Organic Chemistry Second Edition David Klein Chapter 8





- Slides: 85

Organic Chemistry Second Edition David Klein Chapter 8 Alkenes: Structure and Preparation via Elimination Reactions Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

8. 1 Introduction to Elimination • Elimination reactions often compete with substitution reactions. • What are the two main ingredients for a substitution? – A nucleophile and an electrophile with a leaving group • What are the two main ingredients for an elimination? – A base and an electrophile with a leaving group • How is a base both similar and different from a nucleophile? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -2 Klein, Organic Chemistry 2 e

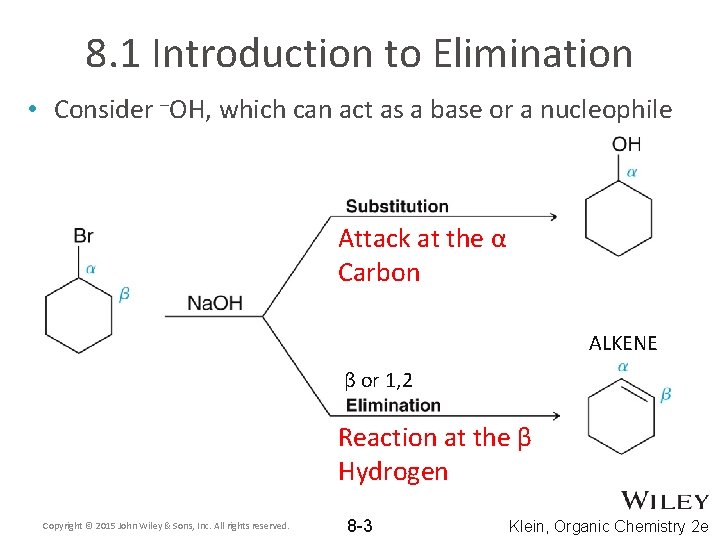
8. 1 Introduction to Elimination • Consider –OH, which can act as a base or a nucleophile Attack at the α Carbon ALKENE β or 1, 2 Reaction at the β Hydrogen Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -3 Klein, Organic Chemistry 2 e

8. 3 Alkene Nomenclature • Alkenes are named using the same procedure we used in Chapter 4 to name alkanes with minor modifications 1. Identify the parent chain, which should include the C=C double bond 2. Identify and Name the substituents 3. Assign a locant (and prefix if necessary) to each substituent. Give the C=C double bond the lowest number possible 4. List the numbered substituents before the parent name in numerical order. 5. The C=C double bond locant is placed either just before the parent name or just before the -ene suffix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -4 Klein, Organic Chemistry 2 e

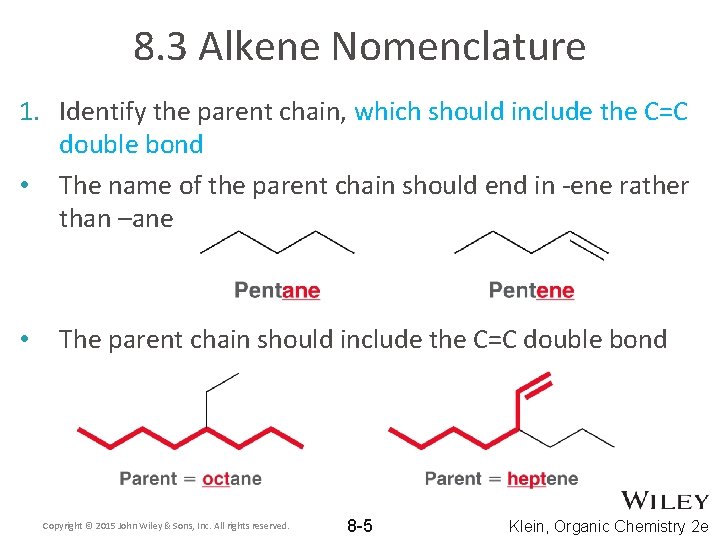
8. 3 Alkene Nomenclature 1. Identify the parent chain, which should include the C=C double bond • The name of the parent chain should end in -ene rather than –ane • The parent chain should include the C=C double bond Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -5 Klein, Organic Chemistry 2 e

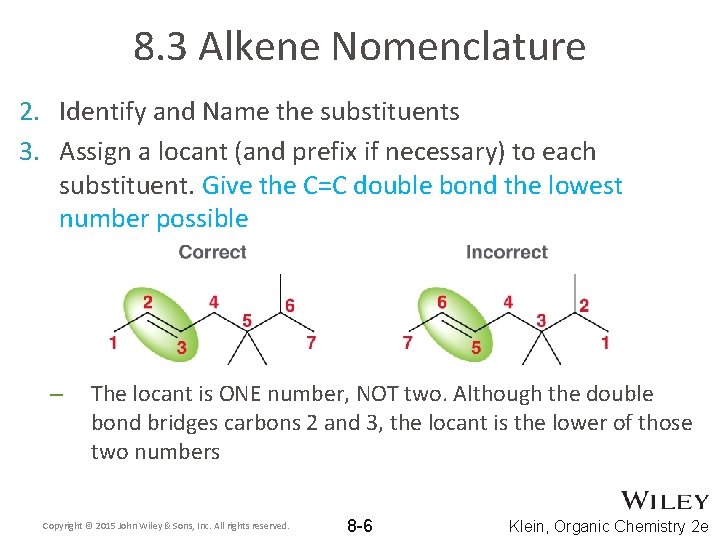
8. 3 Alkene Nomenclature 2. Identify and Name the substituents 3. Assign a locant (and prefix if necessary) to each substituent. Give the C=C double bond the lowest number possible – The locant is ONE number, NOT two. Although the double bond bridges carbons 2 and 3, the locant is the lower of those two numbers Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -6 Klein, Organic Chemistry 2 e

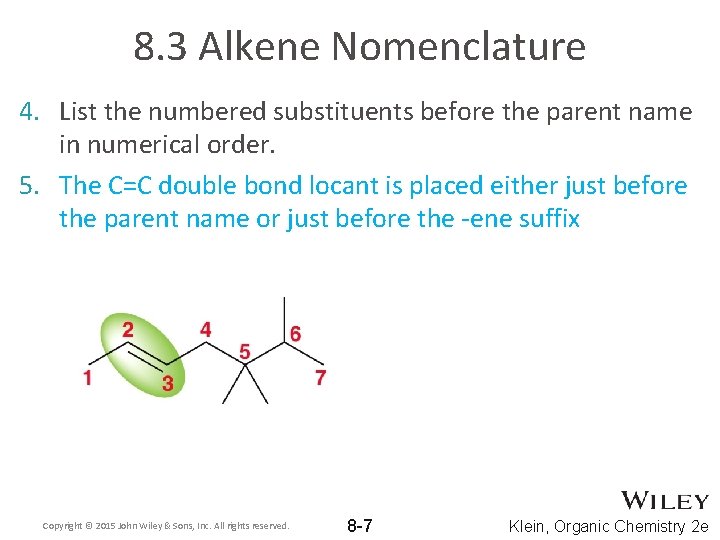
8. 3 Alkene Nomenclature 4. List the numbered substituents before the parent name in numerical order. 5. The C=C double bond locant is placed either just before the parent name or just before the -ene suffix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -7 Klein, Organic Chemistry 2 e


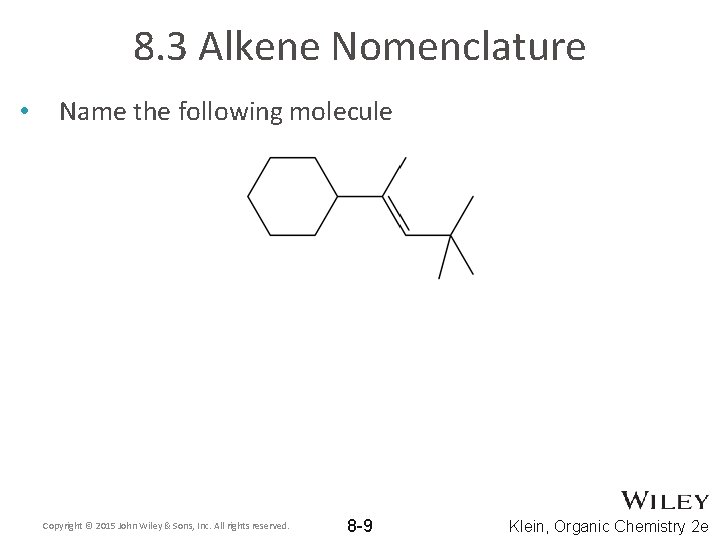
8. 3 Alkene Nomenclature • Name the following molecule Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -9 Klein, Organic Chemistry 2 e

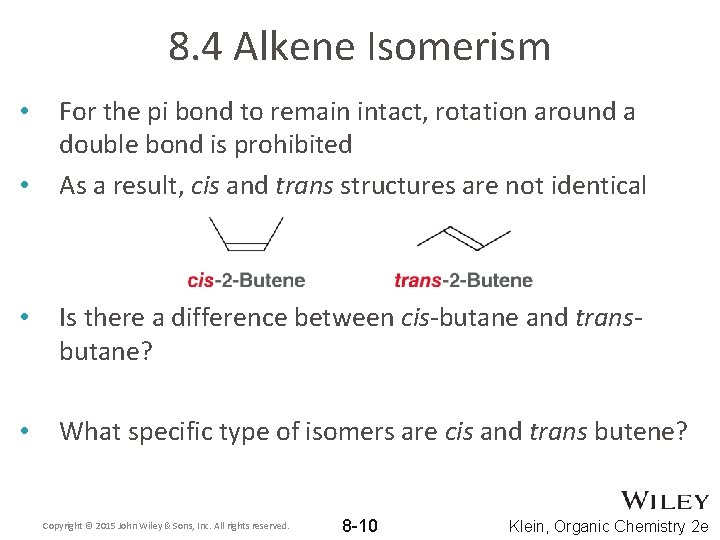
8. 4 Alkene Isomerism • • For the pi bond to remain intact, rotation around a double bond is prohibited As a result, cis and trans structures are not identical • Is there a difference between cis-butane and transbutane? • What specific type of isomers are cis and trans butene? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -10 Klein, Organic Chemistry 2 e

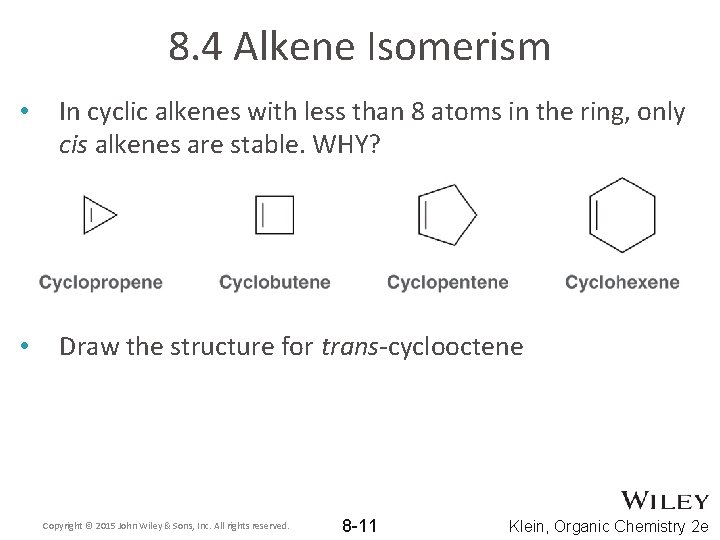
8. 4 Alkene Isomerism • In cyclic alkenes with less than 8 atoms in the ring, only cis alkenes are stable. WHY? • Draw the structure for trans-cyclooctene Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -11 Klein, Organic Chemistry 2 e

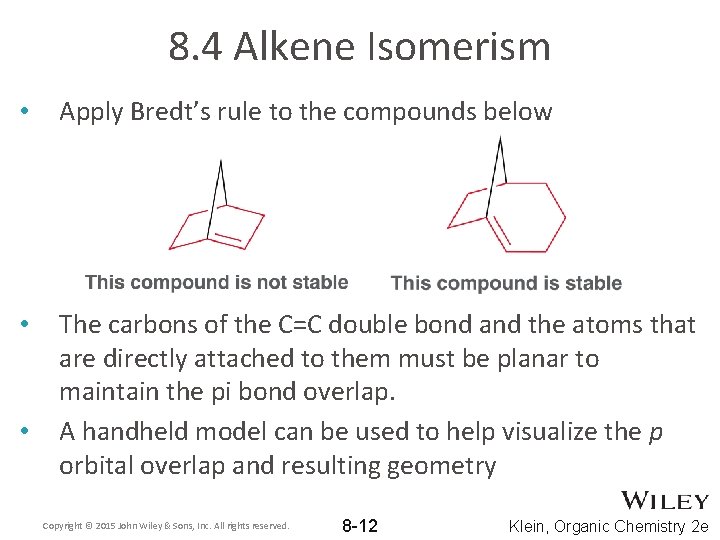
8. 4 Alkene Isomerism • Apply Bredt’s rule to the compounds below • The carbons of the C=C double bond and the atoms that are directly attached to them must be planar to maintain the pi bond overlap. A handheld model can be used to help visualize the p orbital overlap and resulting geometry • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -12 Klein, Organic Chemistry 2 e

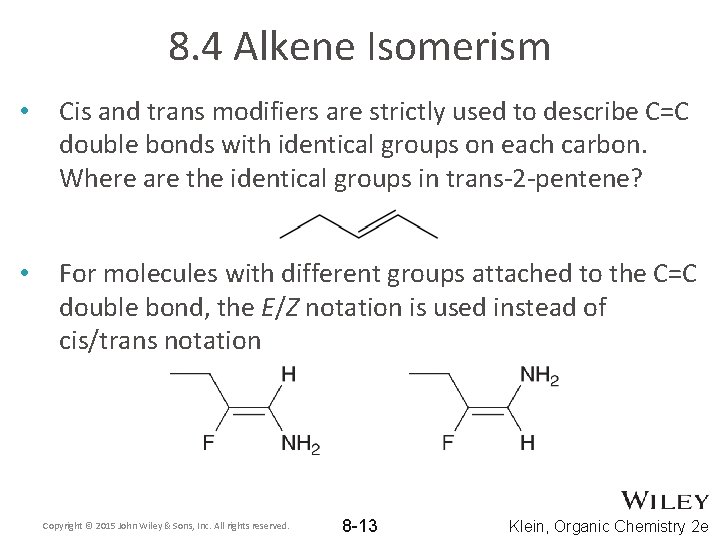
8. 4 Alkene Isomerism • Cis and trans modifiers are strictly used to describe C=C double bonds with identical groups on each carbon. Where are the identical groups in trans-2 -pentene? • For molecules with different groups attached to the C=C double bond, the E/Z notation is used instead of cis/trans notation Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -13 Klein, Organic Chemistry 2 e

