

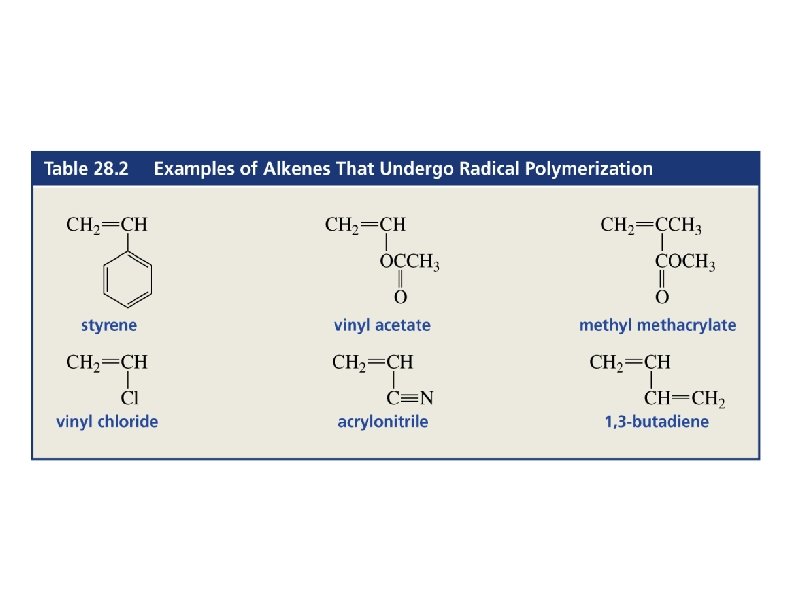
Free Radical Polymerization of Alkenes n Alkenes combine many times to give polymer n Reactivity induced by formation of free radicals

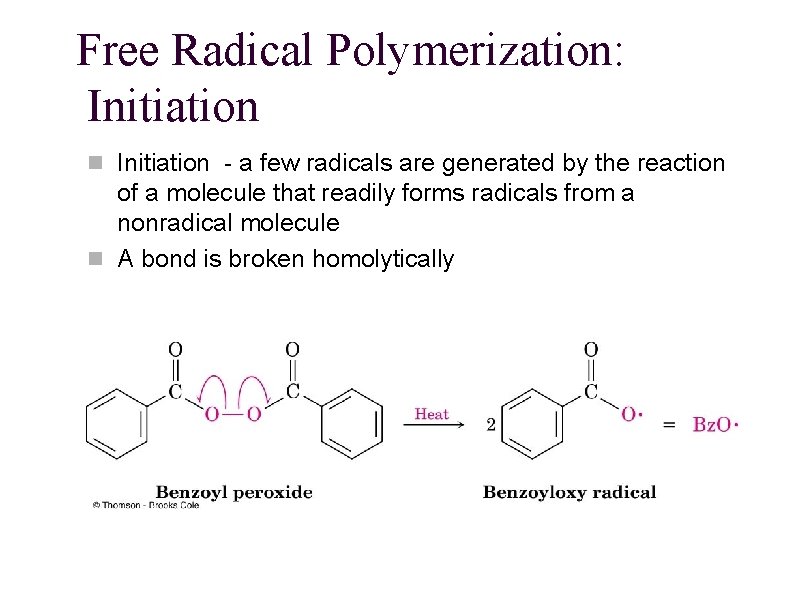
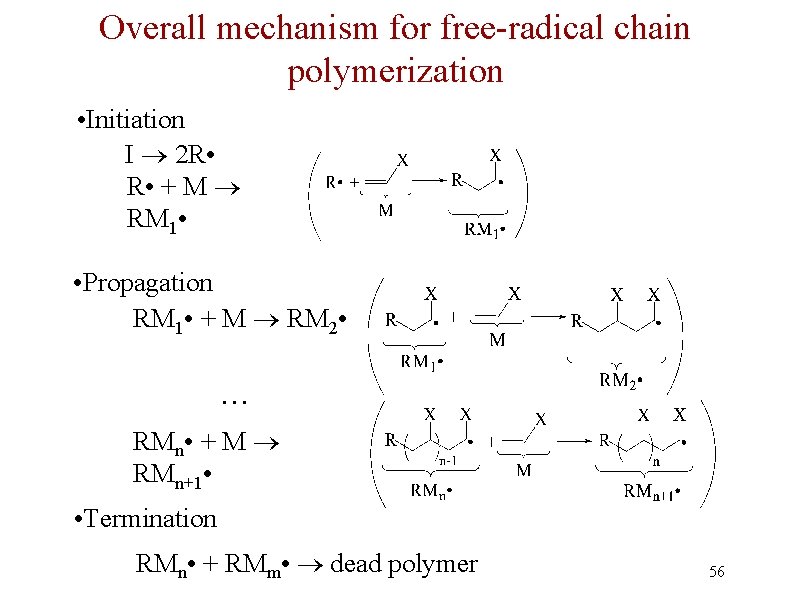
Free Radical Polymerization: Initiation n Initiation - a few radicals are generated by the reaction of a molecule that readily forms radicals from a nonradical molecule n A bond is broken homolytically

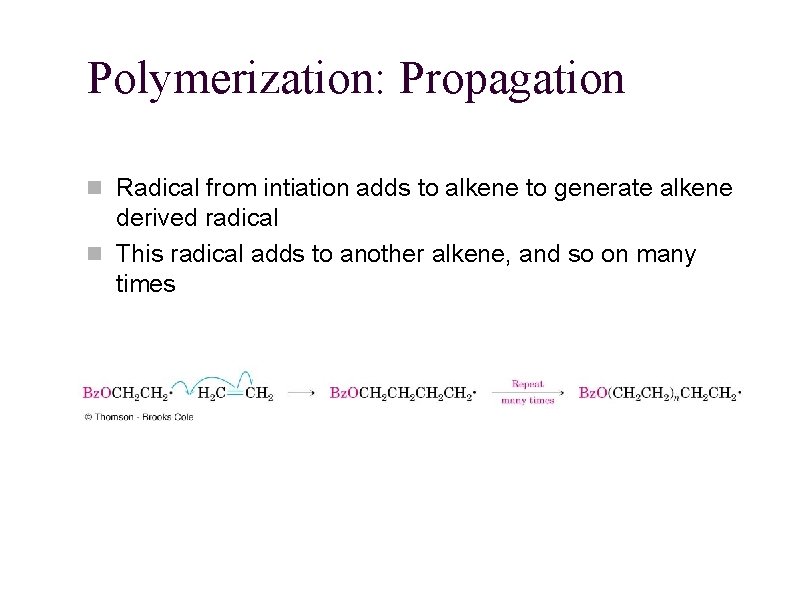
Polymerization: Propagation n Radical from intiation adds to alkene to generate alkene derived radical n This radical adds to another alkene, and so on many times

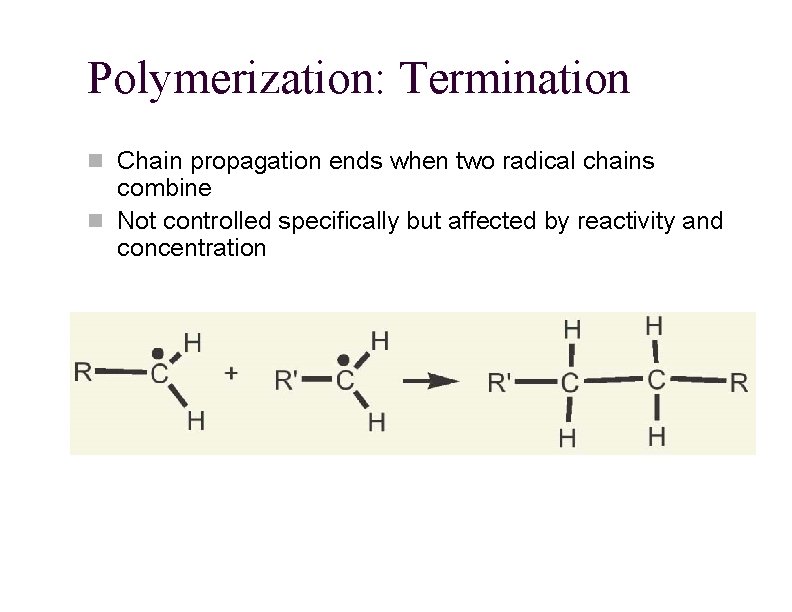
Polymerization: Termination n Chain propagation ends when two radical chains combine n Not controlled specifically but affected by reactivity and concentration

