Organic Chemistry Second Edition David Klein Chapter 7












- Slides: 81

Organic Chemistry Second Edition David Klein Chapter 7 Substitution Reactions Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

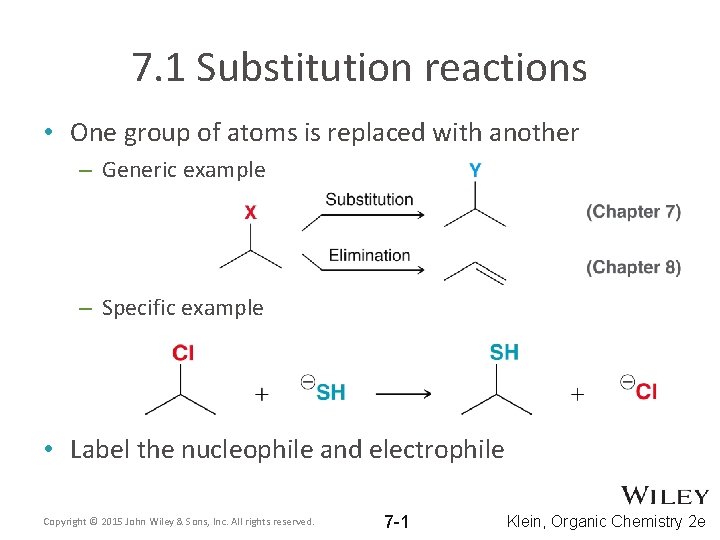
7. 1 Substitution reactions • One group of atoms is replaced with another – Generic example – Specific example • Label the nucleophile and electrophile Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -1 Klein, Organic Chemistry 2 e

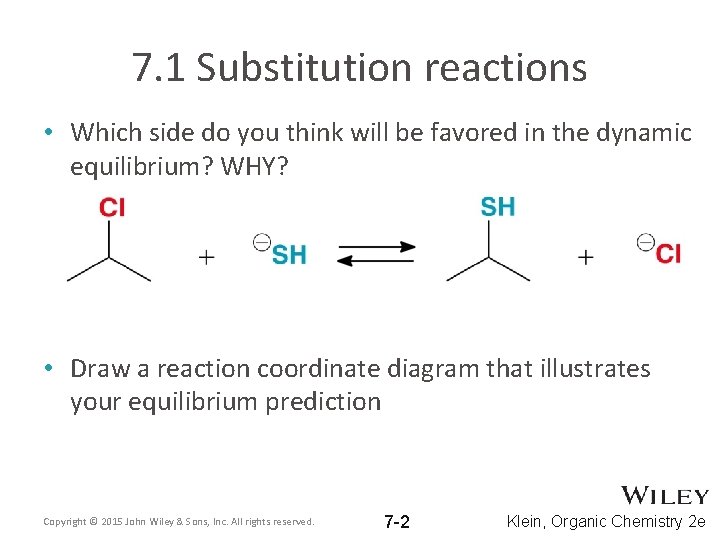
7. 1 Substitution reactions • Which side do you think will be favored in the dynamic equilibrium? WHY? • Draw a reaction coordinate diagram that illustrates your equilibrium prediction Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -2 Klein, Organic Chemistry 2 e

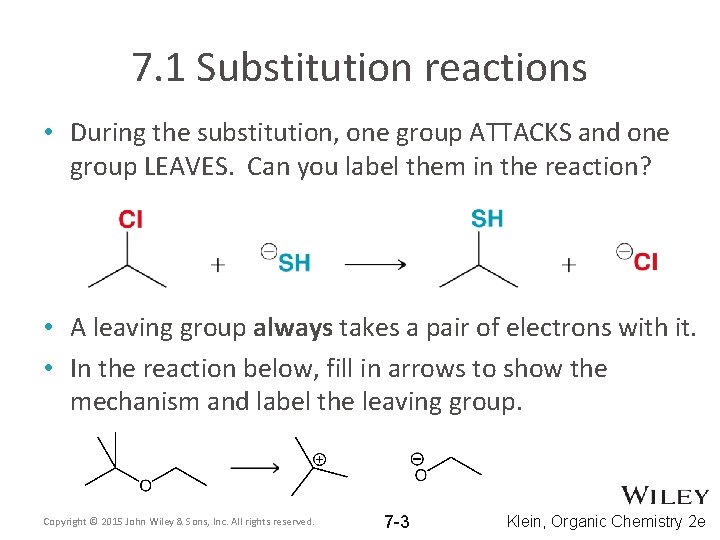
7. 1 Substitution reactions • During the substitution, one group ATTACKS and one group LEAVES. Can you label them in the reaction? • A leaving group always takes a pair of electrons with it. • In the reaction below, fill in arrows to show the mechanism and label the leaving group. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -3 Klein, Organic Chemistry 2 e

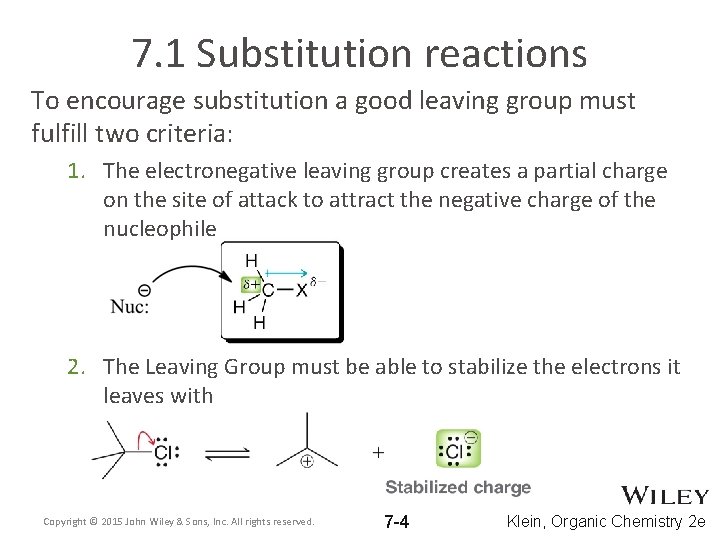
7. 1 Substitution reactions To encourage substitution a good leaving group must fulfill two criteria: 1. The electronegative leaving group creates a partial charge on the site of attack to attract the negative charge of the nucleophile 2. The Leaving Group must be able to stabilize the electrons it leaves with Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -4 Klein, Organic Chemistry 2 e

7. 1 Substitution reactions Can you give some examples of groups of atoms that qualify as good leaving groups according to the two key criteria? 1. Create a positive charge to attract the nucleophile. 2. Be able to stabilize the electrons it leaves with Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -5 Klein, Organic Chemistry 2 e

7. 2 Alkyl Halides • Alkyl halides are compounds where a carbon group (alkyl) is bonded to a halide (F, Cl, Br, or I) • Recall from section 4. 2 the steps we use to name a molecule 1. 2. 3. 4. • Identify and name the parent chain Identify the name of the substituents Assign a locant (number) to each substituents Assemble the name alphabetically The halide group is the key substituent we will name and locate Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -6 Klein, Organic Chemistry 2 e

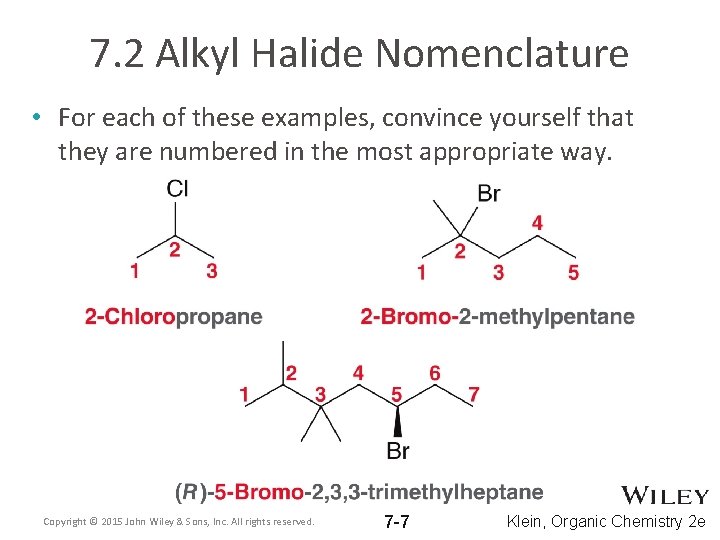
7. 2 Alkyl Halide Nomenclature • For each of these examples, convince yourself that they are numbered in the most appropriate way. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -7 Klein, Organic Chemistry 2 e