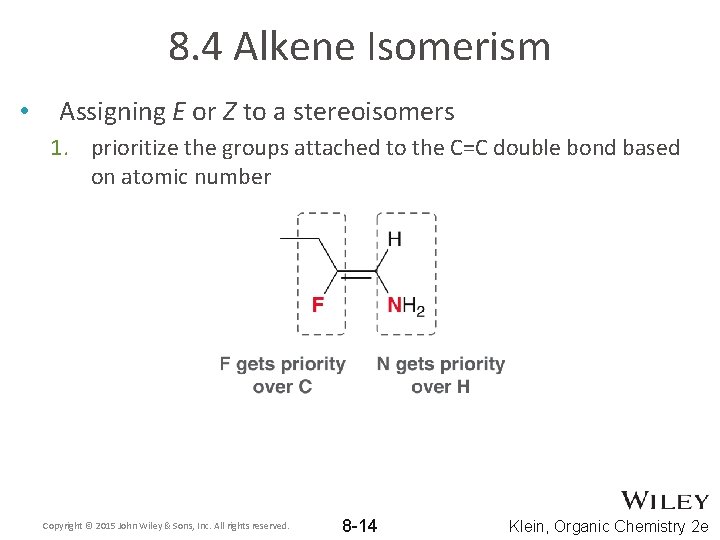
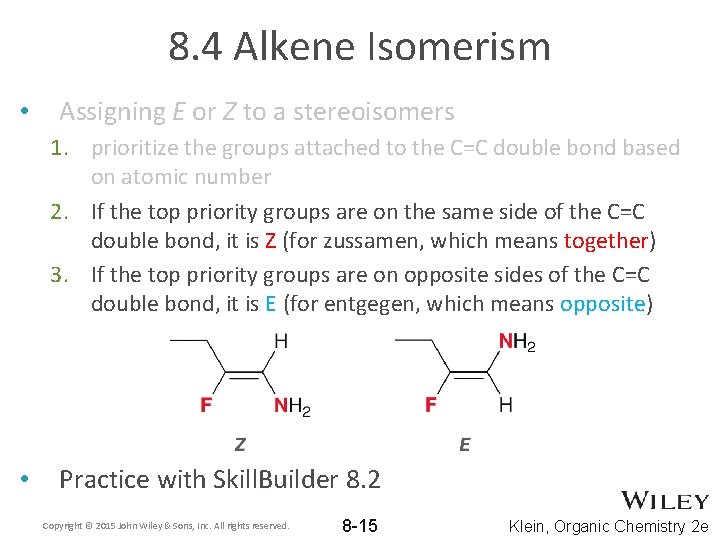
8. 4 Alkene Isomerism • Assigning E or Z to a stereoisomers 1. prioritize the groups attached to the C=C double bond based on atomic number Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -14 Klein, Organic Chemistry 2 e

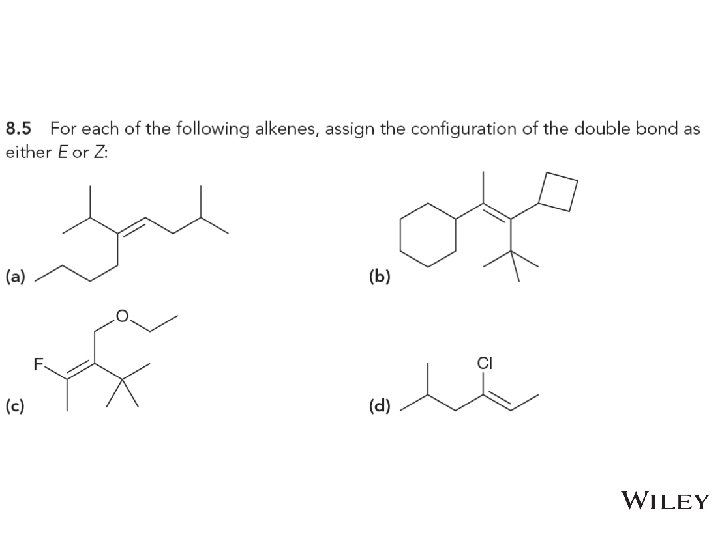
8. 4 Alkene Isomerism • Assigning E or Z to a stereoisomers 1. prioritize the groups attached to the C=C double bond based on atomic number 2. If the top priority groups are on the same side of the C=C double bond, it is Z (for zussamen, which means together) 3. If the top priority groups are on opposite sides of the C=C double bond, it is E (for entgegen, which means opposite) • Practice with Skill. Builder 8. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -15 Klein, Organic Chemistry 2 e


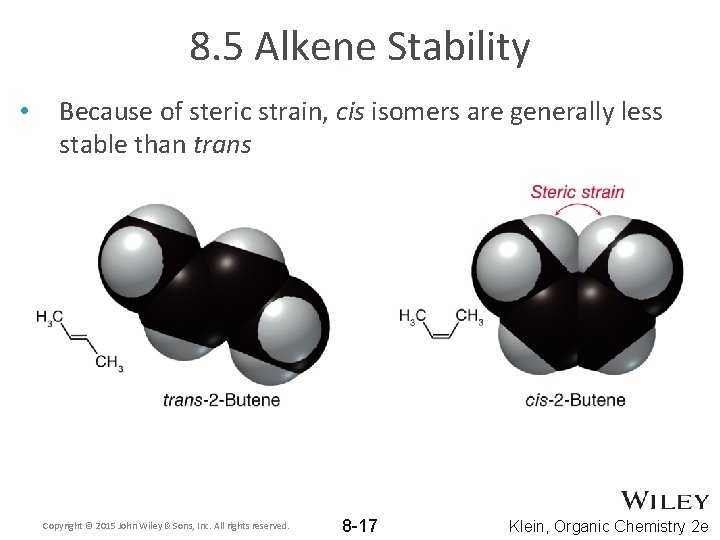
8. 5 Alkene Stability • Because of steric strain, cis isomers are generally less stable than trans Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -17 Klein, Organic Chemistry 2 e

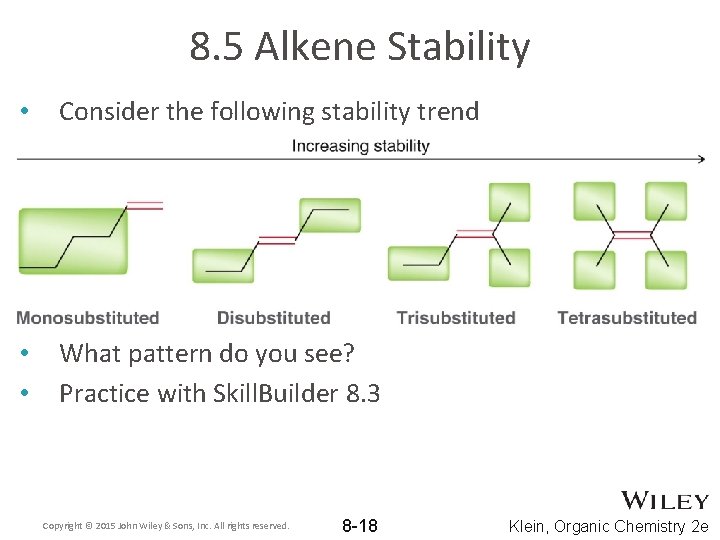
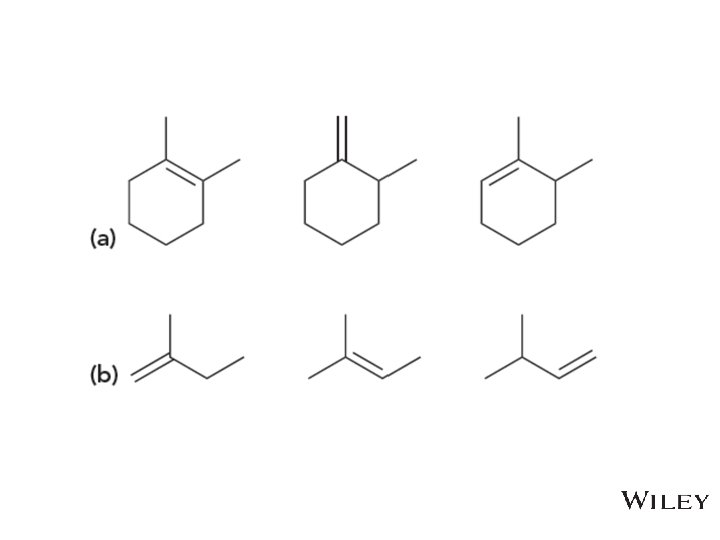
8. 5 Alkene Stability • Consider the following stability trend • • What pattern do you see? Practice with Skill. Builder 8. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -18 Klein, Organic Chemistry 2 e


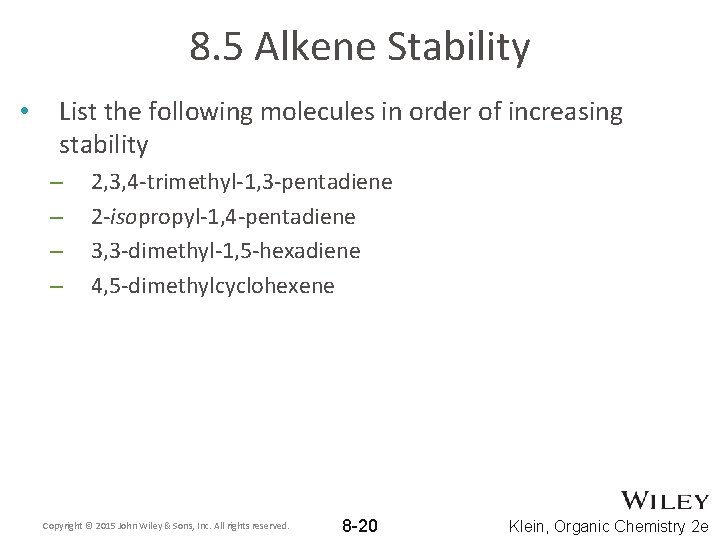
8. 5 Alkene Stability • List the following molecules in order of increasing stability – – 2, 3, 4 -trimethyl-1, 3 -pentadiene 2 -isopropyl-1, 4 -pentadiene 3, 3 -dimethyl-1, 5 -hexadiene 4, 5 -dimethylcyclohexene Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -20 Klein, Organic Chemistry 2 e

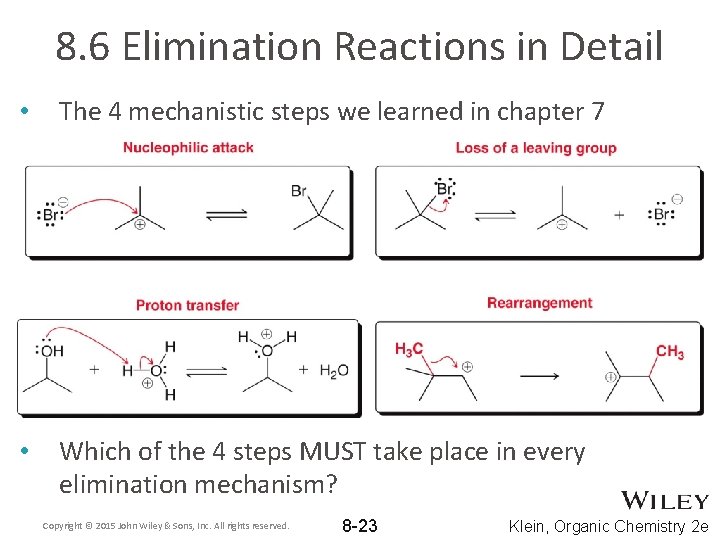
8. 6 Elimination Reactions in Detail • In general, a H atom and a leaving group are eliminated • To understand the mechanism of elimination, first recall the 4 mechanistic steps we learned in chapter 7 – – Nucleophilic attack Loss of a leaving group Proton transfer Rearrangement Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -22 Klein, Organic Chemistry 2 e

8. 6 Elimination Reactions in Detail • The 4 mechanistic steps we learned in chapter 7 • Which of the 4 steps MUST take place in every elimination mechanism? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -23 Klein, Organic Chemistry 2 e

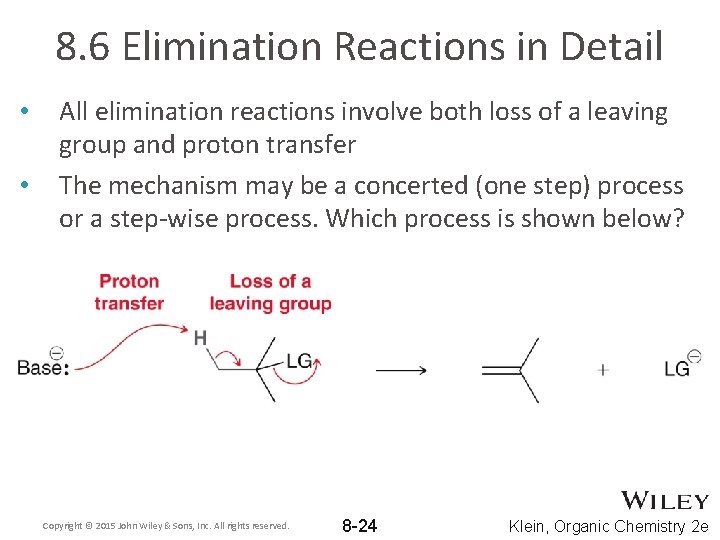
8. 6 Elimination Reactions in Detail • • All elimination reactions involve both loss of a leaving group and proton transfer The mechanism may be a concerted (one step) process or a step-wise process. Which process is shown below? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -24 Klein, Organic Chemistry 2 e