Overall mechanism for free-radical chain polymerization • Initiation I 2 R • + M RM 1 • • Propagation RM 1 • + M RM 2 • … RMn • + M RMn+1 • • Termination RMn • + RMm • dead polymer 56

57

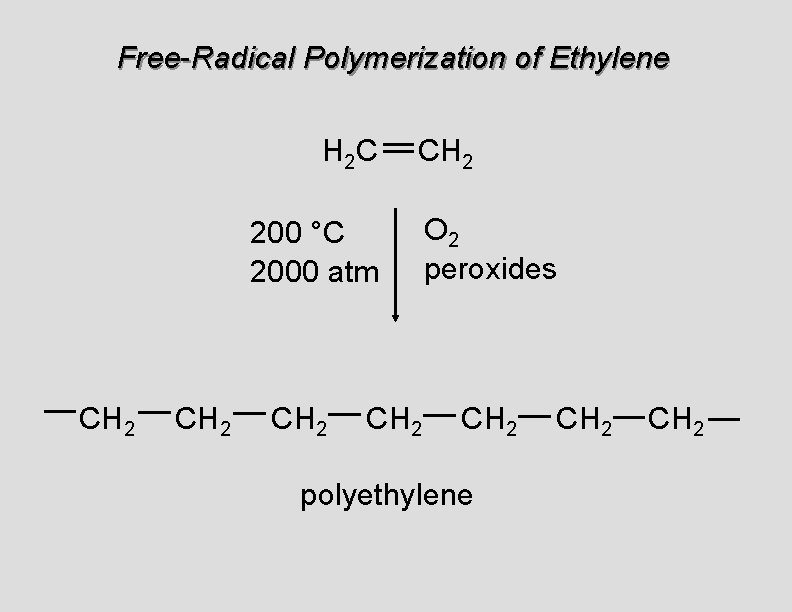
Free-Radical Polymerization of Ethylene H 2 C CH 2 200 °C 2000 atm CH 2 O 2 peroxides CH 2 polyethylene CH 2

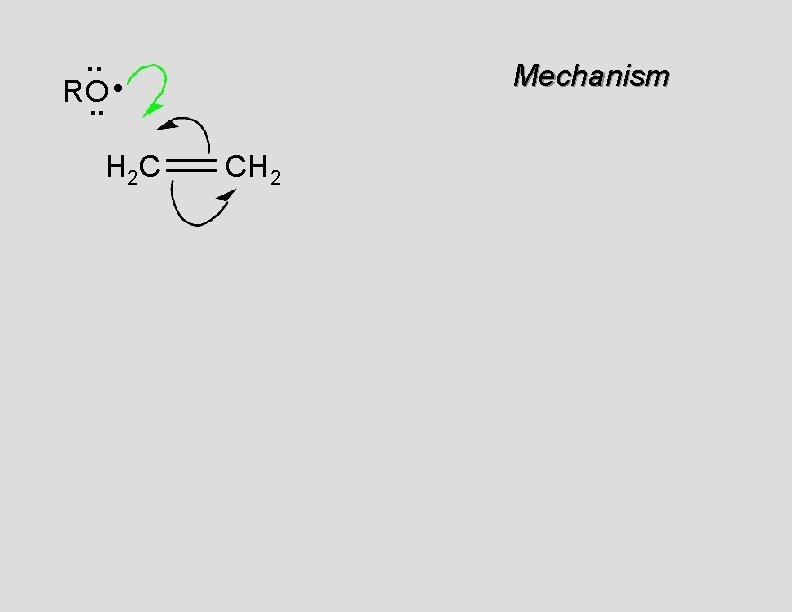
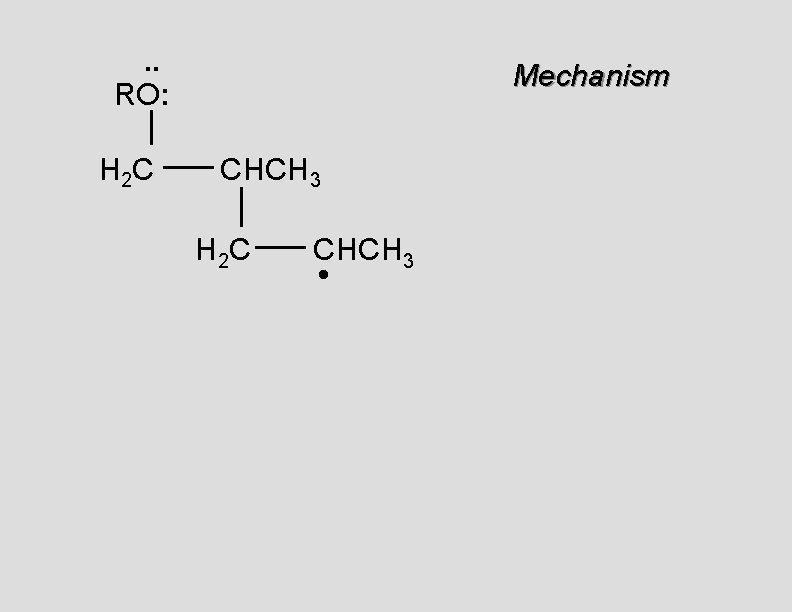
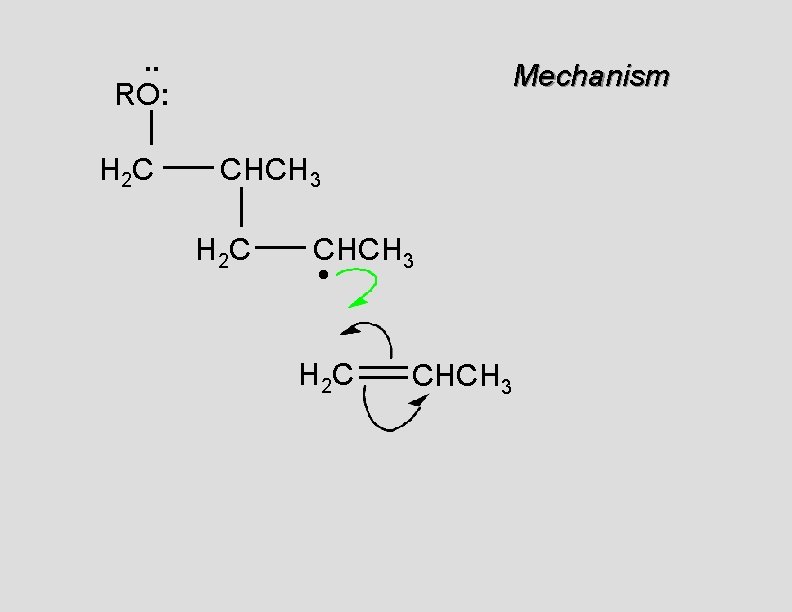
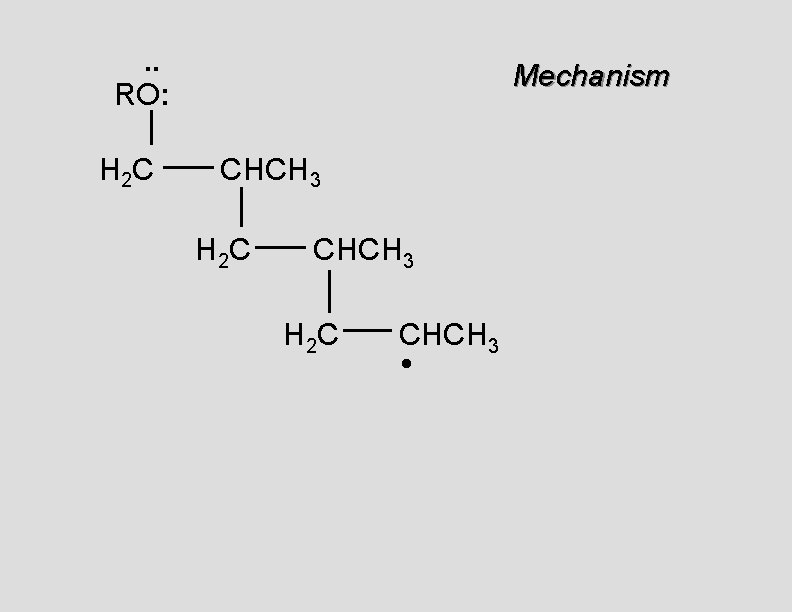
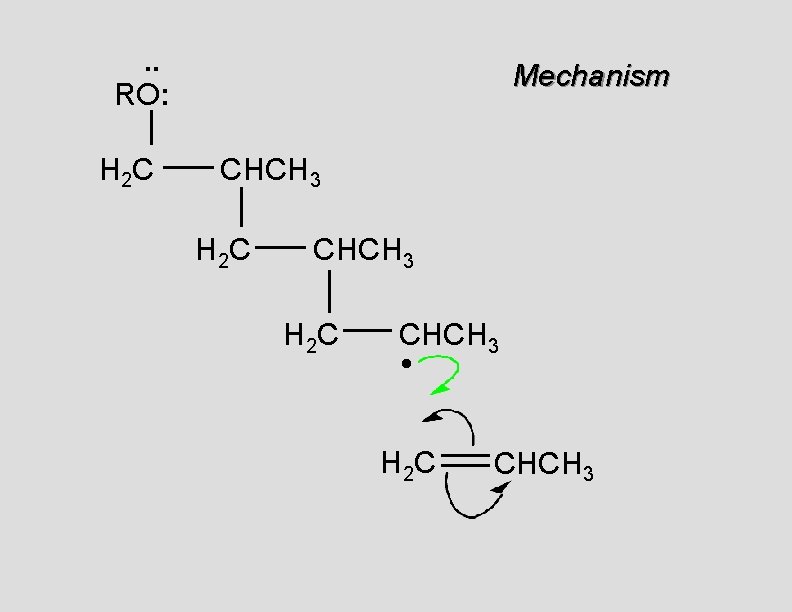
. . • RO. . H 2 C Mechanism CH 2