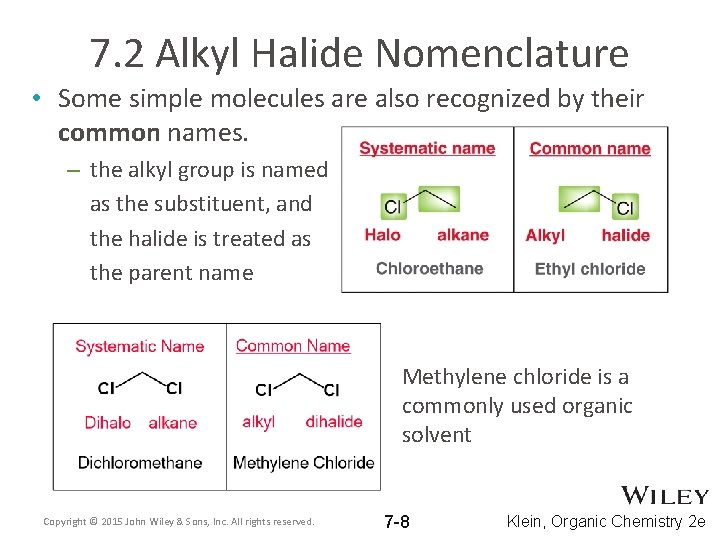
7. 2 Alkyl Halide Nomenclature • Some simple molecules are also recognized by their common names. – the alkyl group is named as the substituent, and the halide is treated as the parent name Methylene chloride is a commonly used organic solvent Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -8 Klein, Organic Chemistry 2 e

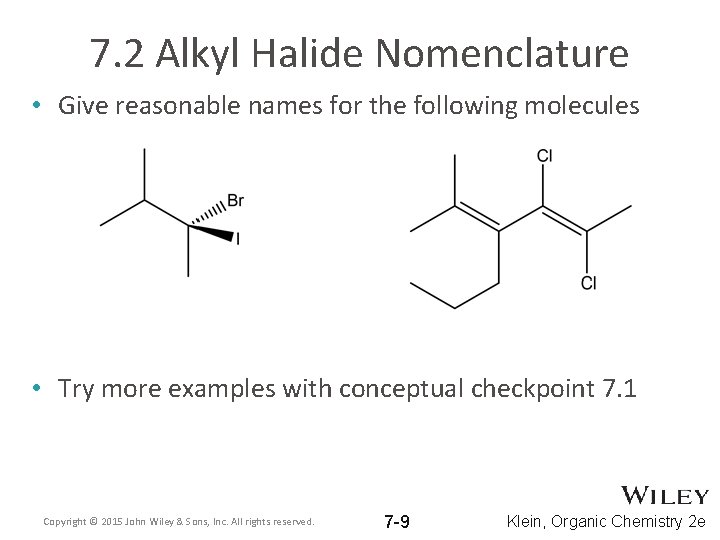
7. 2 Alkyl Halide Nomenclature • Give reasonable names for the following molecules • Try more examples with conceptual checkpoint 7. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -9 Klein, Organic Chemistry 2 e

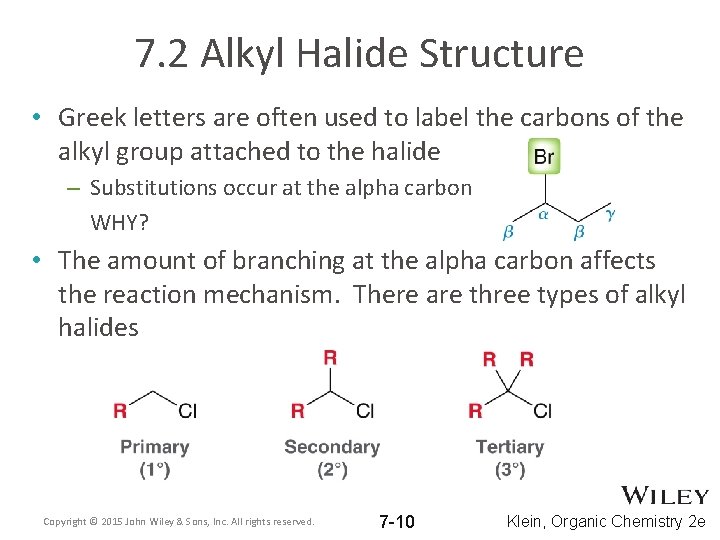
7. 2 Alkyl Halide Structure • Greek letters are often used to label the carbons of the alkyl group attached to the halide – Substitutions occur at the alpha carbon WHY? • The amount of branching at the alpha carbon affects the reaction mechanism. There are three types of alkyl halides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -10 Klein, Organic Chemistry 2 e

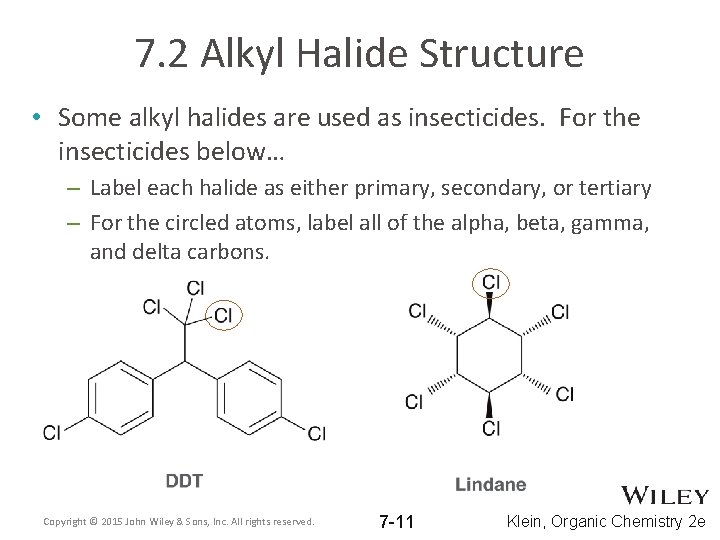
7. 2 Alkyl Halide Structure • Some alkyl halides are used as insecticides. For the insecticides below… – Label each halide as either primary, secondary, or tertiary – For the circled atoms, label all of the alpha, beta, gamma, and delta carbons. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -11 Klein, Organic Chemistry 2 e

7. 2 Alkyl Halide Structure • Halides appear in a wide variety of natural products and synthetic compounds • The structure of the molecule determines its function, and functions include… – – – Insecticides (DDT, etc. ) Dyes (tyrian purple, etc. ) Drugs (anticancer, antidepressants, antimicrobial, etc. ) Food additives (Splenda, etc. ) Many more Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -12 Klein, Organic Chemistry 2 e

7. 2 Alkyl Halide Structure HOW does a molecule’s structure affect its function and properties? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -13 Klein, Organic Chemistry 2 e