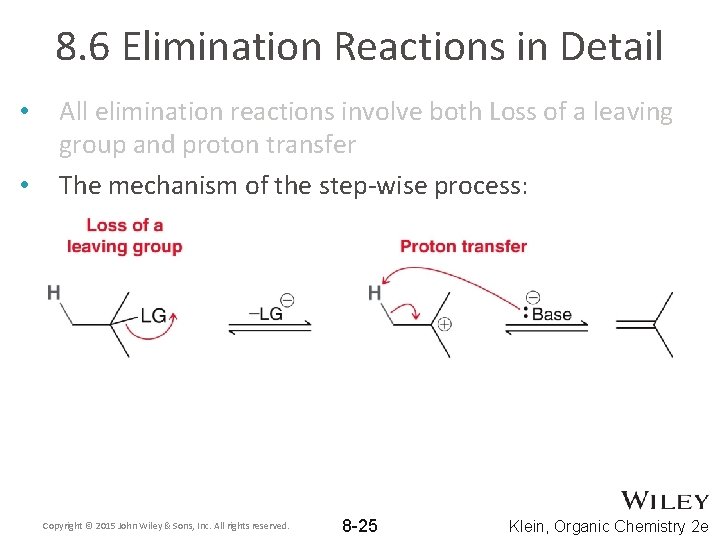
8. 6 Elimination Reactions in Detail • • All elimination reactions involve both Loss of a leaving group and proton transfer The mechanism of the step-wise process: Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -25 Klein, Organic Chemistry 2 e


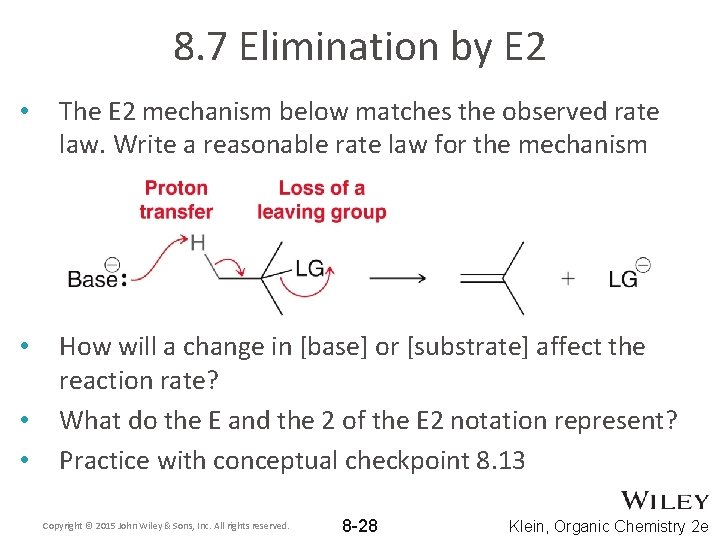
8. 7 Elimination by E 2 • The E 2 mechanism below matches the observed rate law. Write a reasonable rate law for the mechanism • How will a change in [base] or [substrate] affect the reaction rate? What do the E and the 2 of the E 2 notation represent? Practice with conceptual checkpoint 8. 13 • • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -28 Klein, Organic Chemistry 2 e

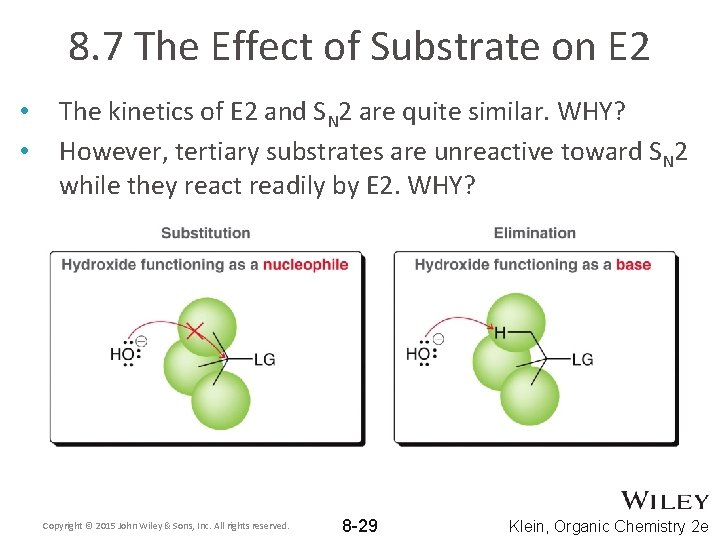
8. 7 The Effect of Substrate on E 2 • • The kinetics of E 2 and SN 2 are quite similar. WHY? However, tertiary substrates are unreactive toward SN 2 while they react readily by E 2. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -29 Klein, Organic Chemistry 2 e

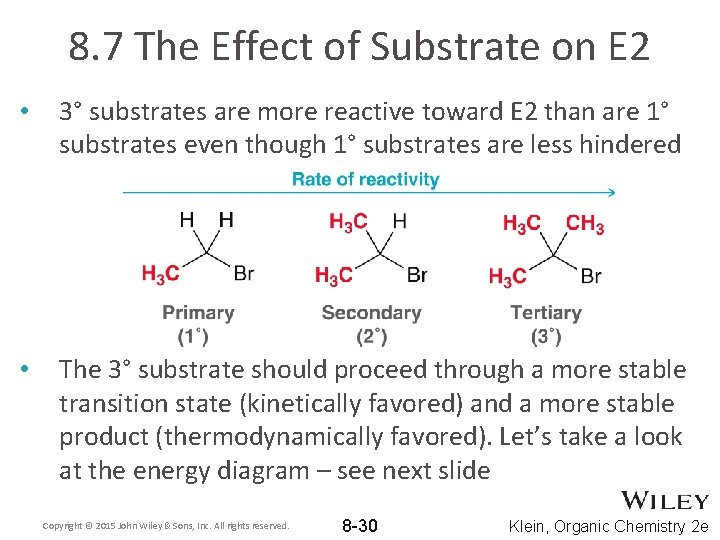
8. 7 The Effect of Substrate on E 2 • 3° substrates are more reactive toward E 2 than are 1° substrates even though 1° substrates are less hindered • The 3° substrate should proceed through a more stable transition state (kinetically favored) and a more stable product (thermodynamically favored). Let’s take a look at the energy diagram – see next slide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -30 Klein, Organic Chemistry 2 e

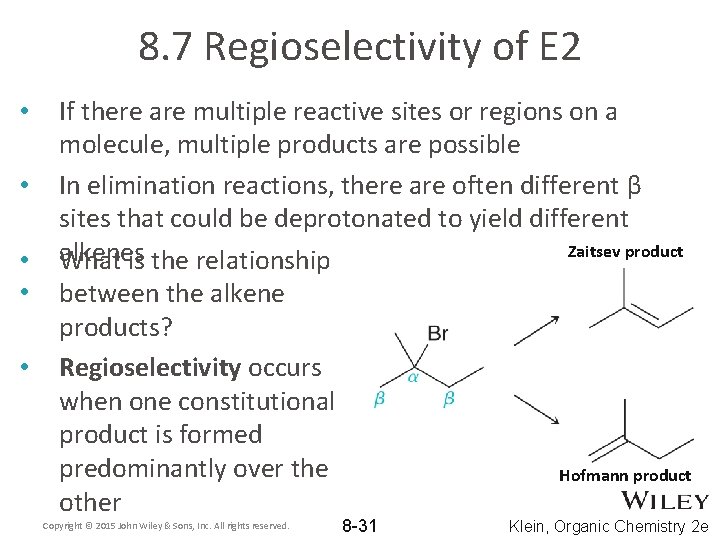
8. 7 Regioselectivity of E 2 • • • If there are multiple reactive sites or regions on a molecule, multiple products are possible In elimination reactions, there are often different β sites that could be deprotonated to yield different Zaitsev product alkenes What is the relationship between the alkene products? Regioselectivity occurs when one constitutional product is formed predominantly over the Hofmann product other Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -31 Klein, Organic Chemistry 2 e

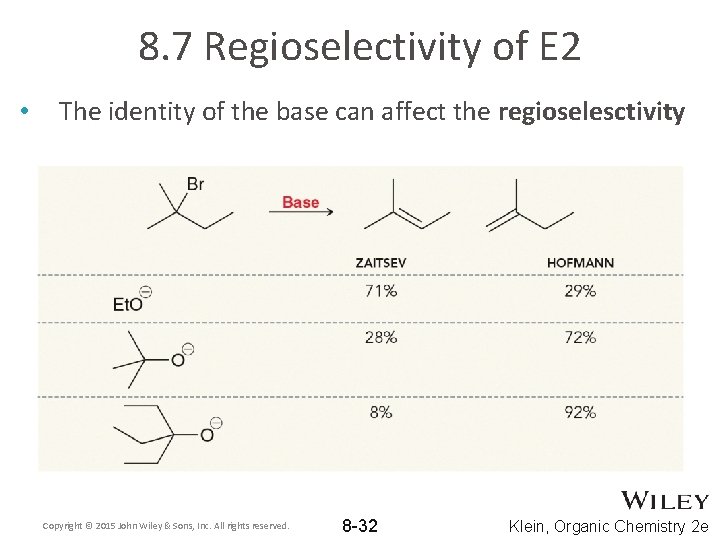
8. 7 Regioselectivity of E 2 • The identity of the base can affect the regioselesctivity Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -32 Klein, Organic Chemistry 2 e

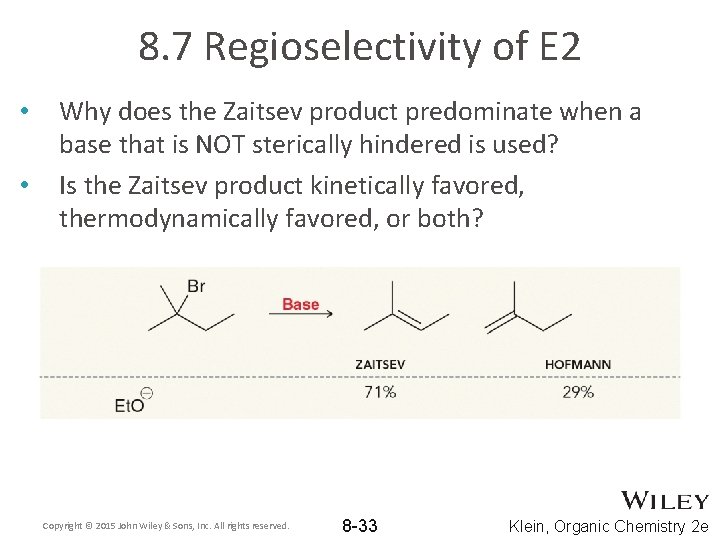
8. 7 Regioselectivity of E 2 • • Why does the Zaitsev product predominate when a base that is NOT sterically hindered is used? Is the Zaitsev product kinetically favored, thermodynamically favored, or both? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -33 Klein, Organic Chemistry 2 e

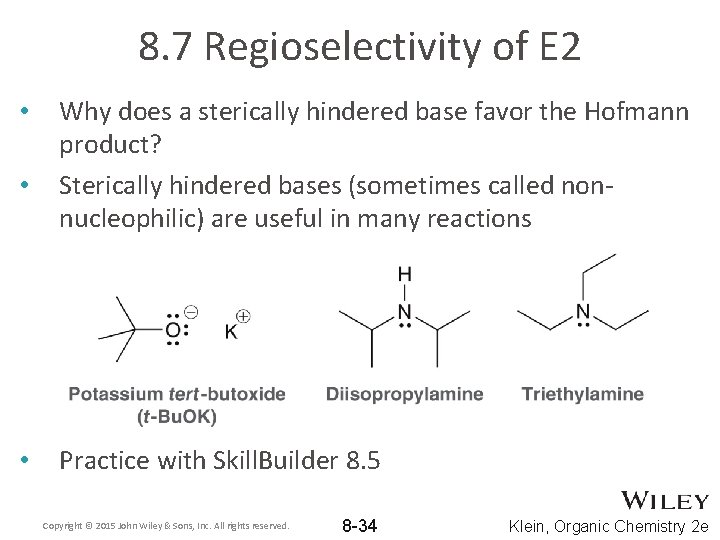
8. 7 Regioselectivity of E 2 • • • Why does a sterically hindered base favor the Hofmann product? Sterically hindered bases (sometimes called nonnucleophilic) are useful in many reactions Practice with Skill. Builder 8. 5 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -34 Klein, Organic Chemistry 2 e


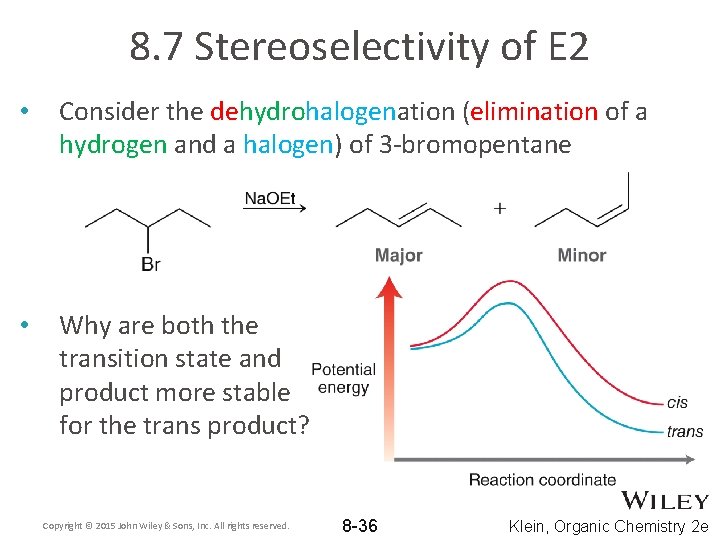
8. 7 Stereoselectivity of E 2 • Consider the dehydrohalogenation (elimination of a hydrogen and a halogen) of 3 -bromopentane • Why are both the transition state and product more stable for the trans product? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -36 Klein, Organic Chemistry 2 e