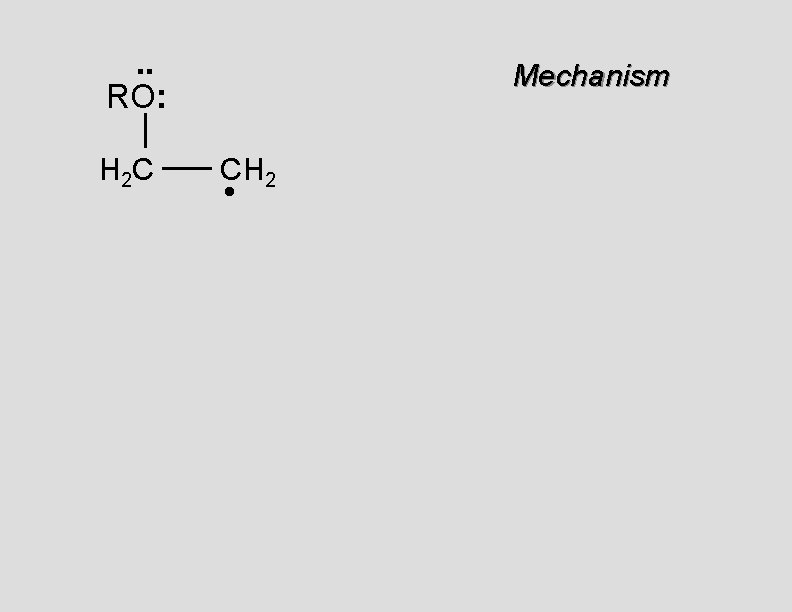
. . RO: H 2 C Mechanism CH 2 •

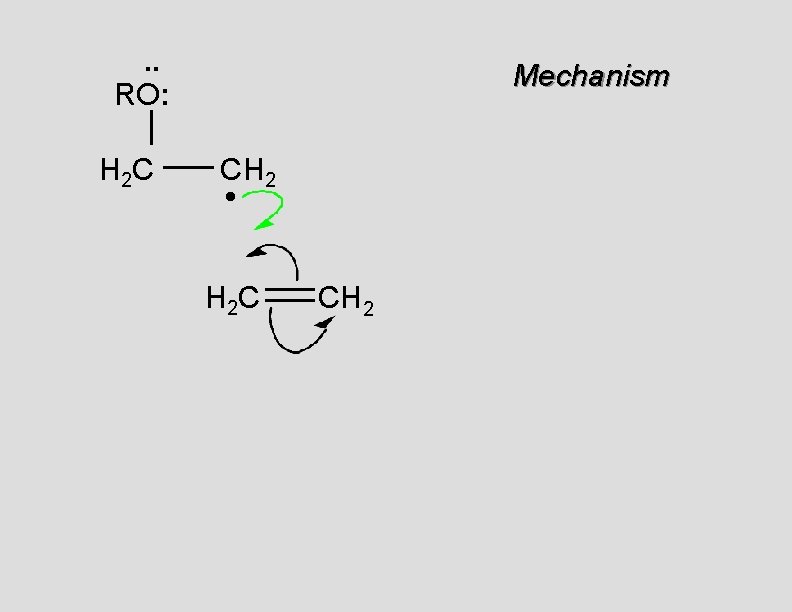
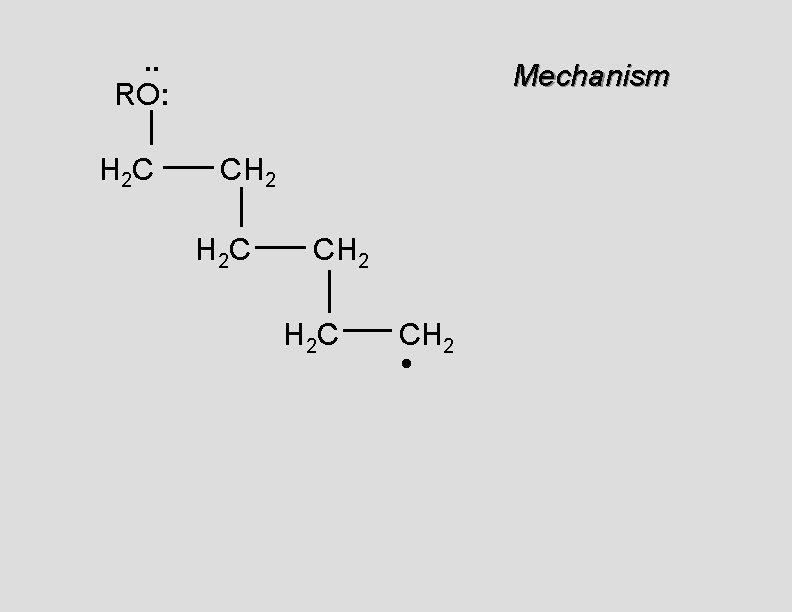
. . RO: H 2 C Mechanism CH 2 • H 2 C CH 2

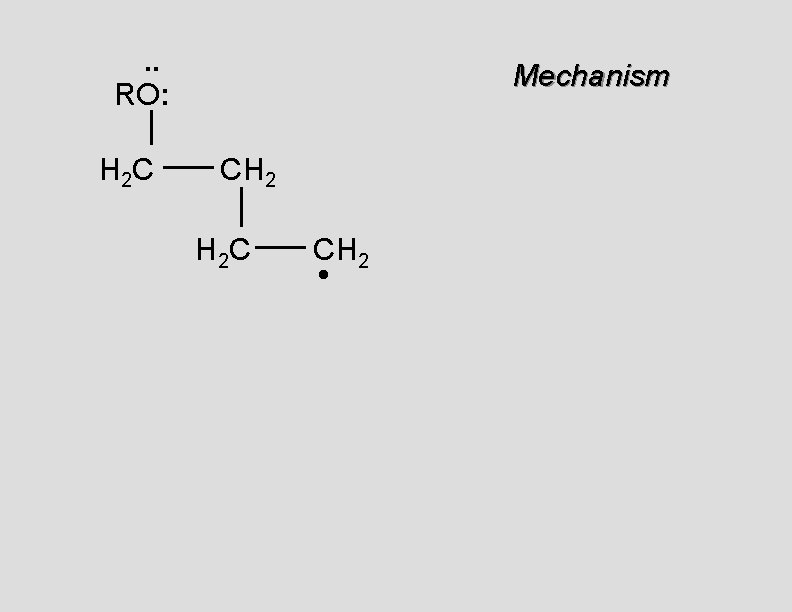
. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 •