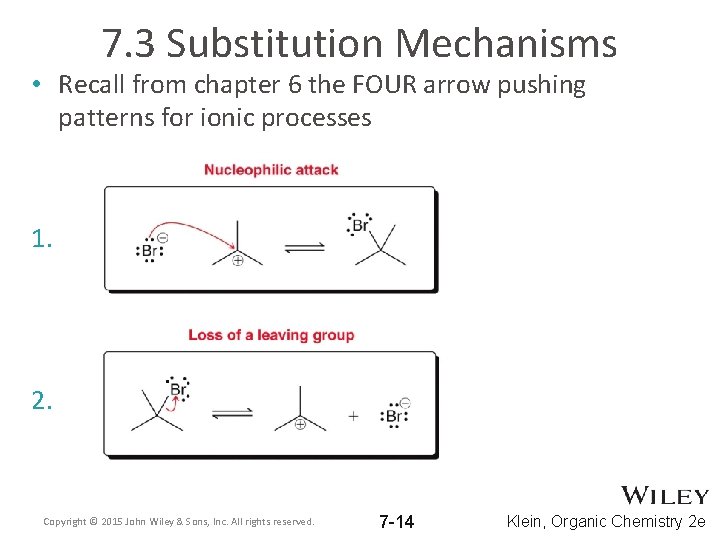
7. 3 Substitution Mechanisms • Recall from chapter 6 the FOUR arrow pushing patterns for ionic processes 1. 2. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -14 Klein, Organic Chemistry 2 e

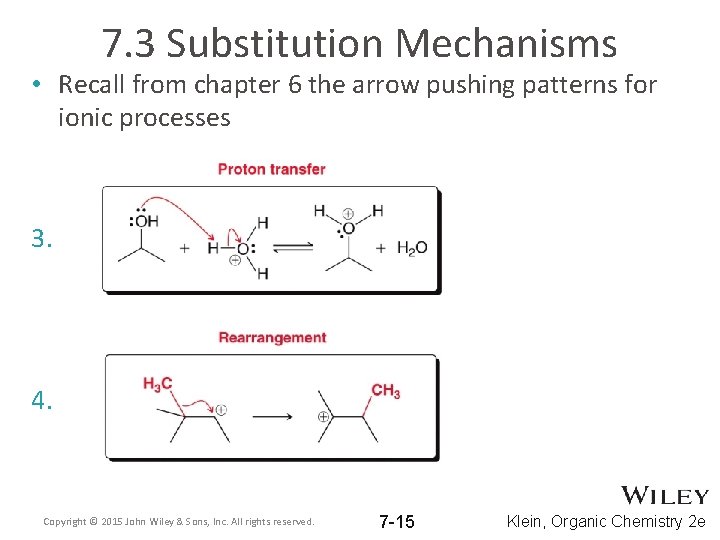
7. 3 Substitution Mechanisms • Recall from chapter 6 the arrow pushing patterns for ionic processes 3. 4. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -15 Klein, Organic Chemistry 2 e

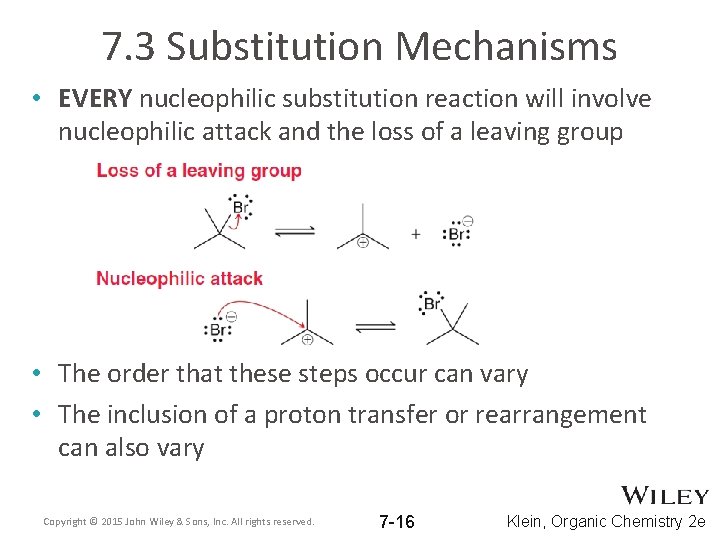
7. 3 Substitution Mechanisms • EVERY nucleophilic substitution reaction will involve nucleophilic attack and the loss of a leaving group • The order that these steps occur can vary • The inclusion of a proton transfer or rearrangement can also vary Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -16 Klein, Organic Chemistry 2 e

7. 3 Substitution Mechanisms • Draw mechanisms for each possibility and critique their likelihood 1. Nucleophilic attack first then loss of leaving group. 2. Loss of leaving group first then nucleophilic attack 3. Both happen simultaneously • Practice arrow pushing with Skill. Builder 7. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -17 Klein, Organic Chemistry 2 e

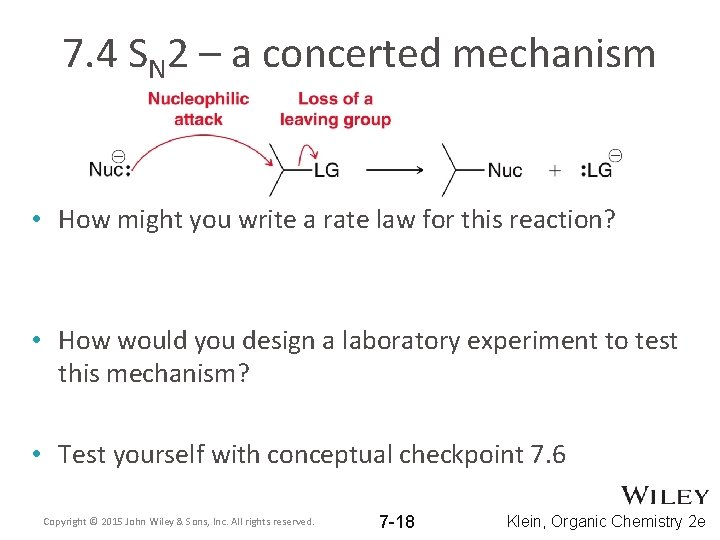
7. 4 SN 2 – a concerted mechanism • How might you write a rate law for this reaction? • How would you design a laboratory experiment to test this mechanism? • Test yourself with conceptual checkpoint 7. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -18 Klein, Organic Chemistry 2 e

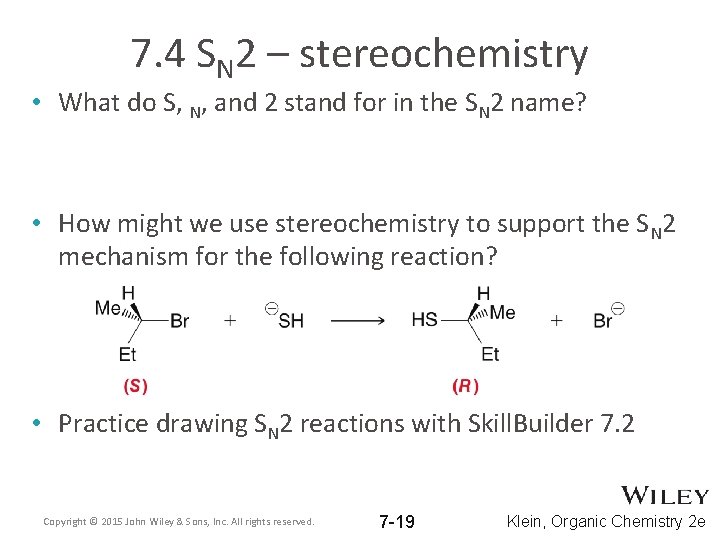
7. 4 SN 2 – stereochemistry • What do S, N, and 2 stand for in the SN 2 name? • How might we use stereochemistry to support the SN 2 mechanism for the following reaction? • Practice drawing SN 2 reactions with Skill. Builder 7. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -19 Klein, Organic Chemistry 2 e