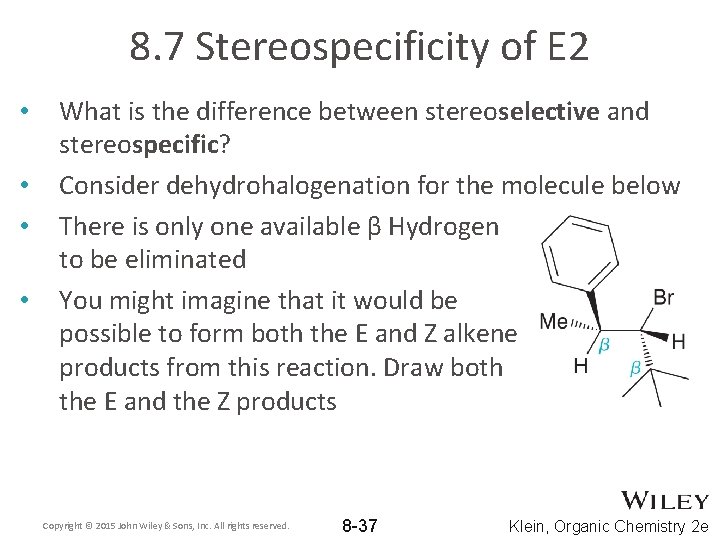
8. 7 Stereospecificity of E 2 • • What is the difference between stereoselective and stereospecific? Consider dehydrohalogenation for the molecule below There is only one available β Hydrogen to be eliminated You might imagine that it would be possible to form both the E and Z alkene products from this reaction. Draw both the E and the Z products Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -37 Klein, Organic Chemistry 2 e

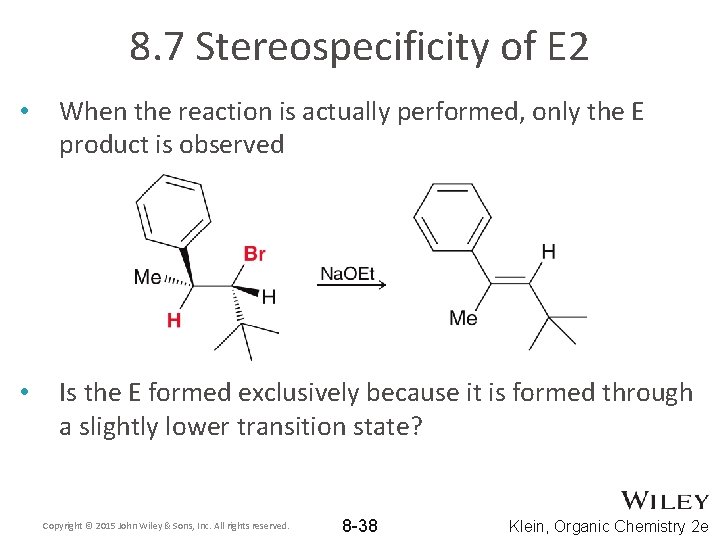
8. 7 Stereospecificity of E 2 • When the reaction is actually performed, only the E product is observed • Is the E formed exclusively because it is formed through a slightly lower transition state? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -38 Klein, Organic Chemistry 2 e

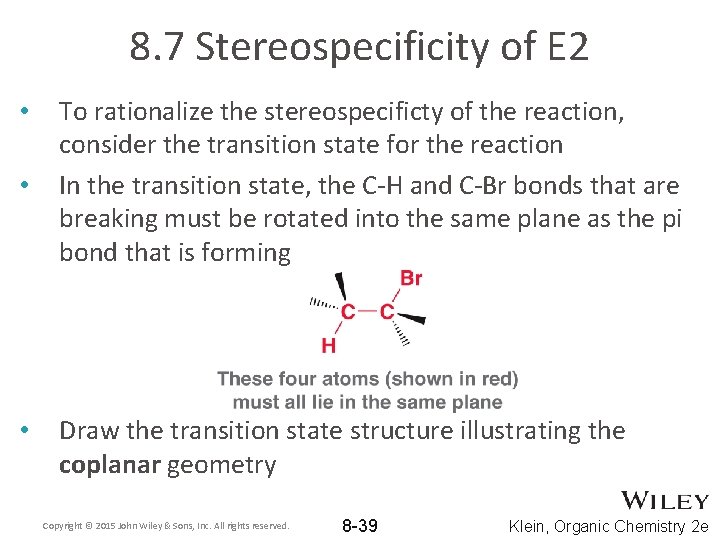
8. 7 Stereospecificity of E 2 • • • To rationalize the stereospecificty of the reaction, consider the transition state for the reaction In the transition state, the C-H and C-Br bonds that are breaking must be rotated into the same plane as the pi bond that is forming Draw the transition state structure illustrating the coplanar geometry Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -39 Klein, Organic Chemistry 2 e

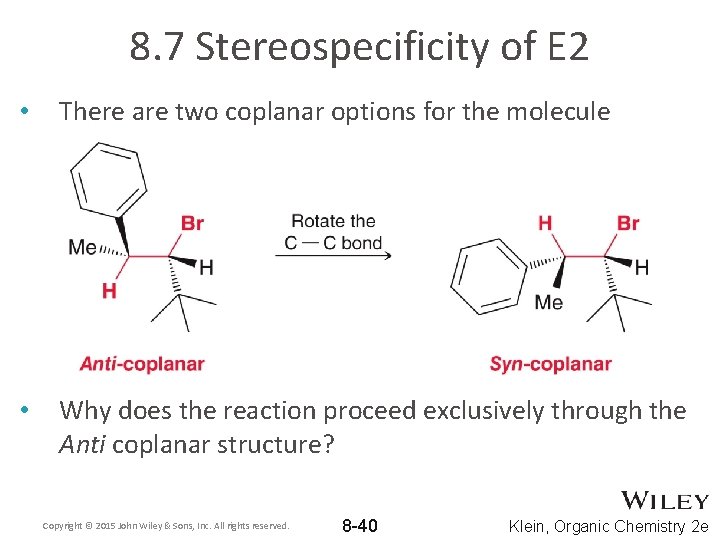
8. 7 Stereospecificity of E 2 • There are two coplanar options for the molecule • Why does the reaction proceed exclusively through the Anti coplanar structure? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -40 Klein, Organic Chemistry 2 e

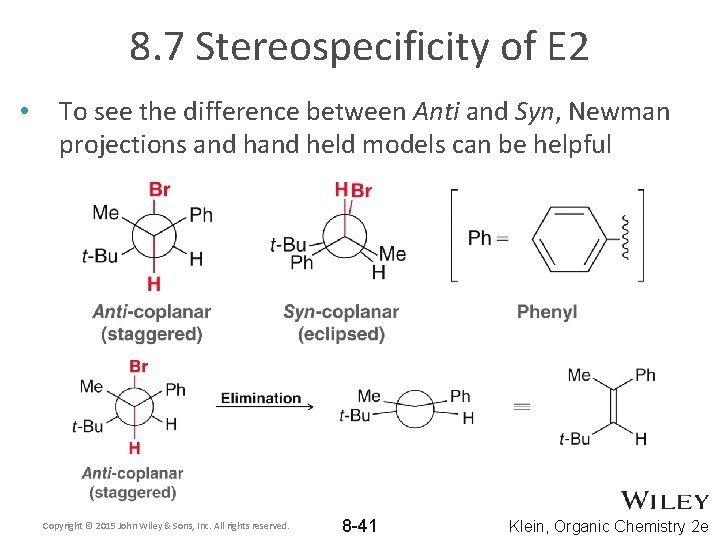
8. 7 Stereospecificity of E 2 • To see the difference between Anti and Syn, Newman projections and held models can be helpful Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -41 Klein, Organic Chemistry 2 e

8. 7 Stereospecificity of E 2 • • Evidence suggests that a strict 180° angle is not necessary for E 2 mechanisms. Similar angles (175– 179°) are sufficient The term, anti-periplanar is generally used instead of anti-coplanar to account for slight deviations from coplanarity Although the E isomer is usually more stable because it is less sterically hindered, the requirement for an antiperiplanar transition state can often lead to the less stable Z isomer Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -42 Klein, Organic Chemistry 2 e

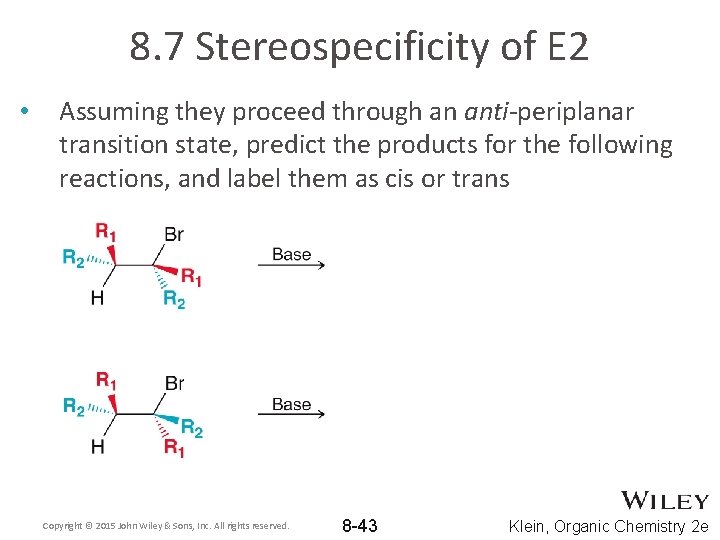
8. 7 Stereospecificity of E 2 • Assuming they proceed through an anti-periplanar transition state, predict the products for the following reactions, and label them as cis or trans Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -43 Klein, Organic Chemistry 2 e


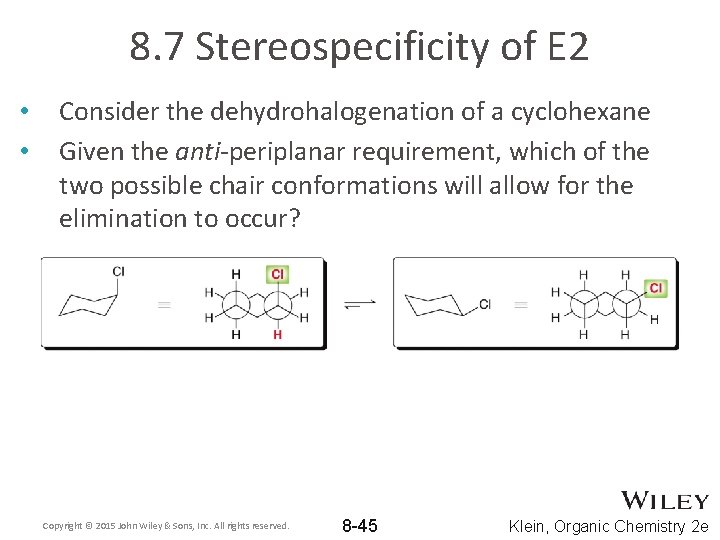
8. 7 Stereospecificity of E 2 • • Consider the dehydrohalogenation of a cyclohexane Given the anti-periplanar requirement, which of the two possible chair conformations will allow for the elimination to occur? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -45 Klein, Organic Chemistry 2 e

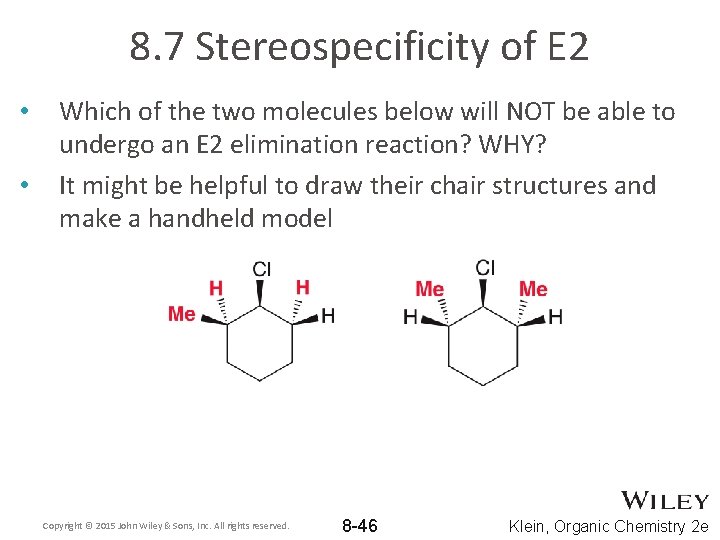
8. 7 Stereospecificity of E 2 • • Which of the two molecules below will NOT be able to undergo an E 2 elimination reaction? WHY? It might be helpful to draw their chair structures and make a handheld model Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -46 Klein, Organic Chemistry 2 e

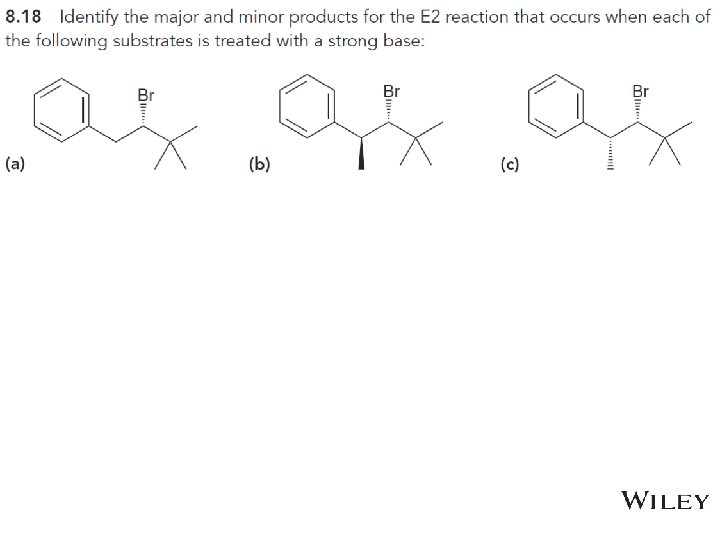
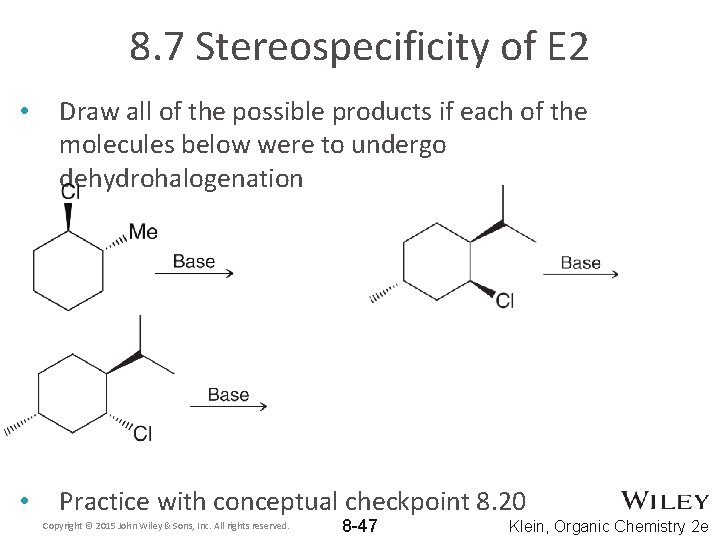
8. 7 Stereospecificity of E 2 • Draw all of the possible products if each of the molecules below were to undergo dehydrohalogenation • Practice with conceptual checkpoint 8. 20 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -47 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