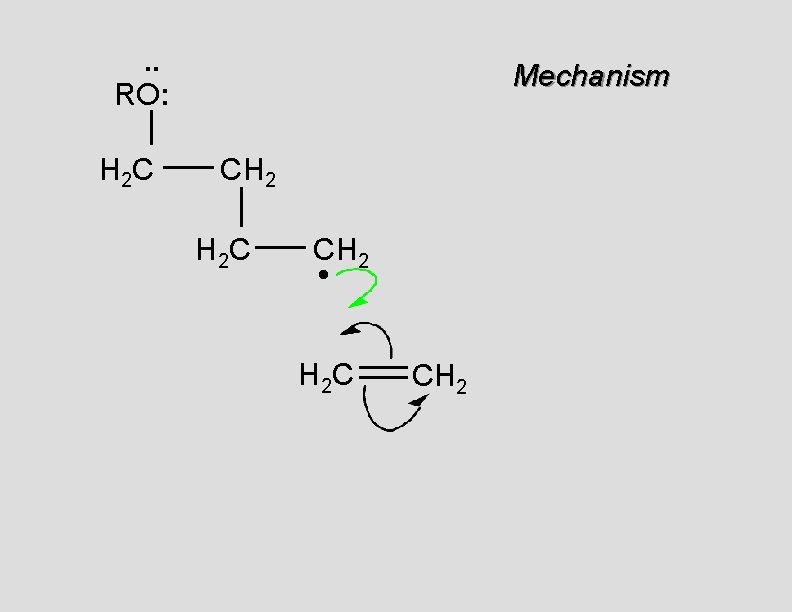
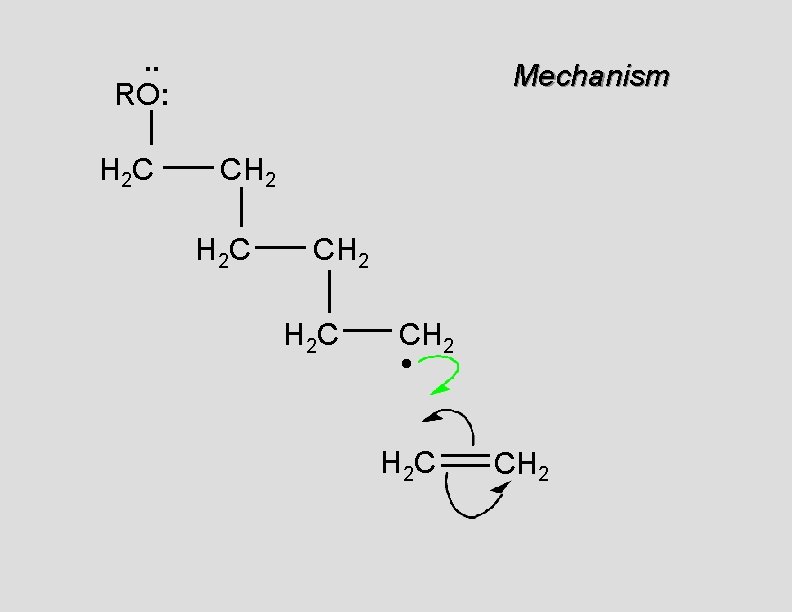
. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 • H 2 C CH 2

. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 •

. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 • H 2 C CH 2

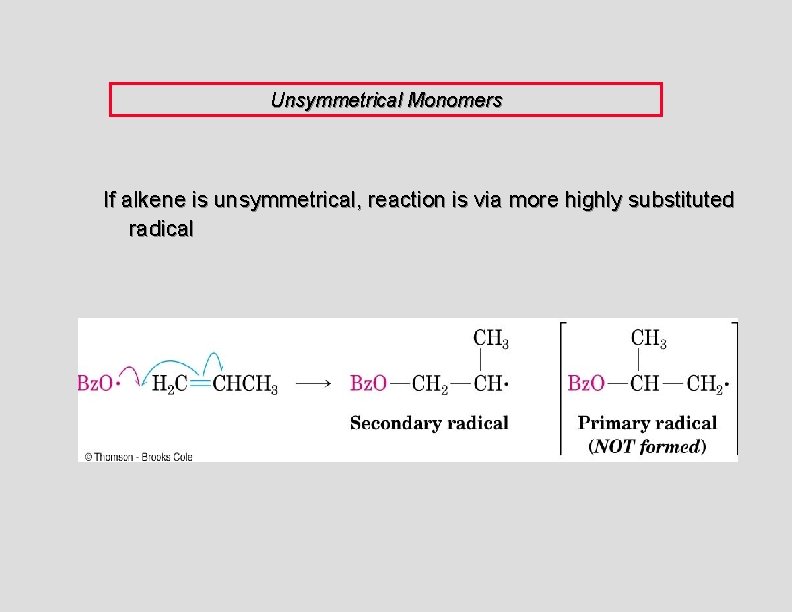
Unsymmetrical Monomers If alkene is unsymmetrical, reaction is via more highly substituted radical

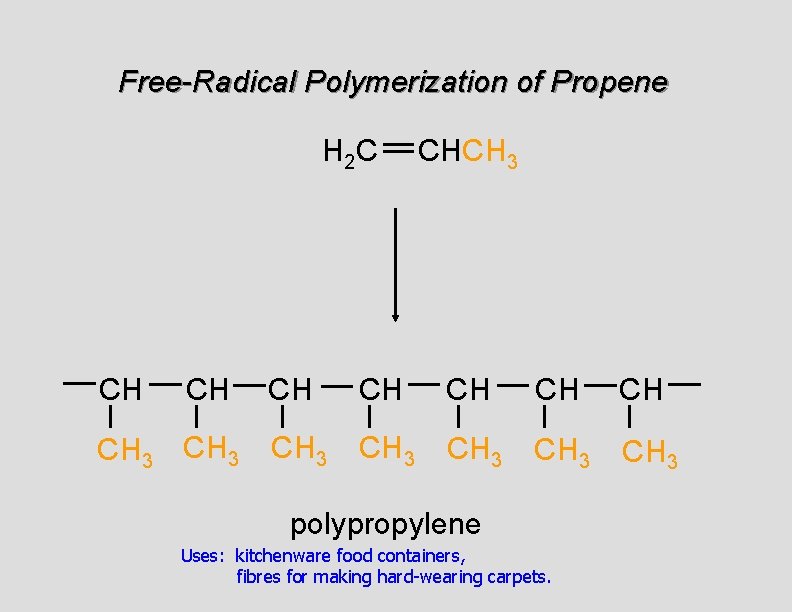
Free-Radical Polymerization of Propene H 2 C CH CH CH 3 CHCH 3 CH CH CH 3 CH 3 polypropylene Uses: kitchenware food containers, fibres for making hard-wearing carpets.