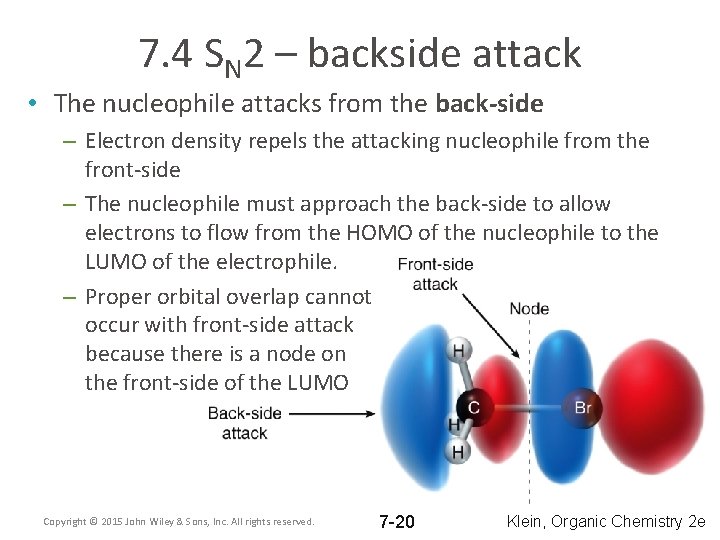
7. 4 SN 2 – backside attack • The nucleophile attacks from the back-side – Electron density repels the attacking nucleophile from the front-side – The nucleophile must approach the back-side to allow electrons to flow from the HOMO of the nucleophile to the LUMO of the electrophile. – Proper orbital overlap cannot occur with front-side attack because there is a node on the front-side of the LUMO Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -20 Klein, Organic Chemistry 2 e

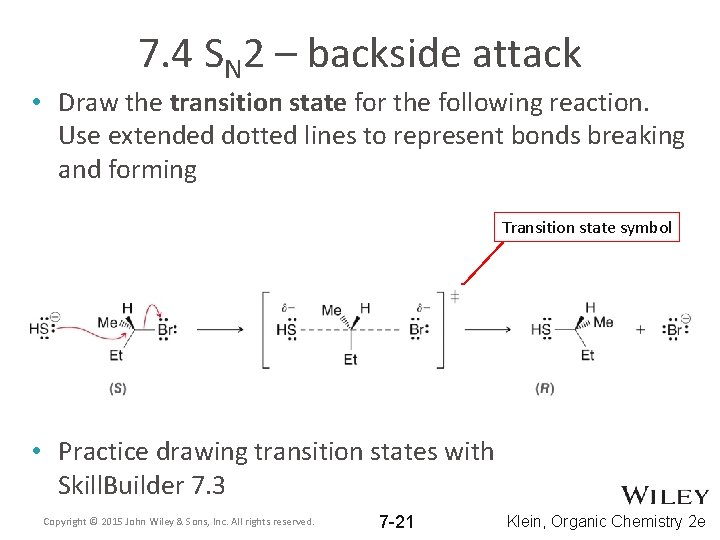
7. 4 SN 2 – backside attack • Draw the transition state for the following reaction. Use extended dotted lines to represent bonds breaking and forming Transition state symbol • Practice drawing transition states with Skill. Builder 7. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -21 Klein, Organic Chemistry 2 e

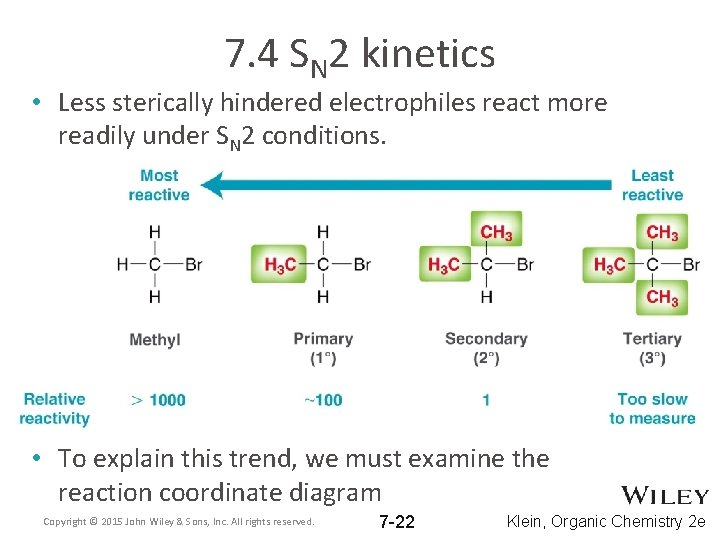
7. 4 SN 2 kinetics • Less sterically hindered electrophiles react more readily under SN 2 conditions. • To explain this trend, we must examine the reaction coordinate diagram Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -22 Klein, Organic Chemistry 2 e

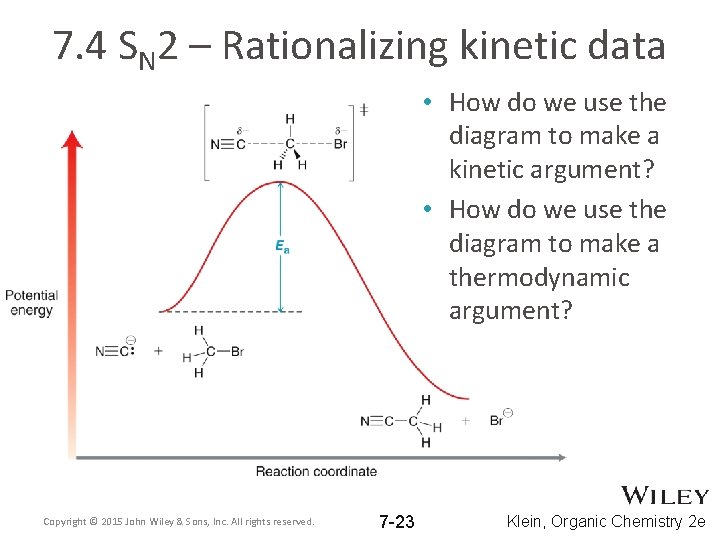
7. 4 SN 2 – Rationalizing kinetic data • How do we use the diagram to make a kinetic argument? • How do we use the diagram to make a thermodynamic argument? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -23 Klein, Organic Chemistry 2 e

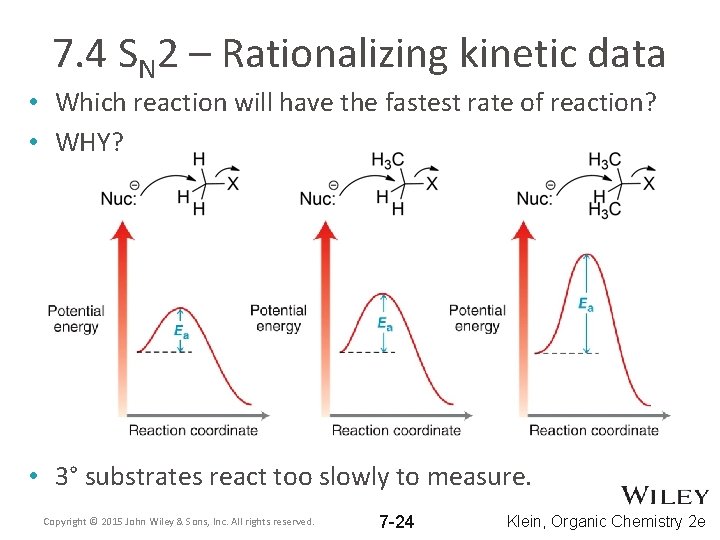
7. 4 SN 2 – Rationalizing kinetic data • Which reaction will have the fastest rate of reaction? • WHY? • 3° substrates react too slowly to measure. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -24 Klein, Organic Chemistry 2 e

7. 4 SN 2 – Rationalizing kinetic data • An example to consider: neopentyl bromide • Draw the structure of neopentyl bromide • Is neopentyl bromide a primary, secondary, or tertiary alkyl bromide? • Should neopentyl bromide react by an SN 2 reaction relatively quickly or relatively slowly? WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -25 Klein, Organic Chemistry 2 e