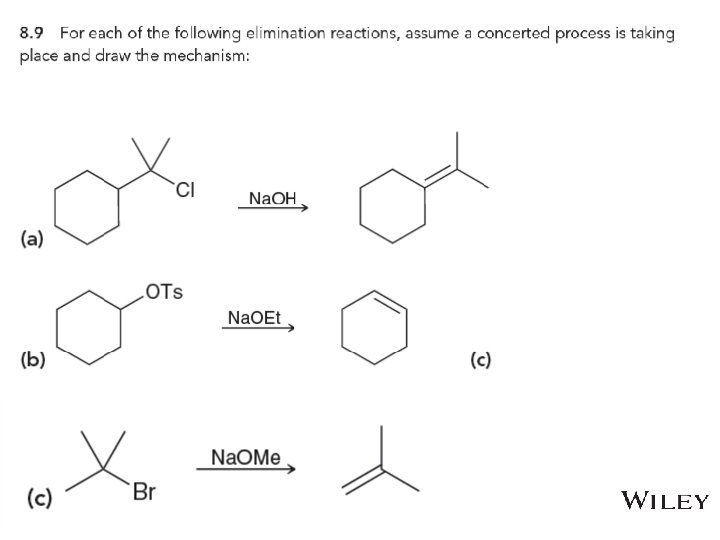
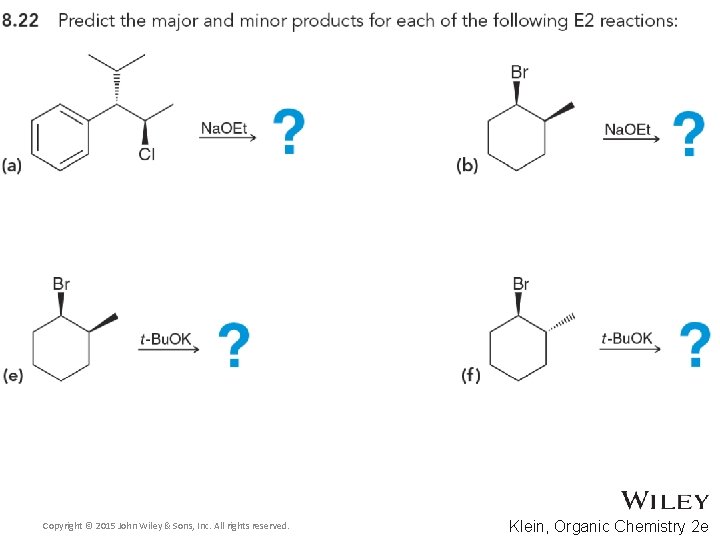
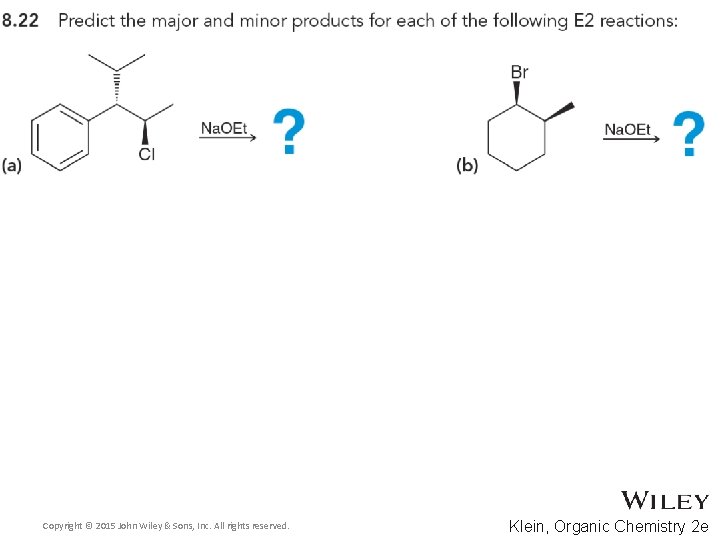
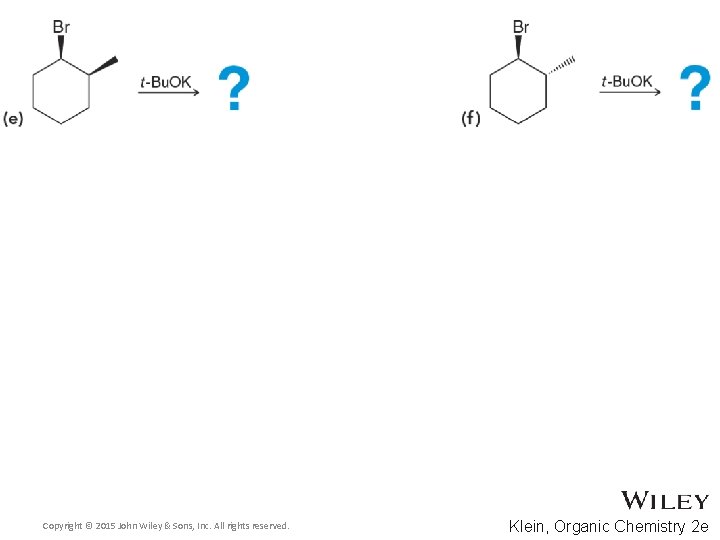
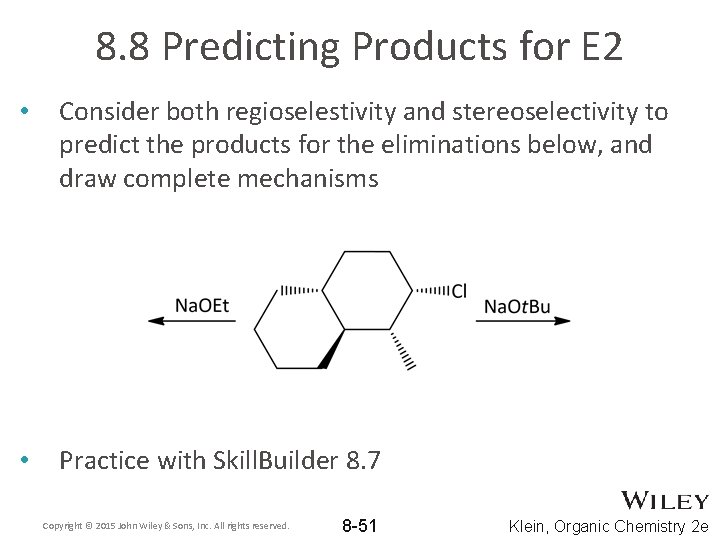
8. 8 Predicting Products for E 2 • Consider both regioselestivity and stereoselectivity to predict the products for the eliminations below, and draw complete mechanisms • Practice with Skill. Builder 8. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -51 Klein, Organic Chemistry 2 e

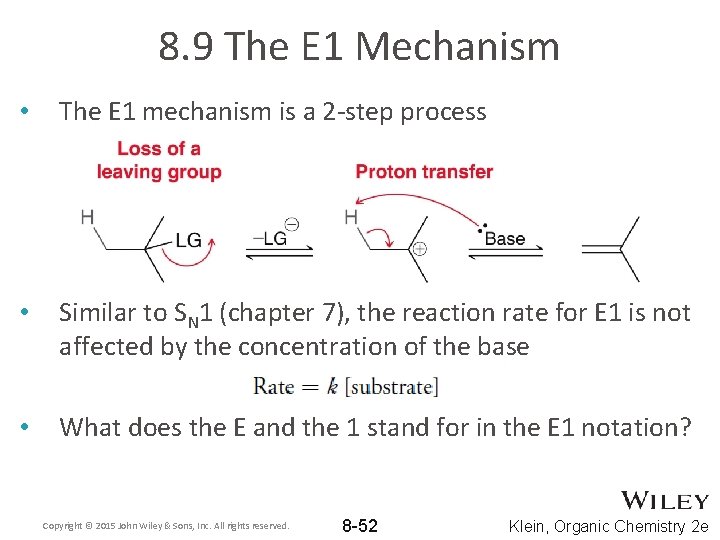
8. 9 The E 1 Mechanism • The E 1 mechanism is a 2 -step process • Similar to SN 1 (chapter 7), the reaction rate for E 1 is not affected by the concentration of the base • What does the E and the 1 stand for in the E 1 notation? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -52 Klein, Organic Chemistry 2 e

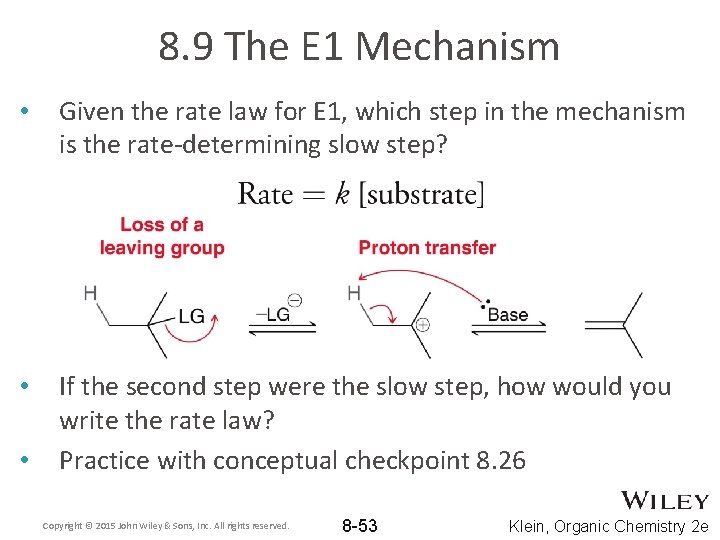
8. 9 The E 1 Mechanism • Given the rate law for E 1, which step in the mechanism is the rate-determining slow step? • If the second step were the slow step, how would you write the rate law? Practice with conceptual checkpoint 8. 26 • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -53 Klein, Organic Chemistry 2 e

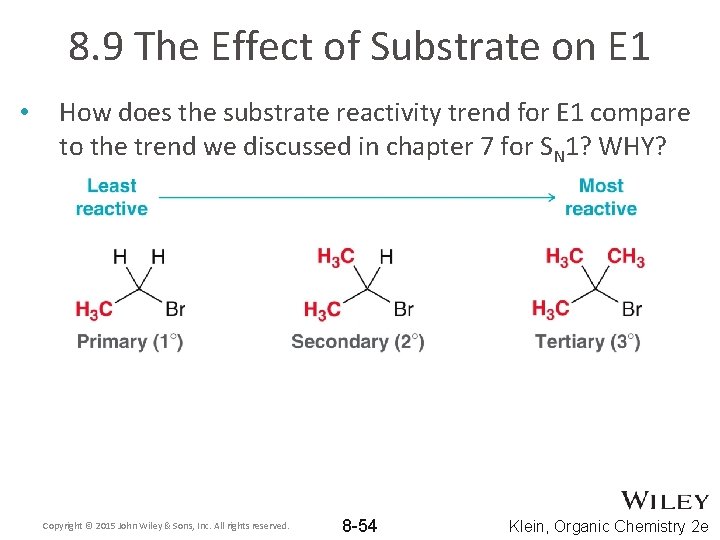
8. 9 The Effect of Substrate on E 1 • How does the substrate reactivity trend for E 1 compare to the trend we discussed in chapter 7 for SN 1? WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -54 Klein, Organic Chemistry 2 e

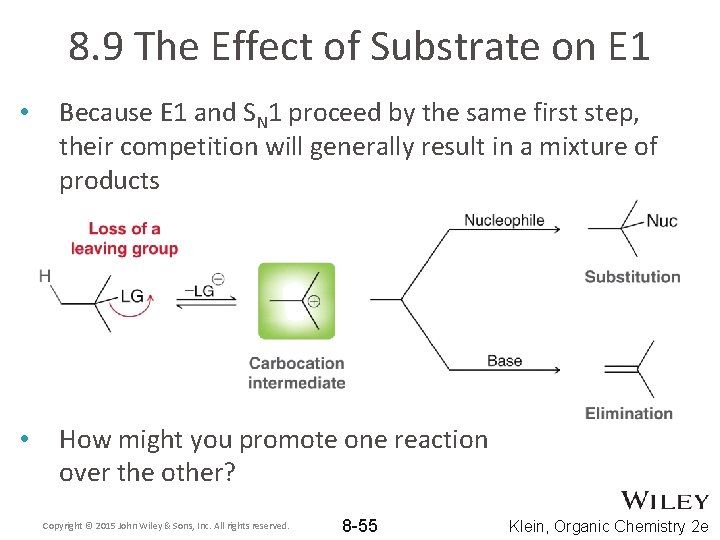
8. 9 The Effect of Substrate on E 1 • Because E 1 and SN 1 proceed by the same first step, their competition will generally result in a mixture of products • How might you promote one reaction over the other? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -55 Klein, Organic Chemistry 2 e