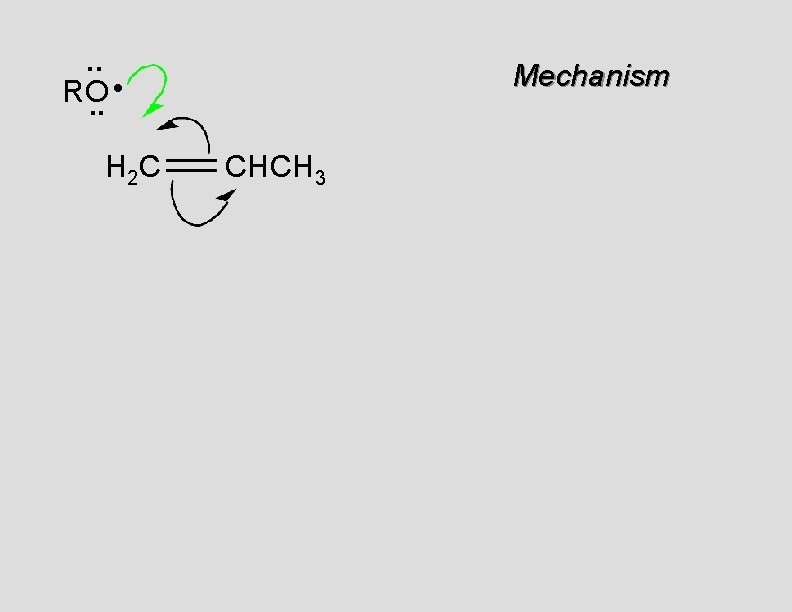
. . • RO. . H 2 C Mechanism CHCH 3

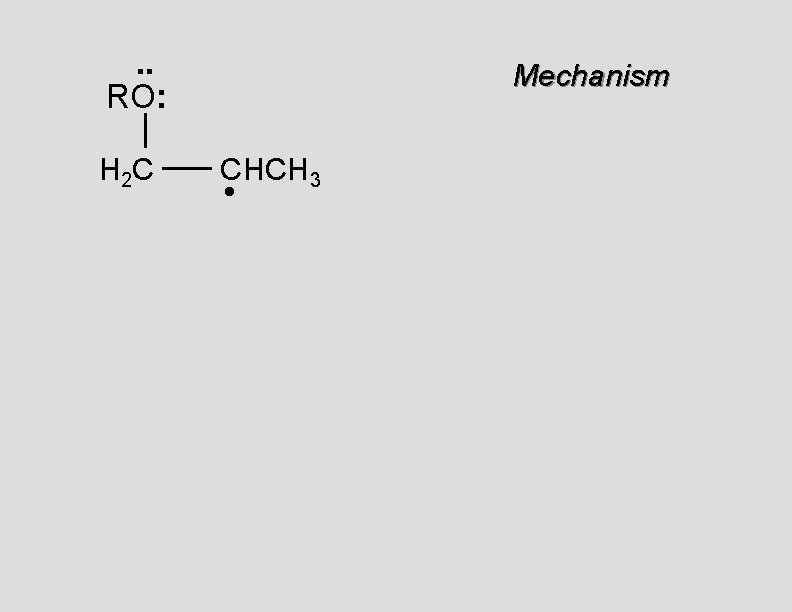
. . RO: H 2 C Mechanism CHCH 3 •

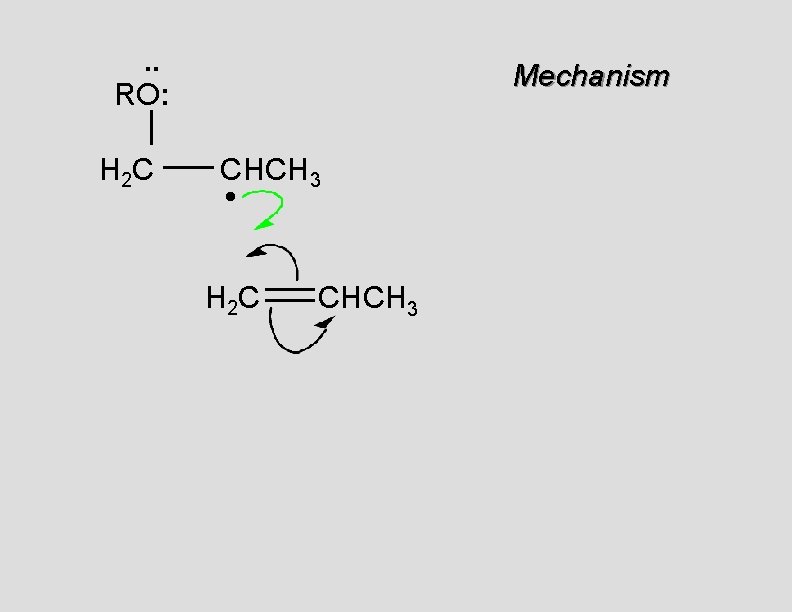
. . RO: H 2 C Mechanism CHCH 3 • H 2 C CHCH 3

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 •

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 • H 2 C CHCH 3

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 •

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 • H 2 C CHCH 3

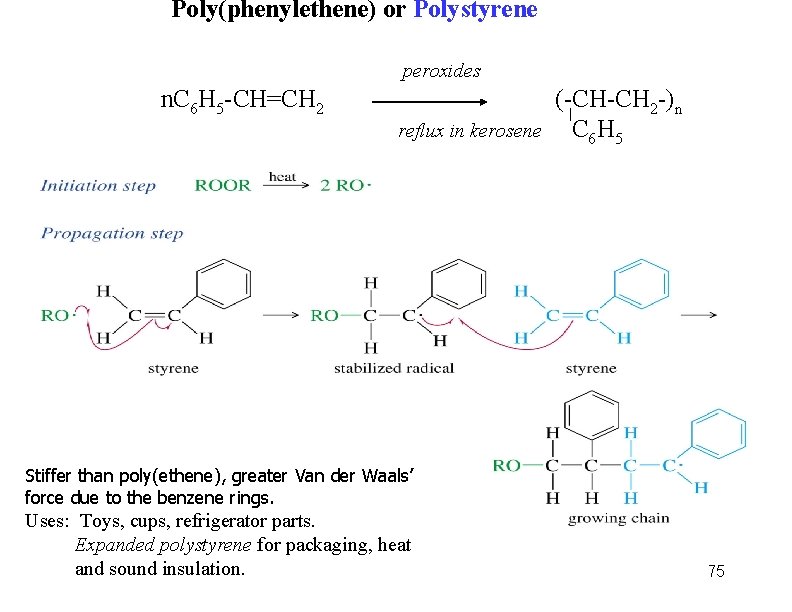
Poly(phenylethene) or Polystyrene peroxides n. C 6 H 5 -CH=CH 2 reflux in kerosene (-CH-CH 2 -)n C 6 H 5 Stiffer than poly(ethene), greater Van der Waals’ force due to the benzene rings. Uses: Toys, cups, refrigerator parts. Expanded polystyrene for packaging, heat and sound insulation. 75

76

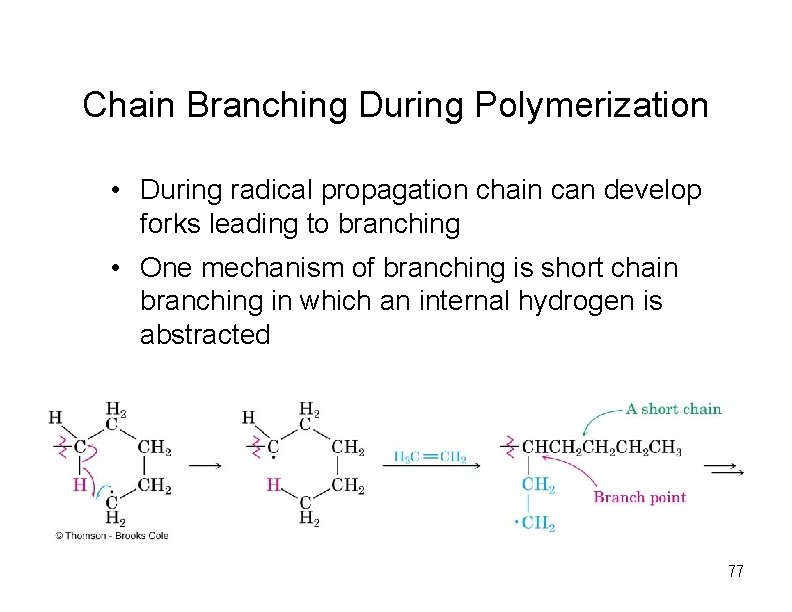
Chain Branching During Polymerization • During radical propagation chain can develop forks leading to branching • One mechanism of branching is short chain branching in which an internal hydrogen is abstracted 77