7. 4 SN 2 – Rationalizing kinetic data • If you memorize rules, you will probably miss questions about exceptions to rules • It is better to understand the concepts than to memorize rules Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -26 Klein, Organic Chemistry 2 e

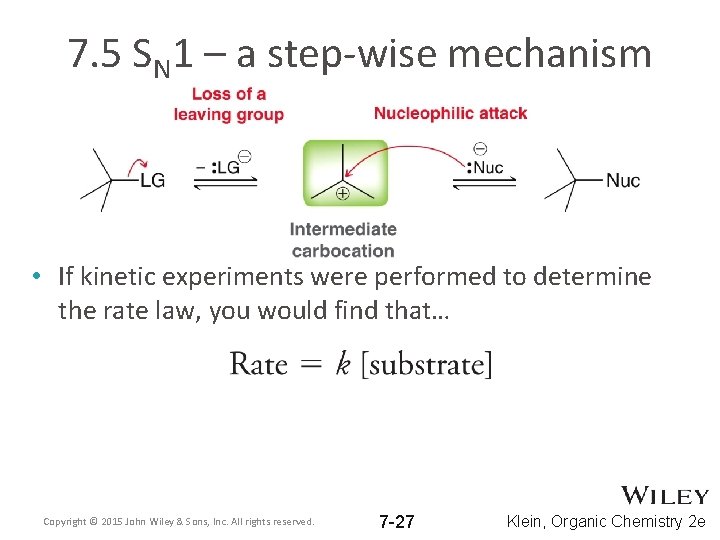
7. 5 SN 1 – a step-wise mechanism • If kinetic experiments were performed to determine the rate law, you would find that… Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -27 Klein, Organic Chemistry 2 e

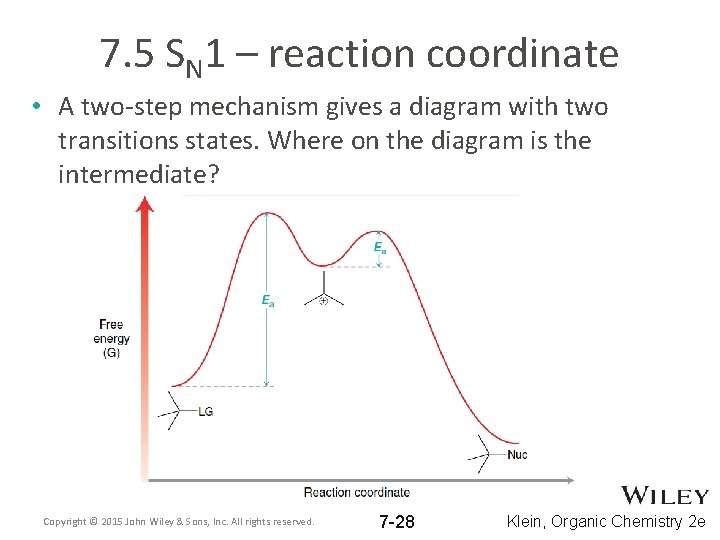
7. 5 SN 1 – reaction coordinate • A two-step mechanism gives a diagram with two transitions states. Where on the diagram is the intermediate? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -28 Klein, Organic Chemistry 2 e

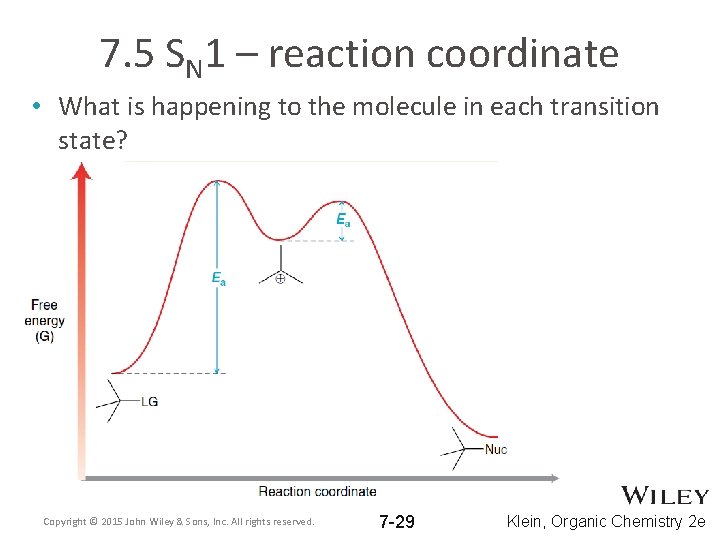
7. 5 SN 1 – reaction coordinate • What is happening to the molecule in each transition state? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -29 Klein, Organic Chemistry 2 e

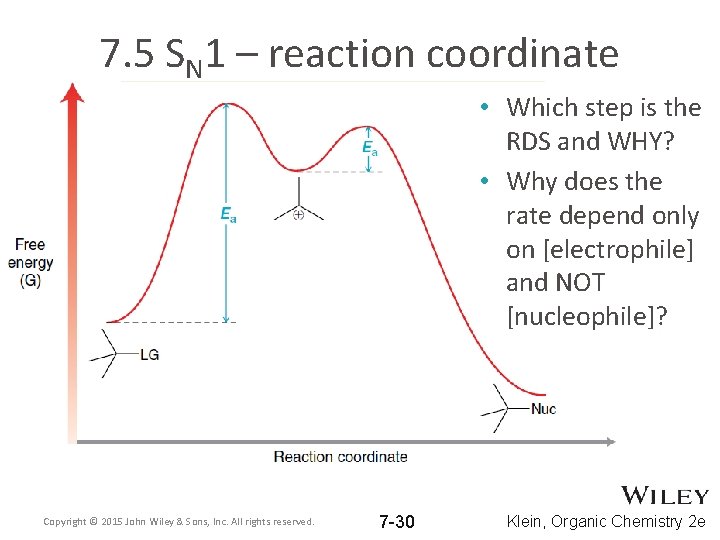
7. 5 SN 1 – reaction coordinate • Which step is the RDS and WHY? • Why does the rate depend only on [electrophile] and NOT [nucleophile]? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -30 Klein, Organic Chemistry 2 e

7. 5 SN 1 – a step-wise mechanism • What do the S, N, and 1 stand for in the SN 1 name? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -31 Klein, Organic Chemistry 2 e

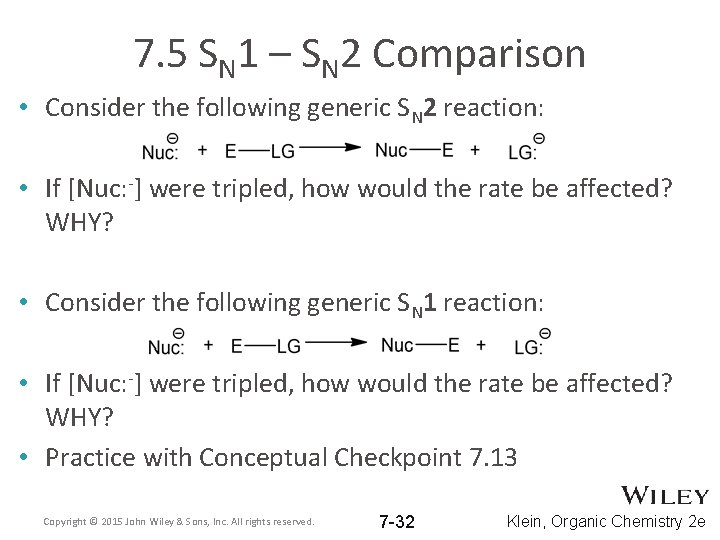
7. 5 SN 1 – SN 2 Comparison • Consider the following generic SN 2 reaction: • If [Nuc: -] were tripled, how would the rate be affected? WHY? • Consider the following generic SN 1 reaction: • If [Nuc: -] were tripled, how would the rate be affected? WHY? • Practice with Conceptual Checkpoint 7. 13 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -32 Klein, Organic Chemistry 2 e