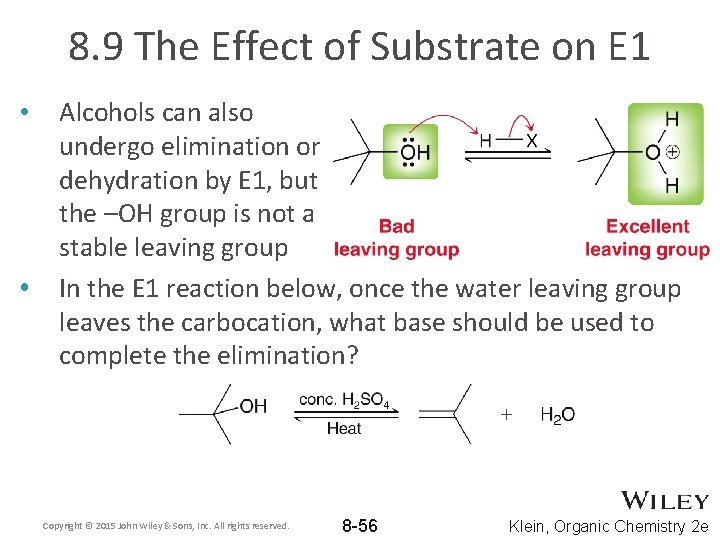
8. 9 The Effect of Substrate on E 1 • • Alcohols can also undergo elimination or dehydration by E 1, but the –OH group is not a stable leaving group In the E 1 reaction below, once the water leaving group leaves the carbocation, what base should be used to complete the elimination? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -56 Klein, Organic Chemistry 2 e

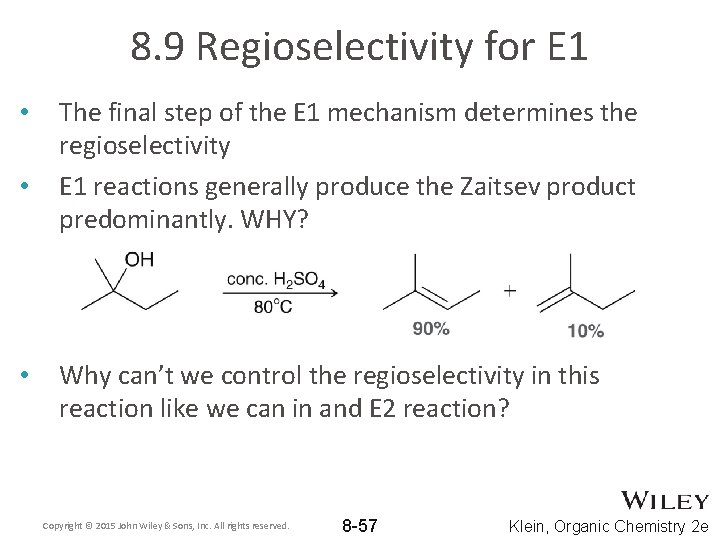
8. 9 Regioselectivity for E 1 • • • The final step of the E 1 mechanism determines the regioselectivity E 1 reactions generally produce the Zaitsev product predominantly. WHY? Why can’t we control the regioselectivity in this reaction like we can in and E 2 reaction? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -57 Klein, Organic Chemistry 2 e

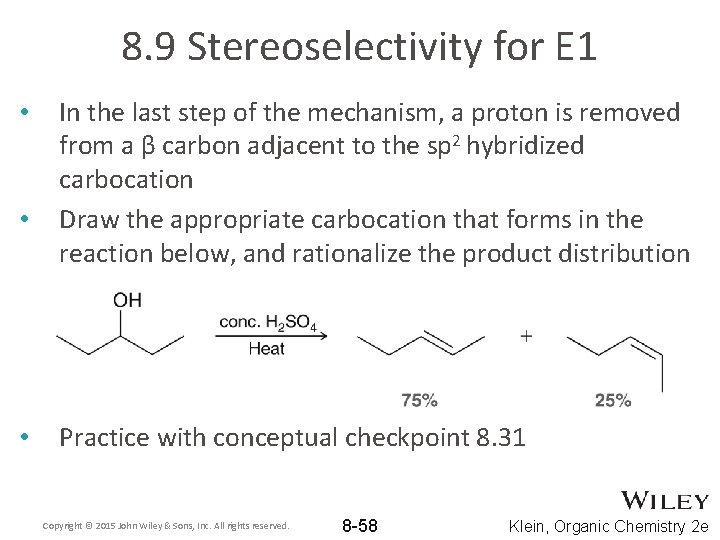
8. 9 Stereoselectivity for E 1 • • • In the last step of the mechanism, a proton is removed from a β carbon adjacent to the sp 2 hybridized carbocation Draw the appropriate carbocation that forms in the reaction below, and rationalize the product distribution Practice with conceptual checkpoint 8. 31 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -58 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

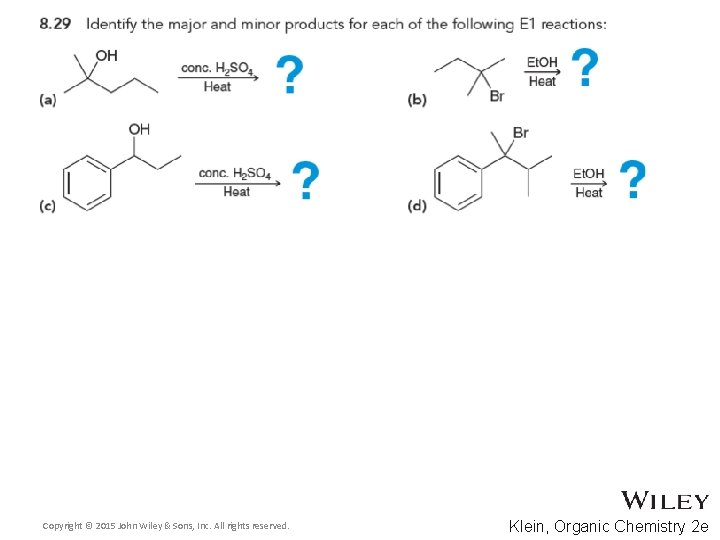
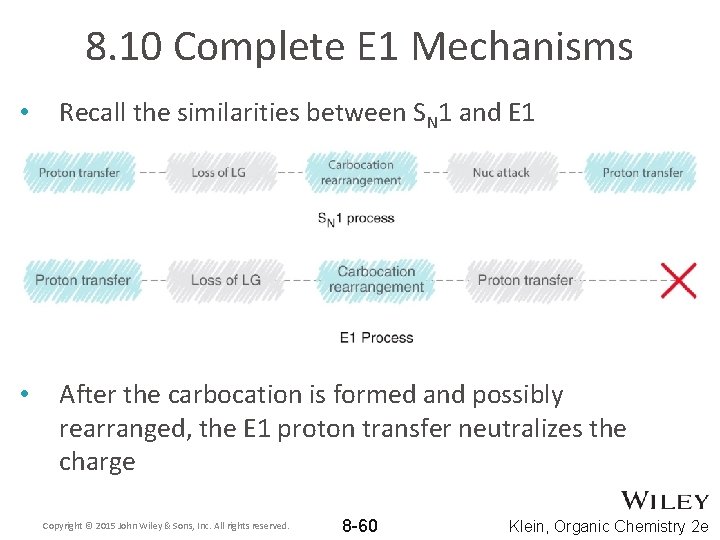
8. 10 Complete E 1 Mechanisms • Recall the similarities between SN 1 and E 1 • After the carbocation is formed and possibly rearranged, the E 1 proton transfer neutralizes the charge Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -60 Klein, Organic Chemistry 2 e

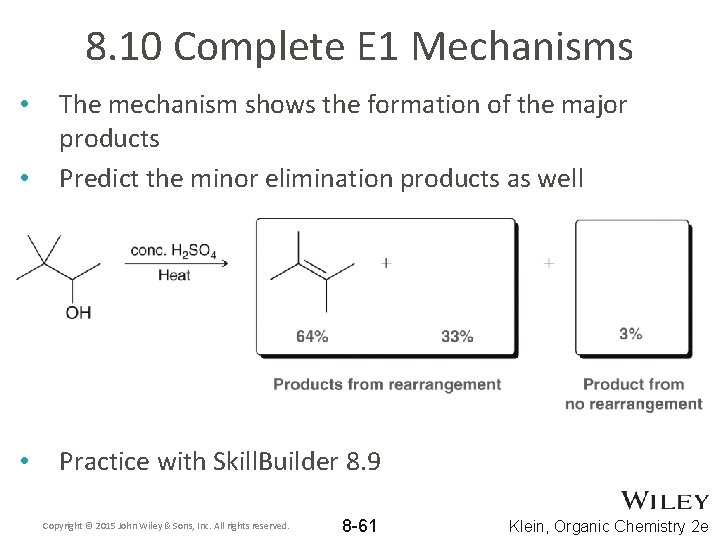
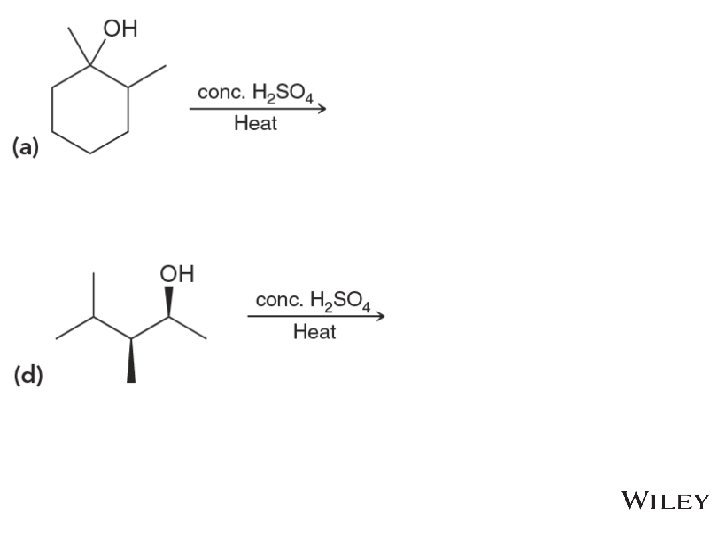
8. 10 Complete E 1 Mechanisms • The mechanism shows the formation of the major products Predict the minor elimination products as well • Practice with Skill. Builder 8. 9 • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -61 Klein, Organic Chemistry 2 e


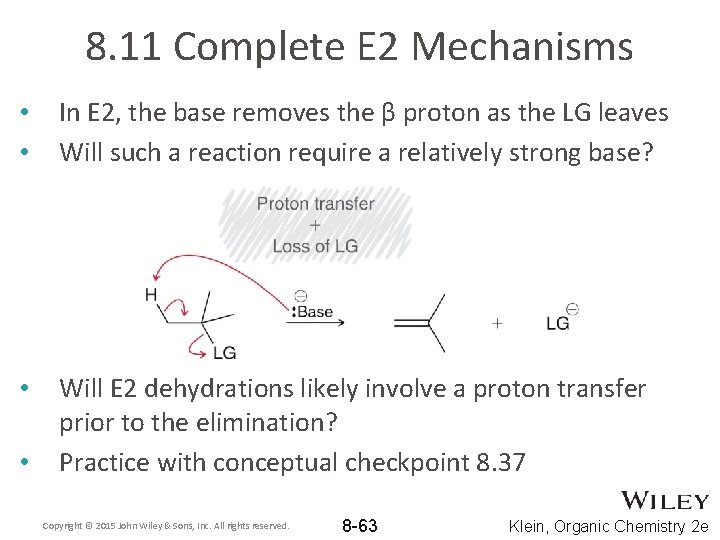
8. 11 Complete E 2 Mechanisms • • In E 2, the base removes the β proton as the LG leaves Will such a reaction require a relatively strong base? • Will E 2 dehydrations likely involve a proton transfer prior to the elimination? Practice with conceptual checkpoint 8. 37 • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -63 Klein, Organic Chemistry 2 e

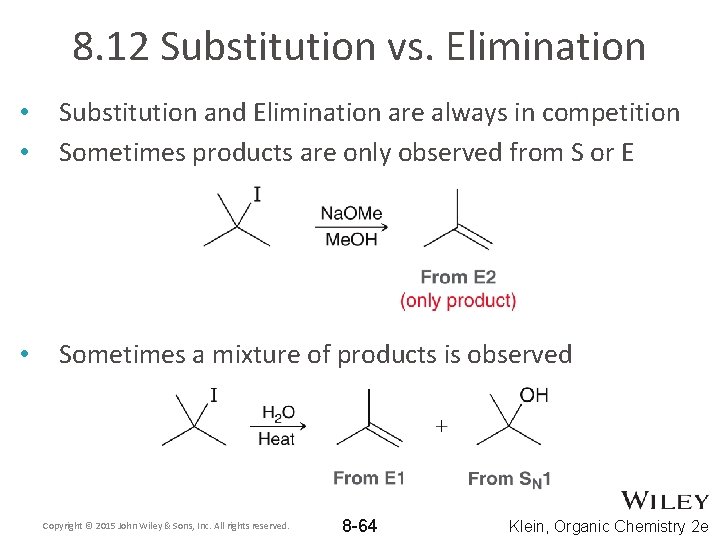
8. 12 Substitution vs. Elimination • • Substitution and Elimination are always in competition Sometimes products are only observed from S or E • Sometimes a mixture of products is observed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -64 Klein, Organic Chemistry 2 e

8. 12 Substitution vs. Elimination To predict whether substitution or elimination will predominate, consider the factors below 1. Determine the function of the reagent. Is it more likely to act as a base, a nucleophile, or both? 2. Analyze the substrate and predict the expected mechanism (SN 1, SN 2, E 1, or E 2) 3. Consider relevant regiochemical and stereochemical requirements • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -65 Klein, Organic Chemistry 2 e