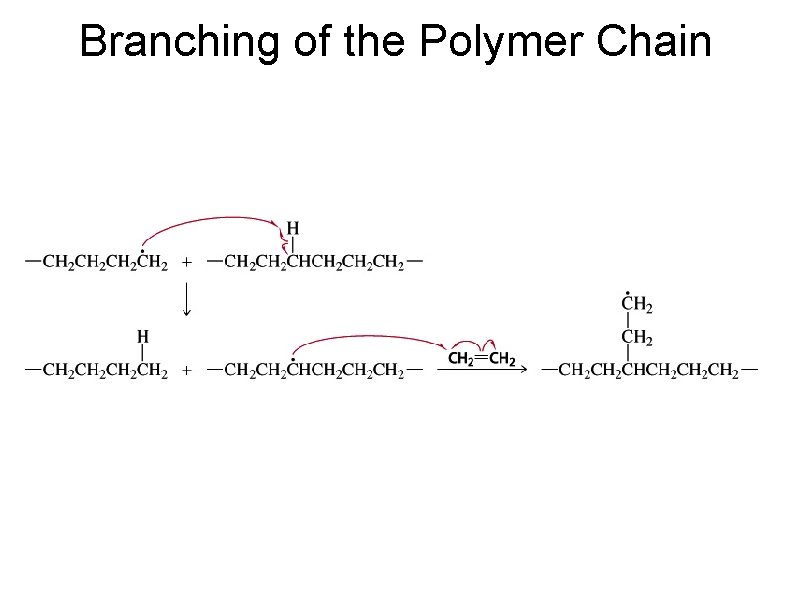
Branching of the Polymer Chain 78

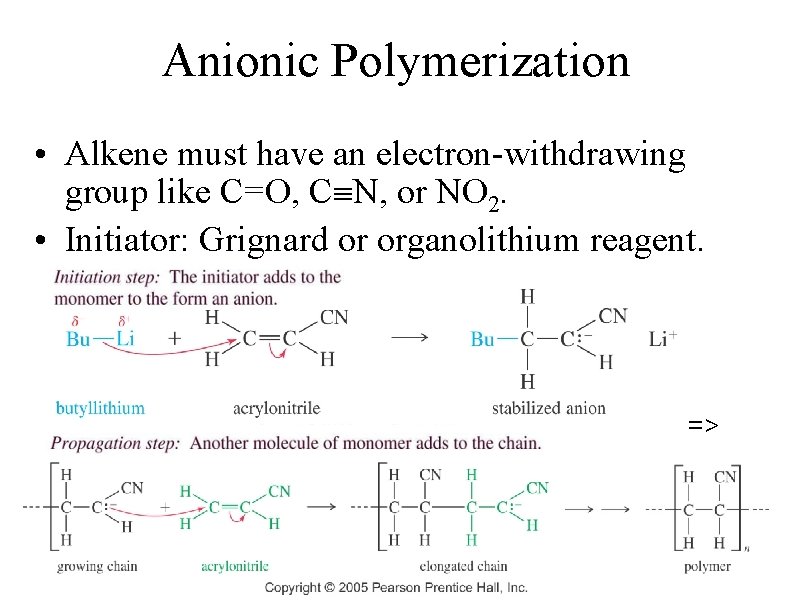
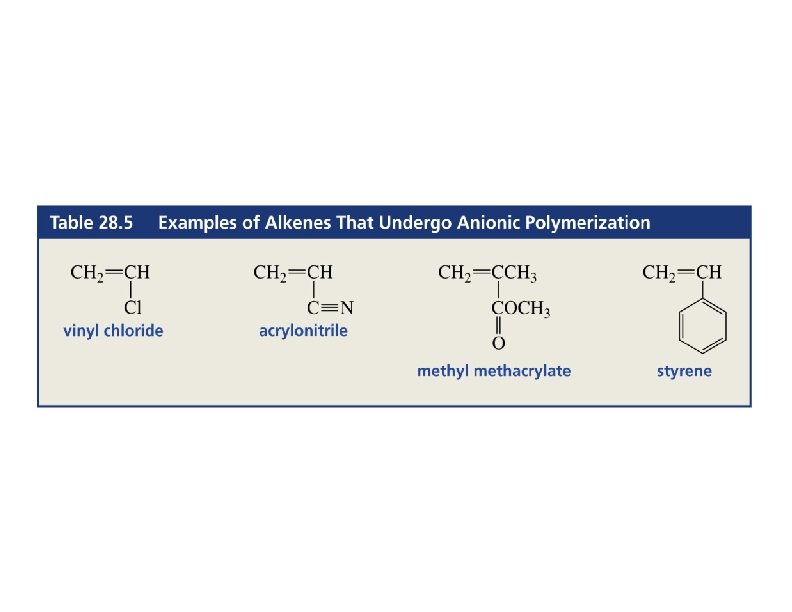
Anionic Polymerization • Alkene must have an electron-withdrawing group like C=O, C N, or NO 2. • Initiator: Grignard or organolithium reagent. => 79

80

81

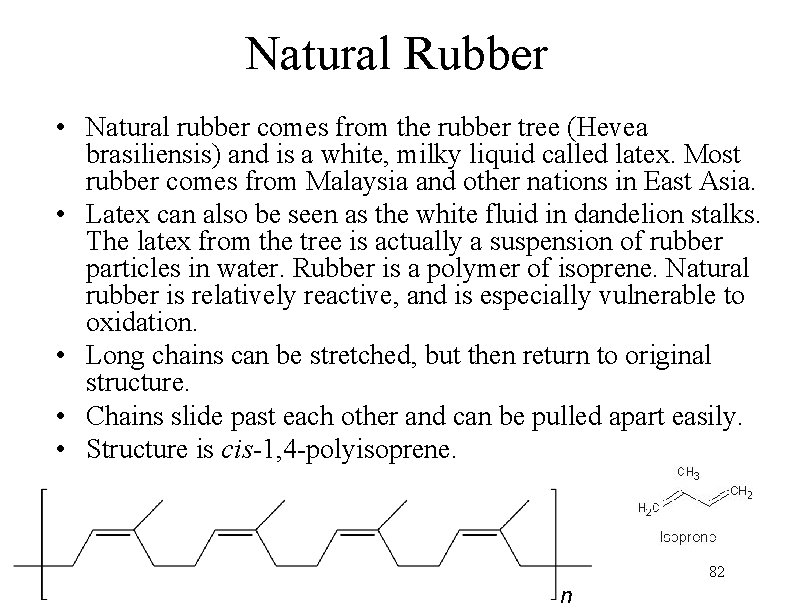
Natural Rubber • Natural rubber comes from the rubber tree (Hevea brasiliensis) and is a white, milky liquid called latex. Most rubber comes from Malaysia and other nations in East Asia. • Latex can also be seen as the white fluid in dandelion stalks. The latex from the tree is actually a suspension of rubber particles in water. Rubber is a polymer of isoprene. Natural rubber is relatively reactive, and is especially vulnerable to oxidation. • Long chains can be stretched, but then return to original structure. • Chains slide past each other and can be pulled apart easily. • Structure is cis-1, 4 -polyisoprene. 82 n

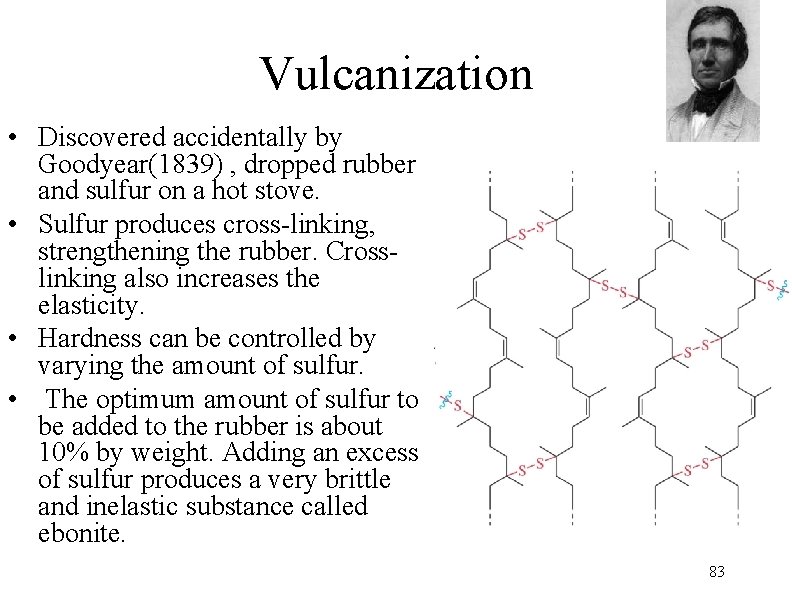
Vulcanization • Discovered accidentally by Goodyear(1839) , dropped rubber and sulfur on a hot stove. • Sulfur produces cross-linking, strengthening the rubber. Crosslinking also increases the elasticity. • Hardness can be controlled by varying the amount of sulfur. • The optimum amount of sulfur to be added to the rubber is about 10% by weight. Adding an excess of sulfur produces a very brittle and inelastic substance called ebonite. 83