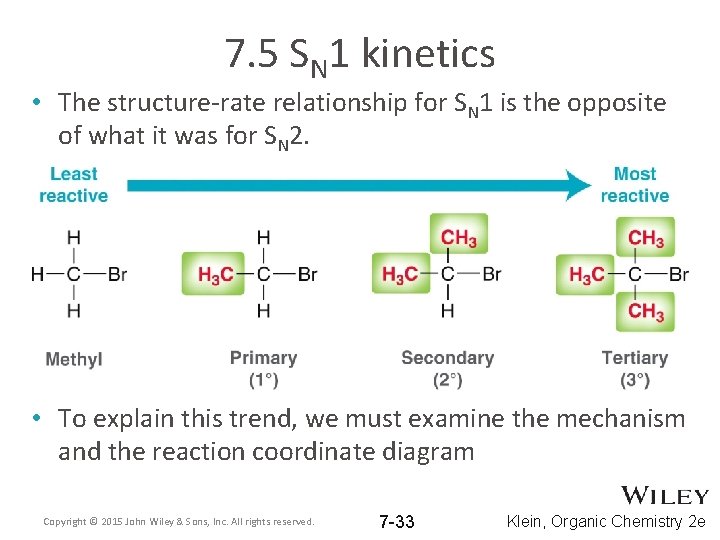
7. 5 SN 1 kinetics • The structure-rate relationship for SN 1 is the opposite of what it was for SN 2. • To explain this trend, we must examine the mechanism and the reaction coordinate diagram Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -33 Klein, Organic Chemistry 2 e

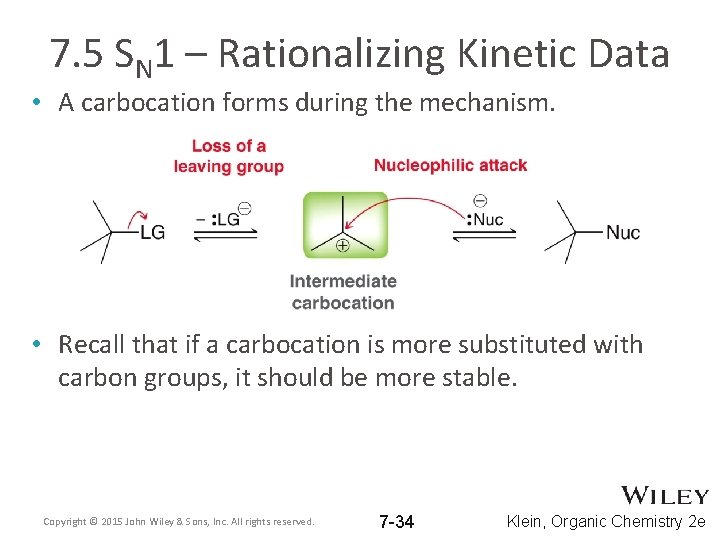
7. 5 SN 1 – Rationalizing Kinetic Data • A carbocation forms during the mechanism. • Recall that if a carbocation is more substituted with carbon groups, it should be more stable. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -34 Klein, Organic Chemistry 2 e

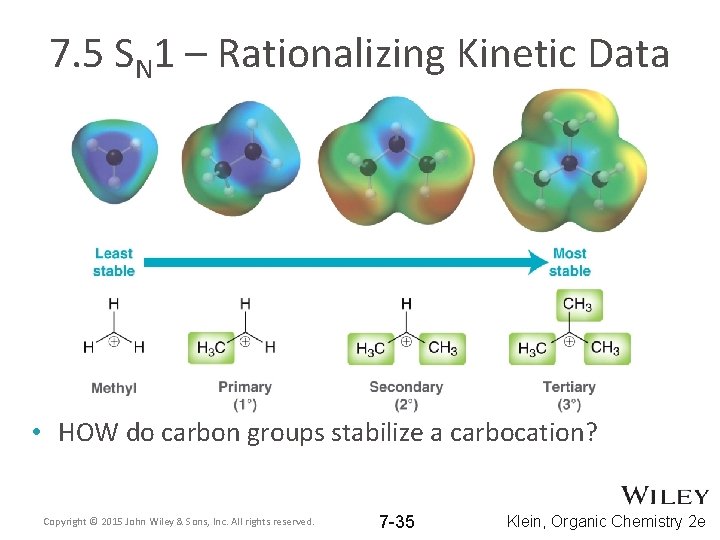
7. 5 SN 1 – Rationalizing Kinetic Data • HOW do carbon groups stabilize a carbocation? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -35 Klein, Organic Chemistry 2 e

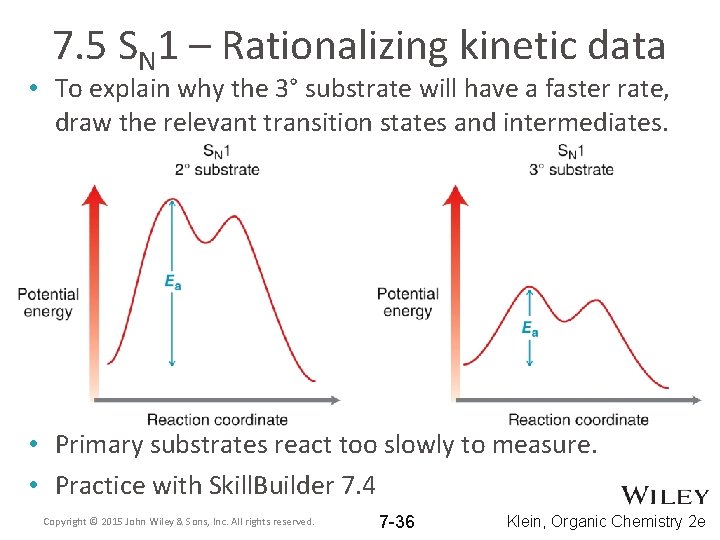
7. 5 SN 1 – Rationalizing kinetic data • To explain why the 3° substrate will have a faster rate, draw the relevant transition states and intermediates. • Primary substrates react too slowly to measure. • Practice with Skill. Builder 7. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -36 Klein, Organic Chemistry 2 e

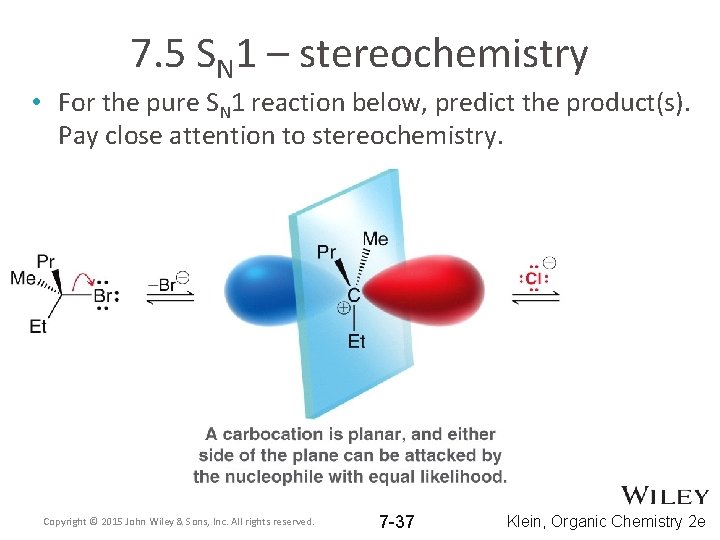
7. 5 SN 1 – stereochemistry • For the pure SN 1 reaction below, predict the product(s). Pay close attention to stereochemistry. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -37 Klein, Organic Chemistry 2 e