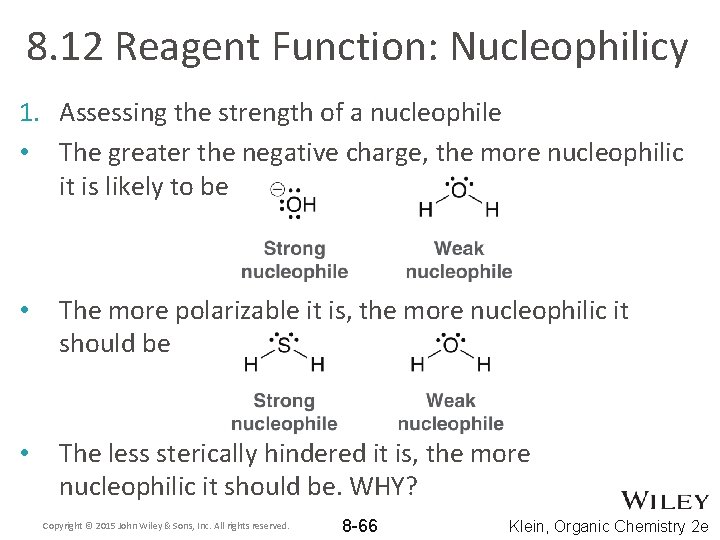
8. 12 Reagent Function: Nucleophilicy 1. Assessing the strength of a nucleophile • The greater the negative charge, the more nucleophilic it is likely to be • The more polarizable it is, the more nucleophilic it should be • The less sterically hindered it is, the more nucleophilic it should be. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -66 Klein, Organic Chemistry 2 e

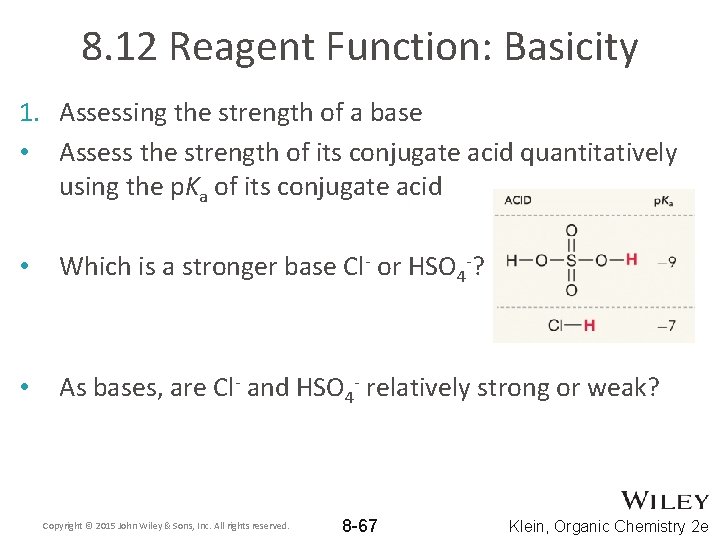
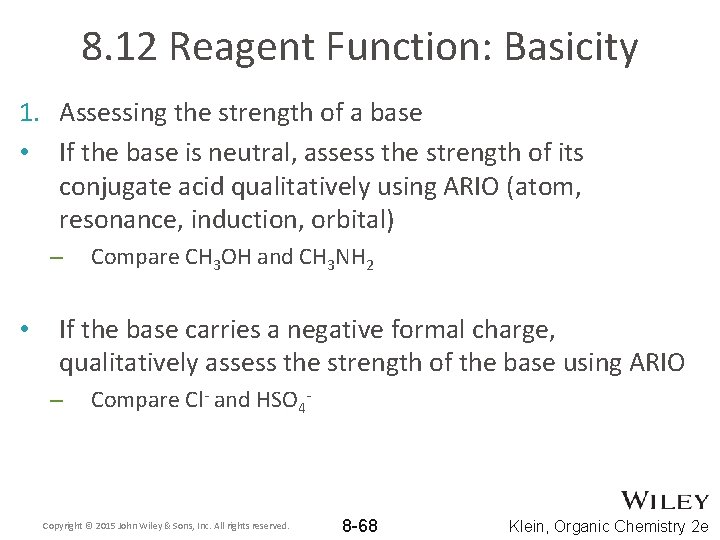
8. 12 Reagent Function: Basicity 1. Assessing the strength of a base • Assess the strength of its conjugate acid quantitatively using the p. Ka of its conjugate acid • Which is a stronger base Cl- or HSO 4 -? • As bases, are Cl- and HSO 4 - relatively strong or weak? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -67 Klein, Organic Chemistry 2 e

8. 12 Reagent Function: Basicity 1. Assessing the strength of a base • If the base is neutral, assess the strength of its conjugate acid qualitatively using ARIO (atom, resonance, induction, orbital) – • Compare CH 3 OH and CH 3 NH 2 If the base carries a negative formal charge, qualitatively assess the strength of the base using ARIO – Compare Cl- and HSO 4 - Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -68 Klein, Organic Chemistry 2 e

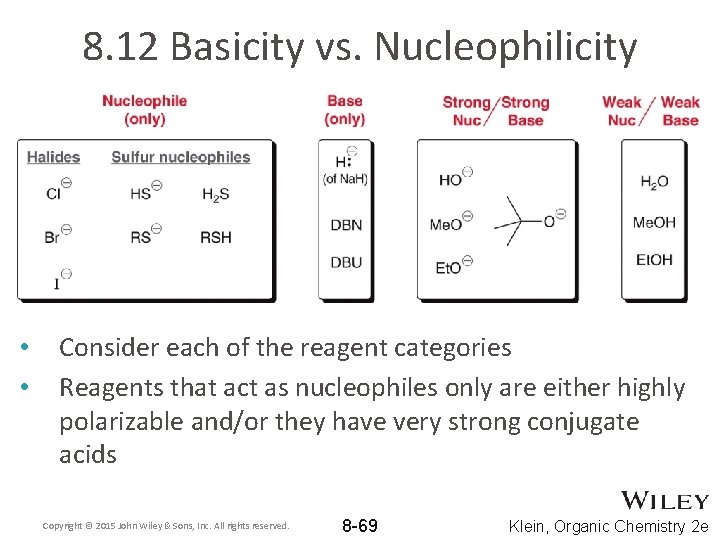
8. 12 Basicity vs. Nucleophilicity • • Consider each of the reagent categories Reagents that act as nucleophiles only are either highly polarizable and/or they have very strong conjugate acids Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -69 Klein, Organic Chemistry 2 e

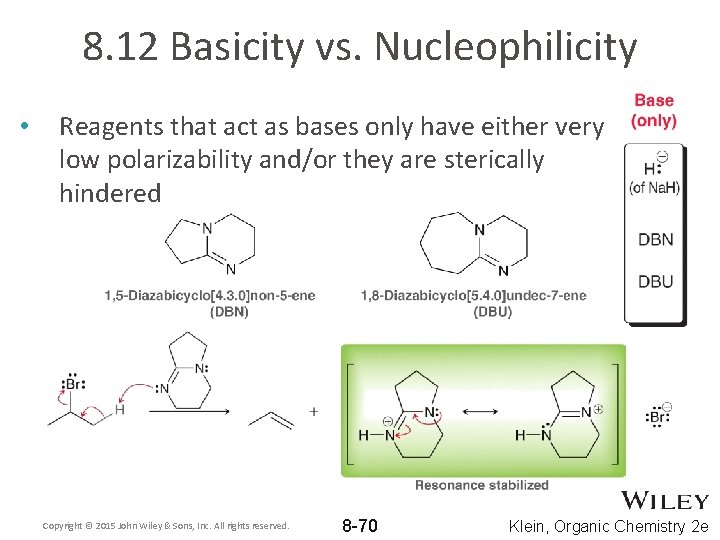
8. 12 Basicity vs. Nucleophilicity • Reagents that act as bases only have either very low polarizability and/or they are sterically hindered Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -70 Klein, Organic Chemistry 2 e

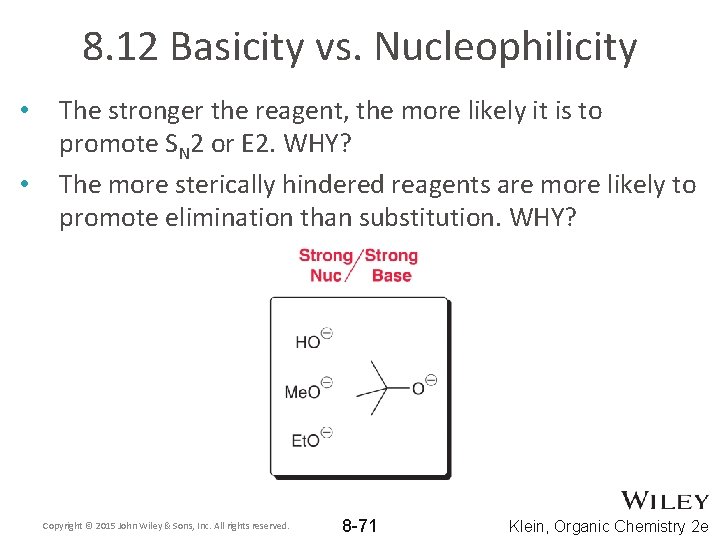
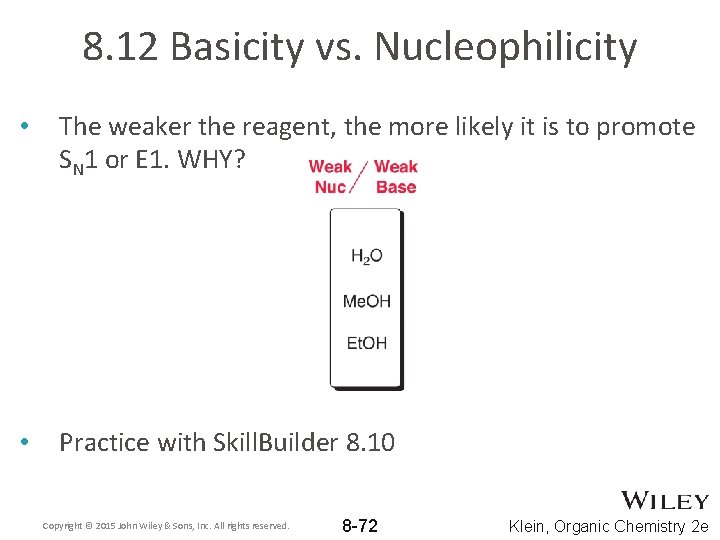
8. 12 Basicity vs. Nucleophilicity • • The stronger the reagent, the more likely it is to promote SN 2 or E 2. WHY? The more sterically hindered reagents are more likely to promote elimination than substitution. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -71 Klein, Organic Chemistry 2 e

8. 12 Basicity vs. Nucleophilicity • The weaker the reagent, the more likely it is to promote SN 1 or E 1. WHY? • Practice with Skill. Builder 8. 10 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -72 Klein, Organic Chemistry 2 e

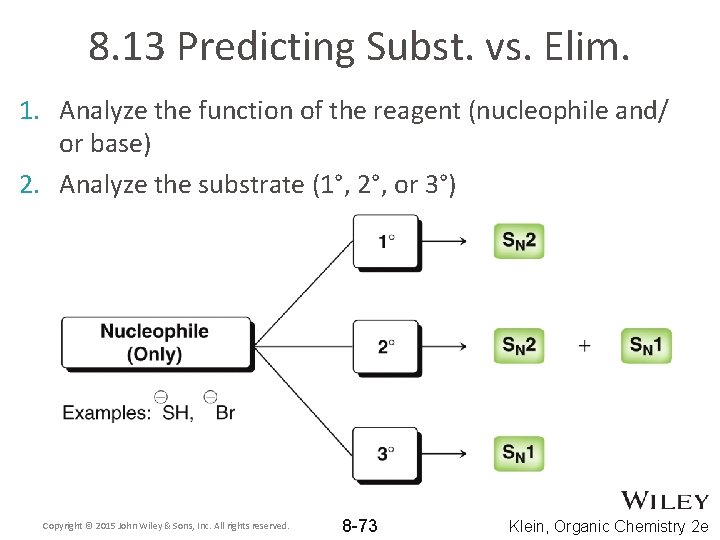
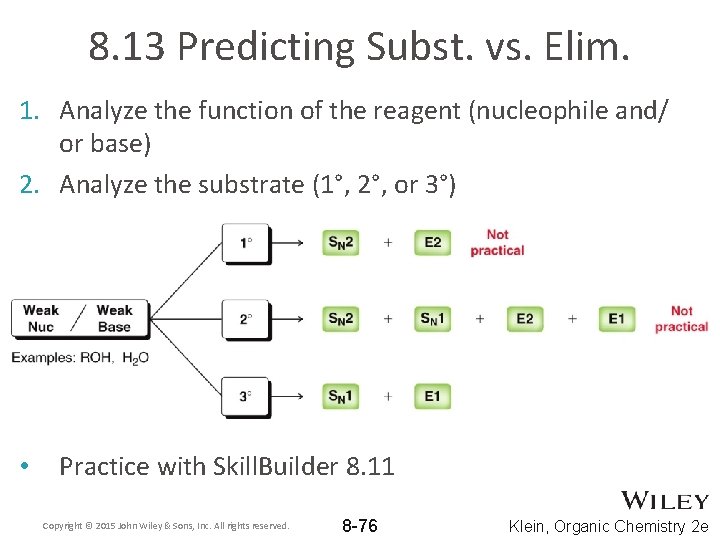
8. 13 Predicting Subst. vs. Elim. 1. Analyze the function of the reagent (nucleophile and/ or base) 2. Analyze the substrate (1°, 2°, or 3°) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -73 Klein, Organic Chemistry 2 e

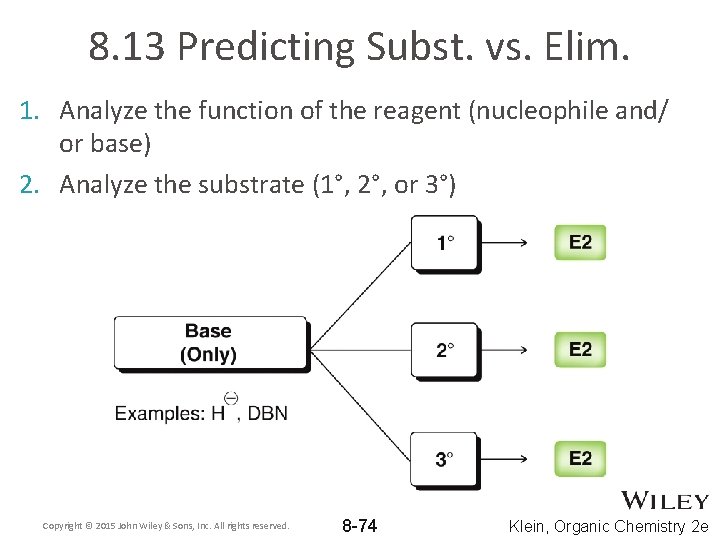
8. 13 Predicting Subst. vs. Elim. 1. Analyze the function of the reagent (nucleophile and/ or base) 2. Analyze the substrate (1°, 2°, or 3°) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -74 Klein, Organic Chemistry 2 e