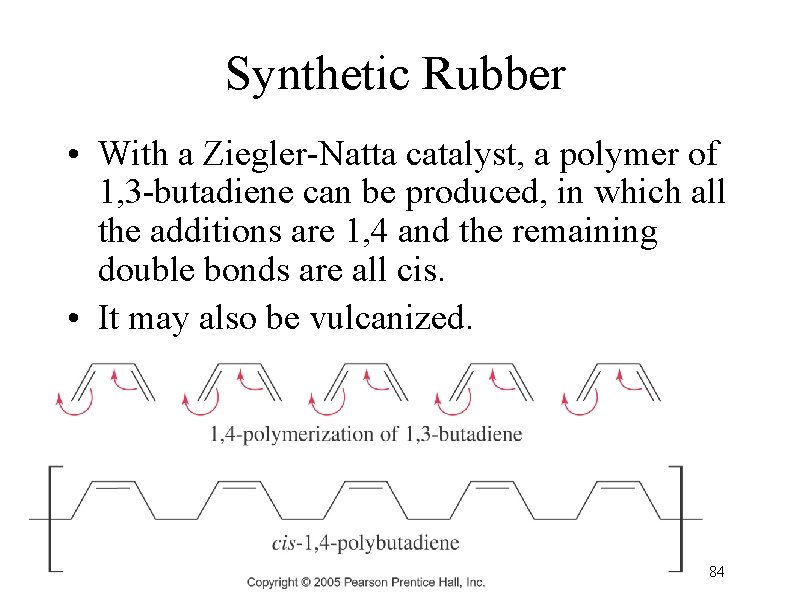
Synthetic Rubber • With a Ziegler-Natta catalyst, a polymer of 1, 3 -butadiene can be produced, in which all the additions are 1, 4 and the remaining double bonds are all cis. • It may also be vulcanized. 84

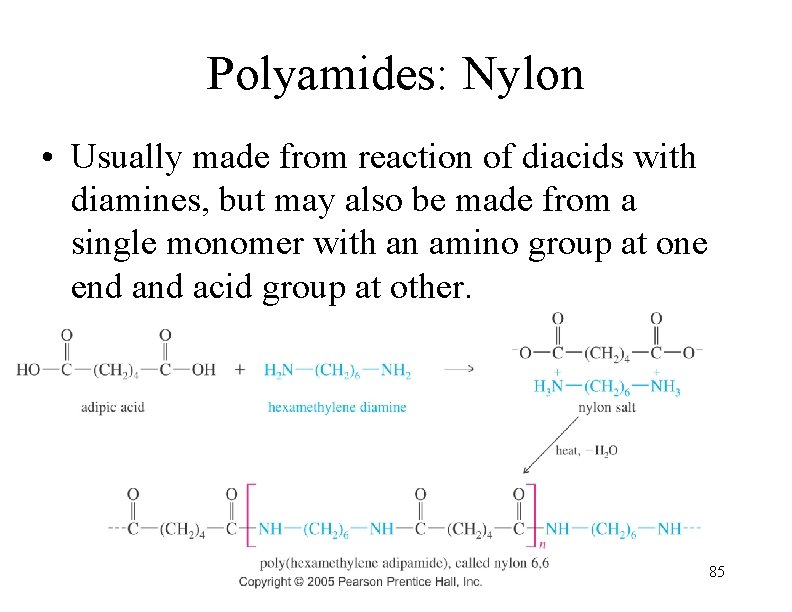
Polyamides: Nylon • Usually made from reaction of diacids with diamines, but may also be made from a single monomer with an amino group at one end acid group at other. 85

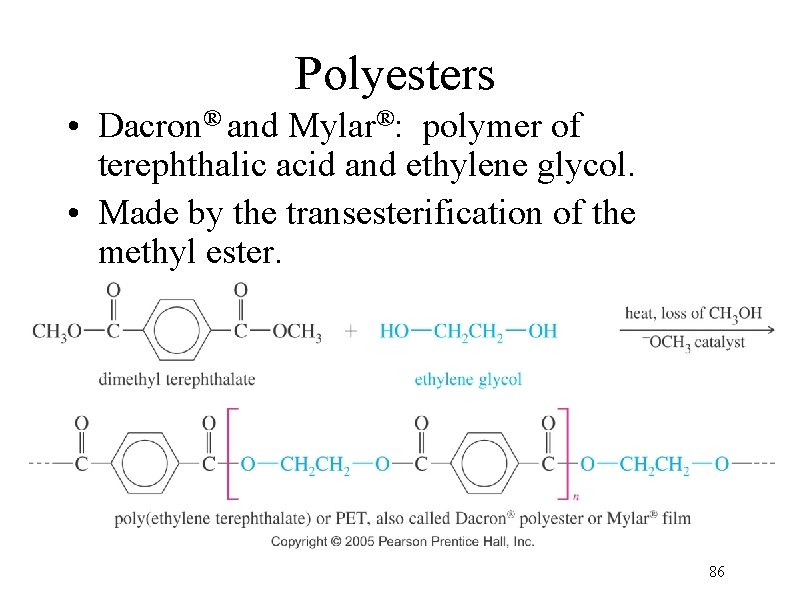
Polyesters • Dacron® and Mylar®: polymer of terephthalic acid and ethylene glycol. • Made by the transesterification of the methyl ester. 86

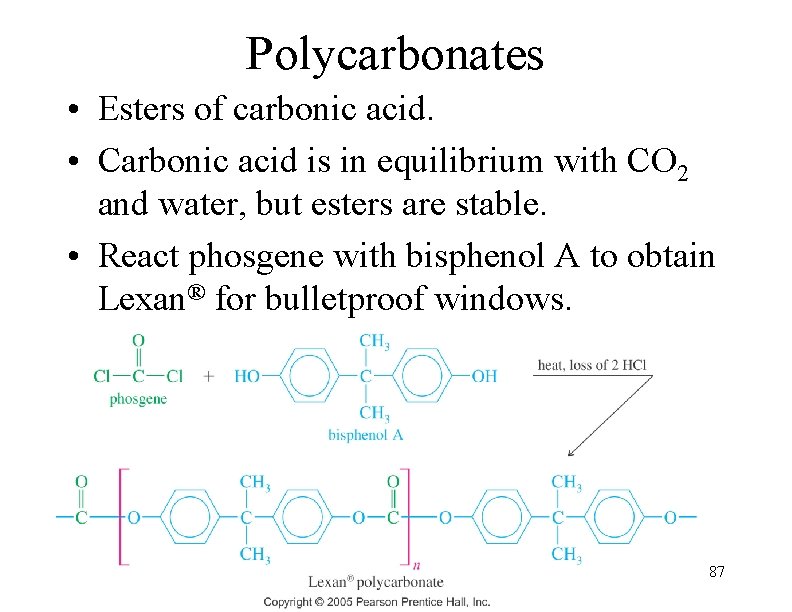
Polycarbonates • Esters of carbonic acid. • Carbonic acid is in equilibrium with CO 2 and water, but esters are stable. • React phosgene with bisphenol A to obtain Lexan® for bulletproof windows. 87

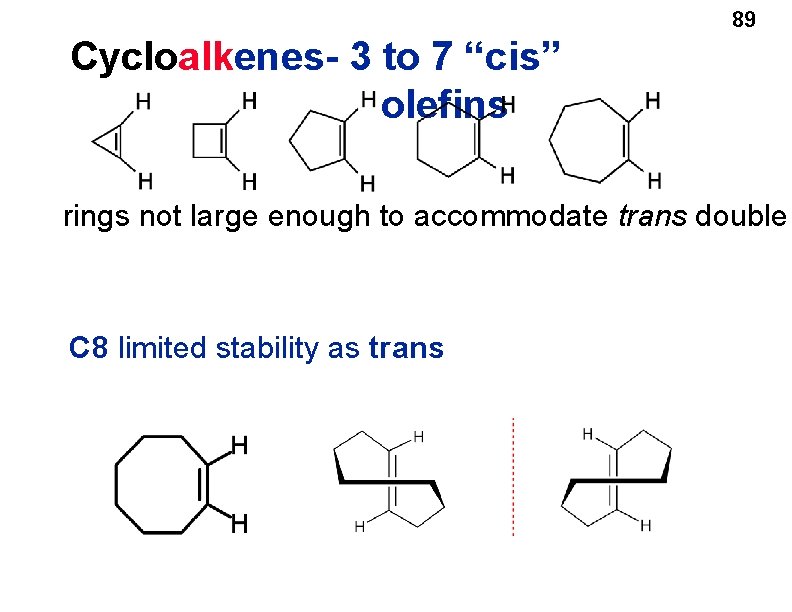
Cycloalkenes • Cyclopropene and cyclobutene have angle strain. • Larger cycloalkenes, such as cyclopentene and cyclohexene, can incorporate a double bond into the ring with little or no angle strain. 88