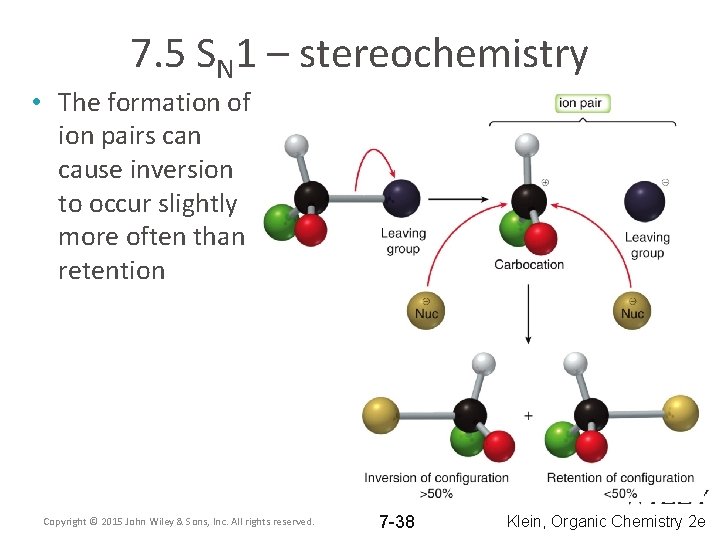
7. 5 SN 1 – stereochemistry • The formation of ion pairs can cause inversion to occur slightly more often than retention Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -38 Klein, Organic Chemistry 2 e

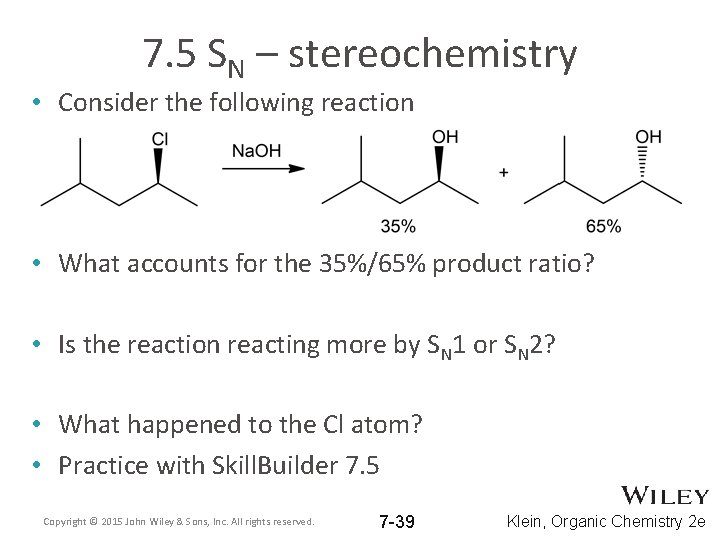
7. 5 SN – stereochemistry • Consider the following reaction • What accounts for the 35%/65% product ratio? • Is the reaction reacting more by SN 1 or SN 2? • What happened to the Cl atom? • Practice with Skill. Builder 7. 5 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -39 Klein, Organic Chemistry 2 e

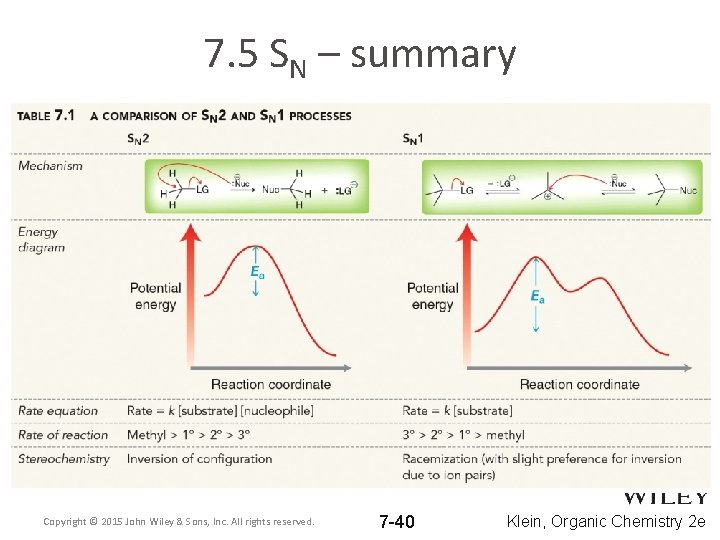
7. 5 SN – summary Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -40 Klein, Organic Chemistry 2 e

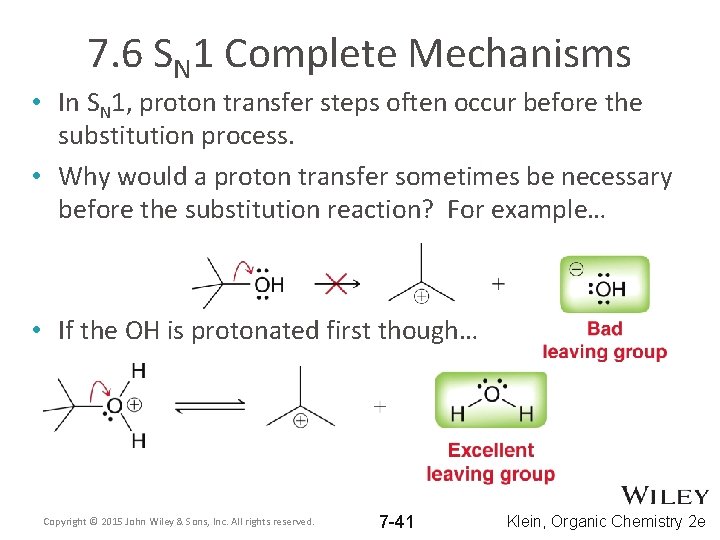
7. 6 SN 1 Complete Mechanisms • In SN 1, proton transfer steps often occur before the substitution process. • Why would a proton transfer sometimes be necessary before the substitution reaction? For example… • If the OH is protonated first though… Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -41 Klein, Organic Chemistry 2 e

7. 6 SN 1 Complete Mechanisms • Would it also be helpful to protonate an OH group in an SN 2 substitution? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -42 Klein, Organic Chemistry 2 e

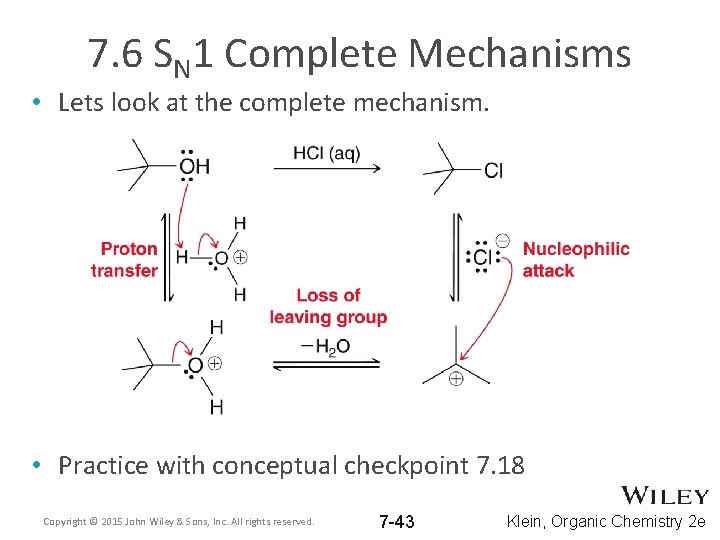
7. 6 SN 1 Complete Mechanisms • Lets look at the complete mechanism. • Practice with conceptual checkpoint 7. 18 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -43 Klein, Organic Chemistry 2 e