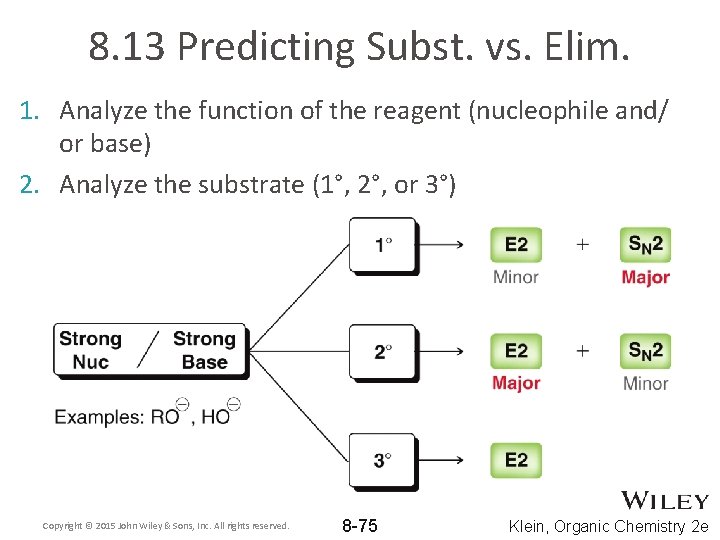
8. 13 Predicting Subst. vs. Elim. 1. Analyze the function of the reagent (nucleophile and/ or base) 2. Analyze the substrate (1°, 2°, or 3°) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -75 Klein, Organic Chemistry 2 e

8. 13 Predicting Subst. vs. Elim. 1. Analyze the function of the reagent (nucleophile and/ or base) 2. Analyze the substrate (1°, 2°, or 3°) • Practice with Skill. Builder 8. 11 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -76 Klein, Organic Chemistry 2 e

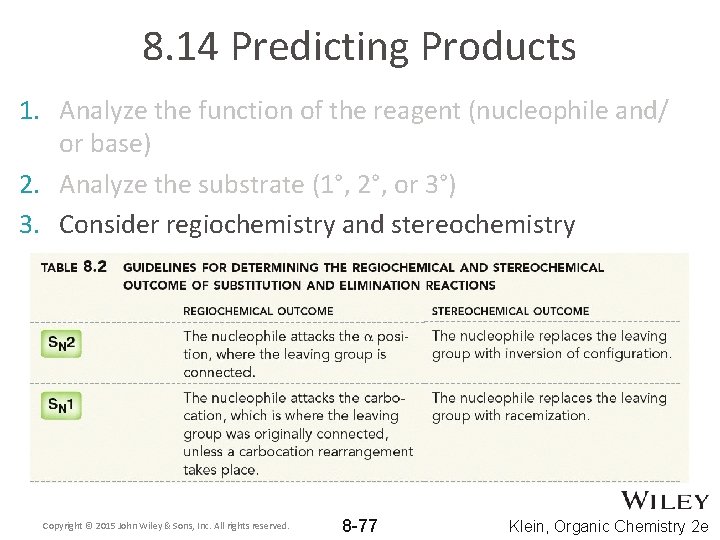
8. 14 Predicting Products 1. Analyze the function of the reagent (nucleophile and/ or base) 2. Analyze the substrate (1°, 2°, or 3°) 3. Consider regiochemistry and stereochemistry Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -77 Klein, Organic Chemistry 2 e

8. 14 Predicting Products 3. Consider regiochemistry and stereochemistry • Practice with Skill. Builder 8. 12 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -78 Klein, Organic Chemistry 2 e

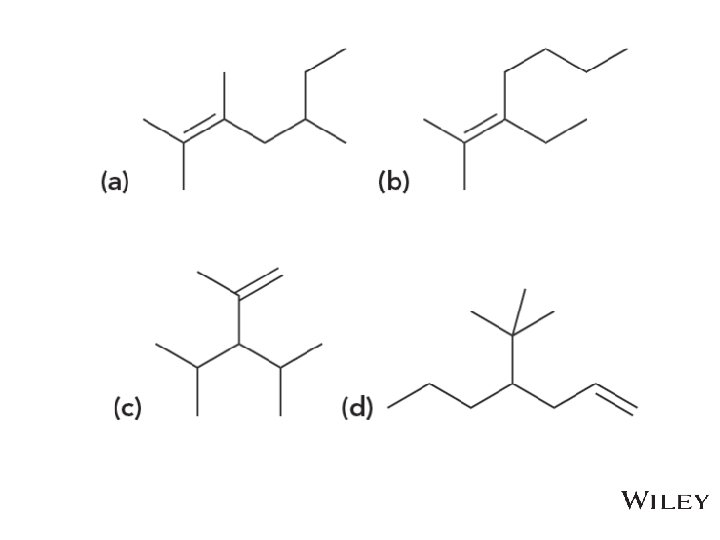
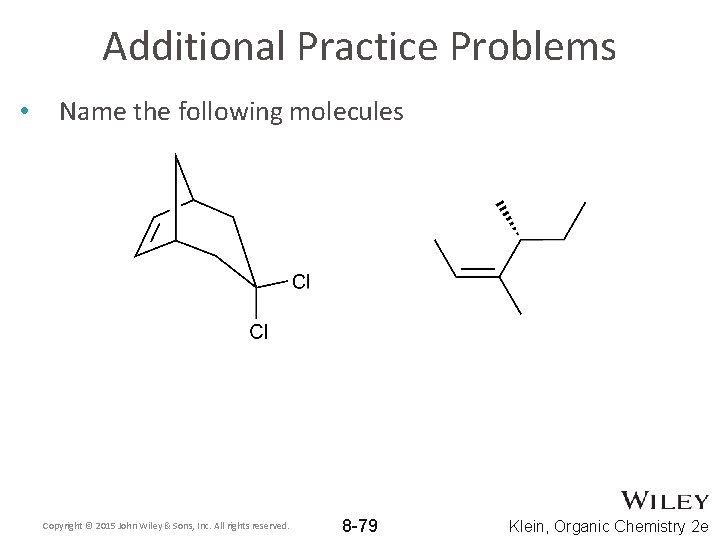
Additional Practice Problems • Name the following molecules Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -79 Klein, Organic Chemistry 2 e

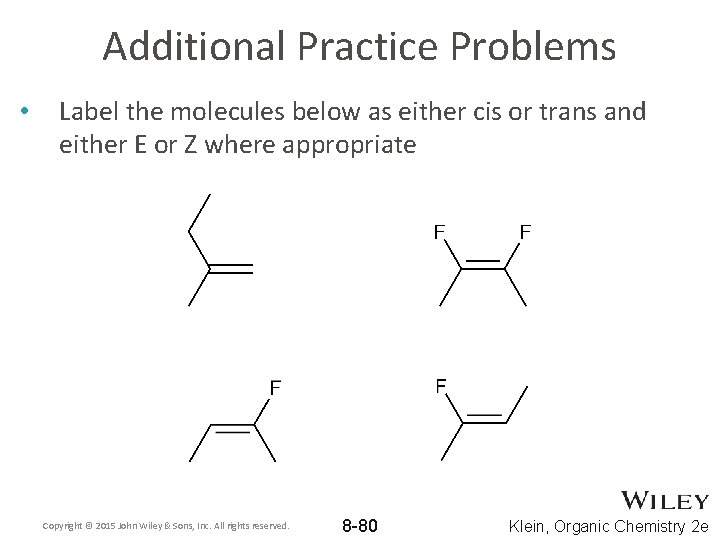
Additional Practice Problems • Label the molecules below as either cis or trans and either E or Z where appropriate Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -80 Klein, Organic Chemistry 2 e

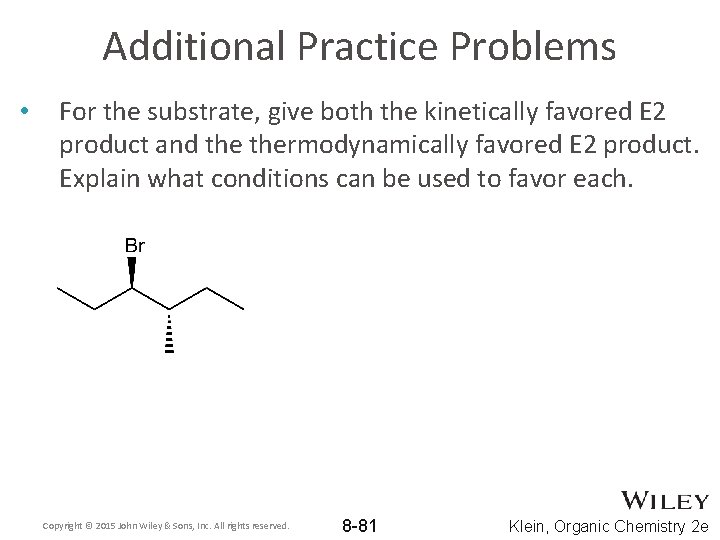
Additional Practice Problems • For the substrate, give both the kinetically favored E 2 product and thermodynamically favored E 2 product. Explain what conditions can be used to favor each. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -81 Klein, Organic Chemistry 2 e

Additional Practice Problems • Since tertiary substrates react more readily than secondary or primary in both E 1 and E 2 mechanisms, what factor(s) usually controls which mechanism will dominate and why? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -82 Klein, Organic Chemistry 2 e

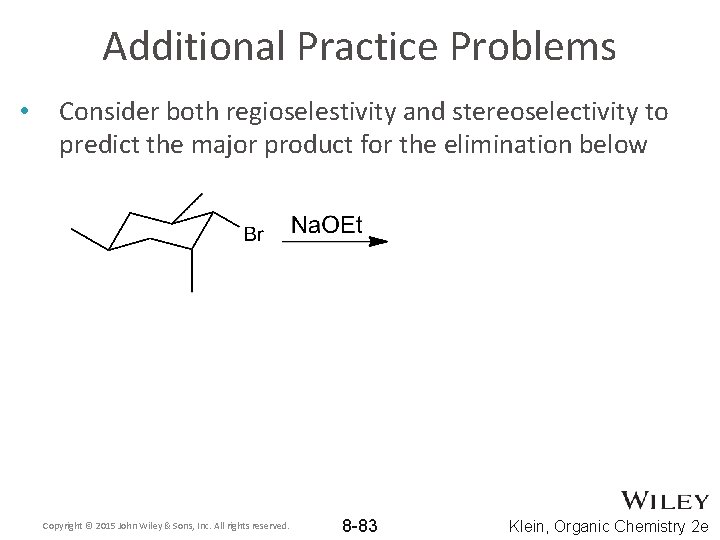
Additional Practice Problems • Consider both regioselestivity and stereoselectivity to predict the major product for the elimination below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -83 Klein, Organic Chemistry 2 e

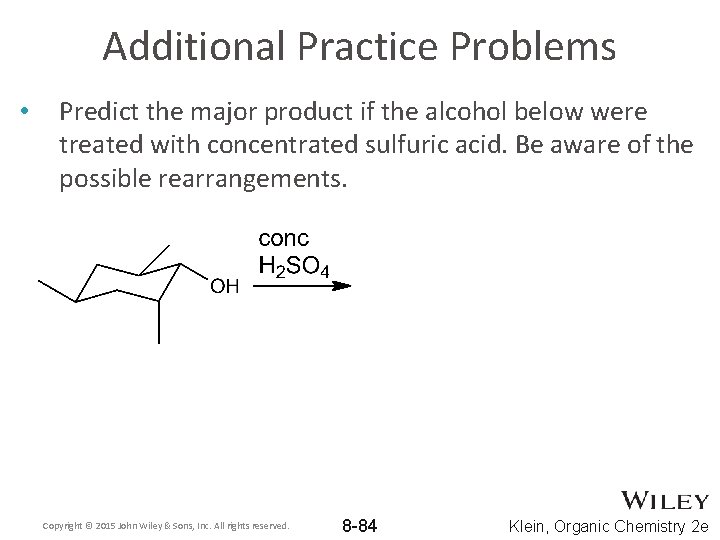
Additional Practice Problems • Predict the major product if the alcohol below were treated with concentrated sulfuric acid. Be aware of the possible rearrangements. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -84 Klein, Organic Chemistry 2 e

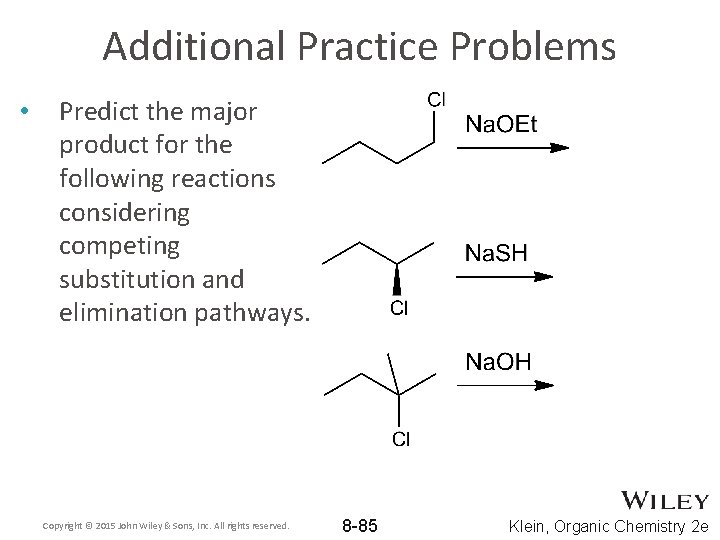
Additional Practice Problems • Predict the major product for the following reactions considering competing substitution and elimination pathways. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 8 -85 Klein, Organic Chemistry 2 e