89 Cycloalkenes- 3 to 7 “cis” olefins rings not large enough to accommodate trans double C 8 limited stability as trans

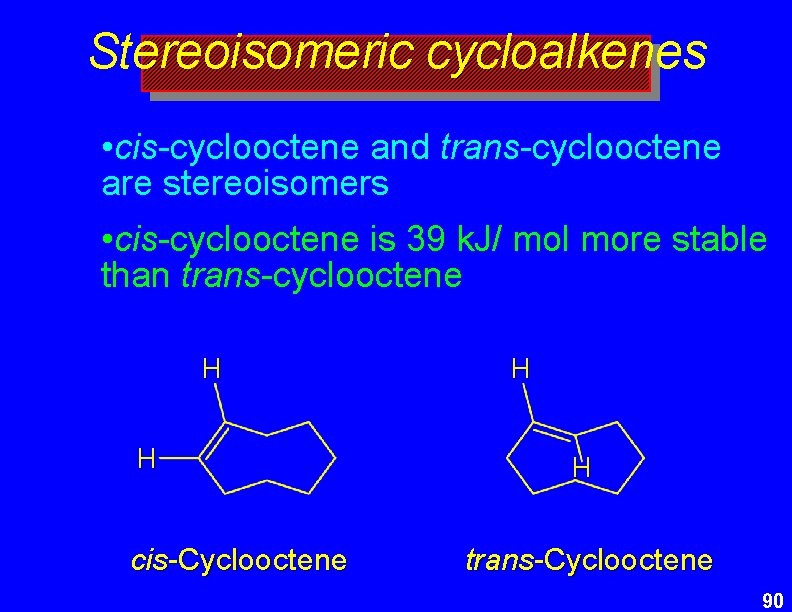
Stereoisomeric cycloalkenes • cis-cyclooctene and trans-cyclooctene are stereoisomers • cis-cyclooctene is 39 k. J/ mol more stable than trans-cyclooctene H H cis-Cyclooctene H H trans-Cyclooctene 90

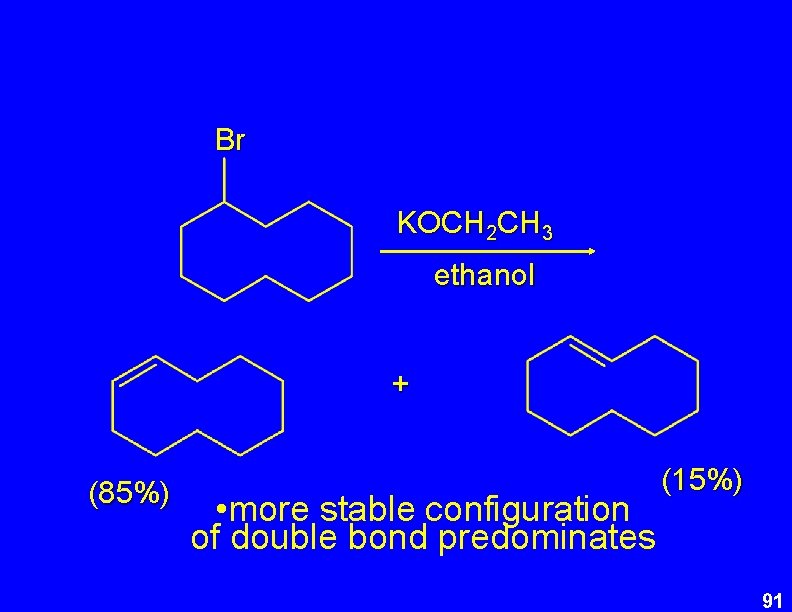
Br KOCH 2 CH 3 ethanol + (85%) • more stable configuration of double bond predominates (15%) 91

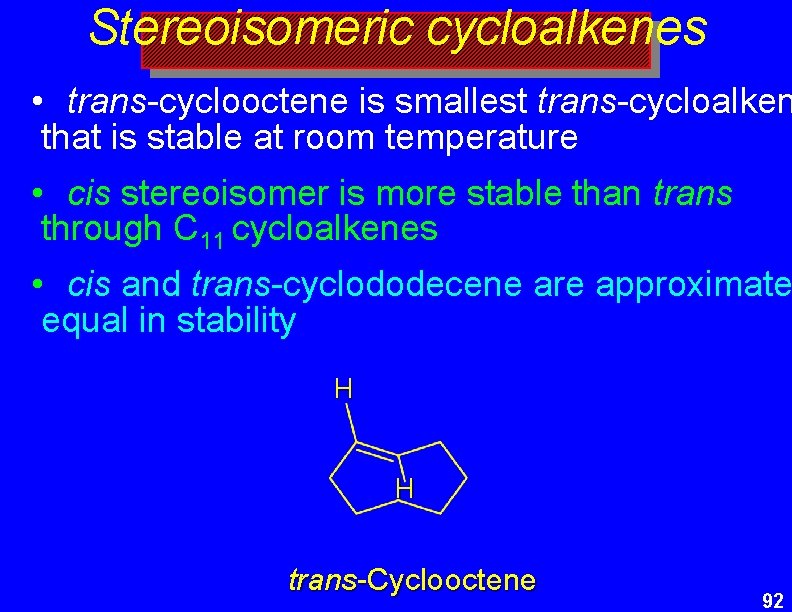
Stereoisomeric cycloalkenes • trans-cyclooctene is smallest trans-cycloalken that is stable at room temperature • cis stereoisomer is more stable than trans through C 11 cycloalkenes • cis and trans-cyclododecene are approximate equal in stability H H trans-Cyclooctene 92

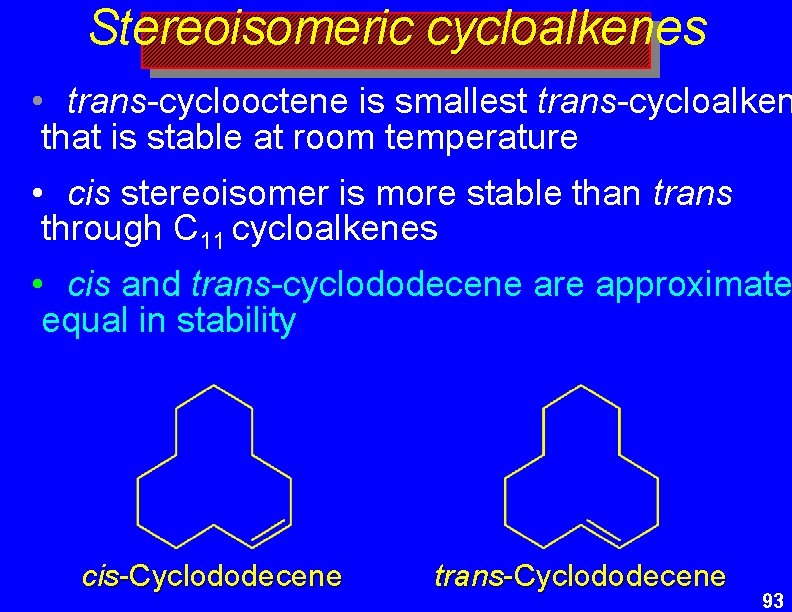
Stereoisomeric cycloalkenes • trans-cyclooctene is smallest trans-cycloalken that is stable at room temperature • cis stereoisomer is more stable than trans through C 11 cycloalkenes • cis and trans-cyclododecene are approximate equal in stability cis-Cyclododecene trans-Cyclododecene 93