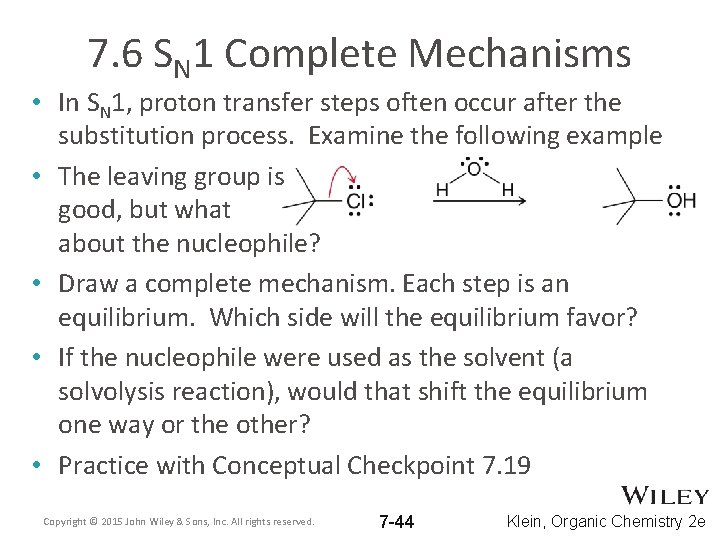
7. 6 SN 1 Complete Mechanisms • In SN 1, proton transfer steps often occur after the substitution process. Examine the following example • The leaving group is good, but what about the nucleophile? • Draw a complete mechanism. Each step is an equilibrium. Which side will the equilibrium favor? • If the nucleophile were used as the solvent (a solvolysis reaction), would that shift the equilibrium one way or the other? • Practice with Conceptual Checkpoint 7. 19 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -44 Klein, Organic Chemistry 2 e

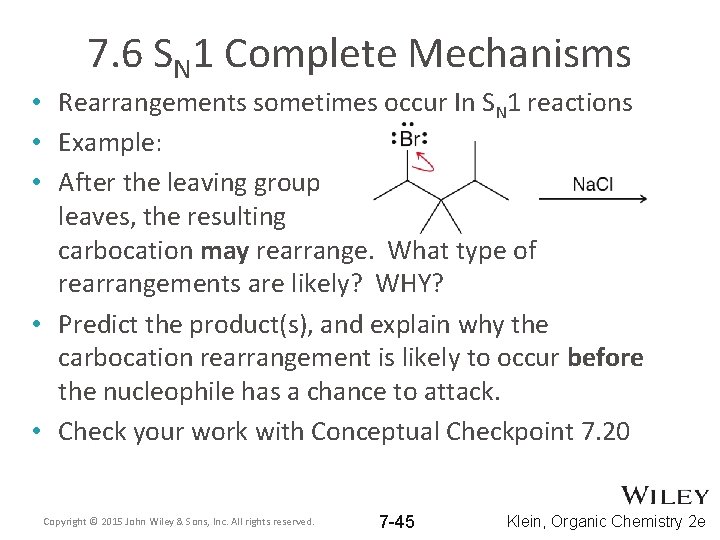
7. 6 SN 1 Complete Mechanisms • Rearrangements sometimes occur In SN 1 reactions • Example: • After the leaving group leaves, the resulting carbocation may rearrange. What type of rearrangements are likely? WHY? • Predict the product(s), and explain why the carbocation rearrangement is likely to occur before the nucleophile has a chance to attack. • Check your work with Conceptual Checkpoint 7. 20 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -45 Klein, Organic Chemistry 2 e

7. 6 SN 1 Complete Mechanisms Summary of considerations to make • Will proton transfers be necessary? – look at the quality of the leaving group – Look at the stability of the final product • Will the mechanism be SN 1 or SN 2? – look at how crowded the electrophilic site is – Look at how stable the resulting carbocation would be • Are rearrangements likely? – look for ways to improve the stability of the carbocation • Will the product have inversion or racemization? – SN 1=racemization while SN 2=inversion Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -46 Klein, Organic Chemistry 2 e

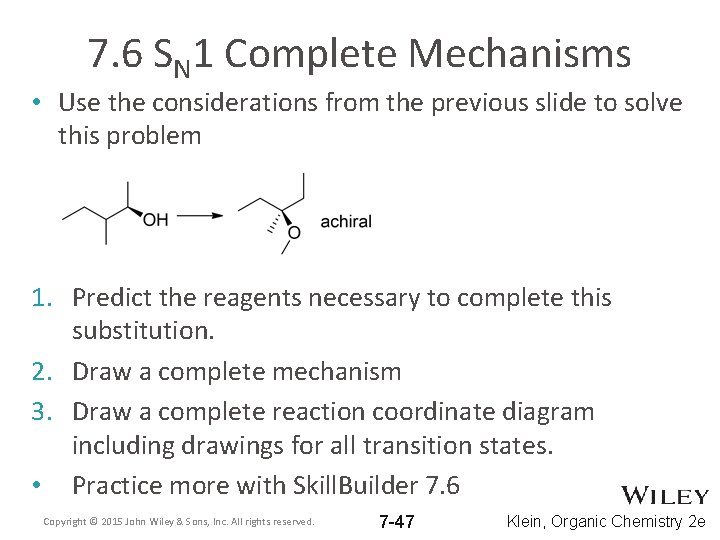
7. 6 SN 1 Complete Mechanisms • Use the considerations from the previous slide to solve this problem 1. Predict the reagents necessary to complete this substitution. 2. Draw a complete mechanism 3. Draw a complete reaction coordinate diagram including drawings for all transition states. • Practice more with Skill. Builder 7. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -47 Klein, Organic Chemistry 2 e

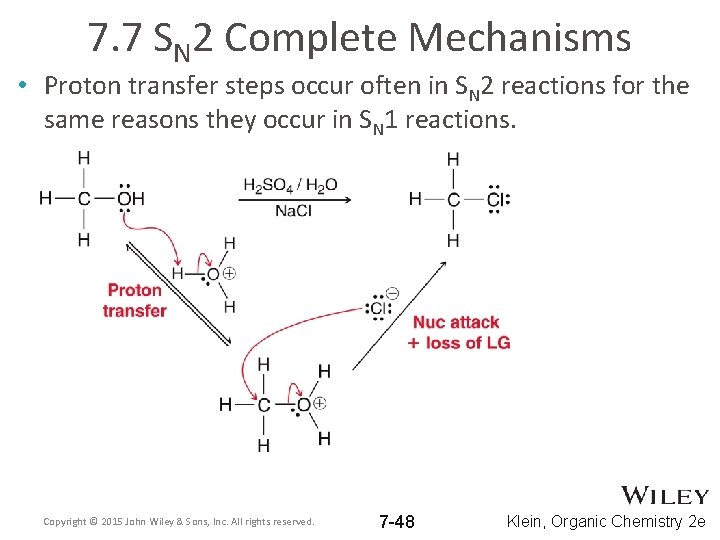
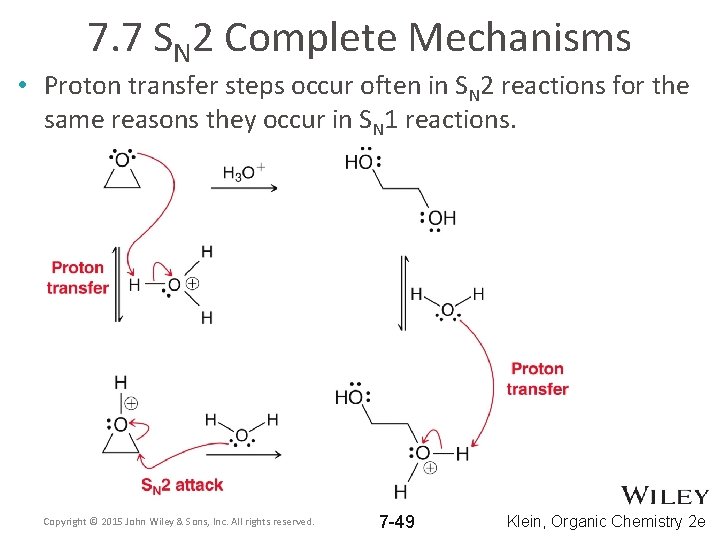
7. 7 SN 2 Complete Mechanisms • Proton transfer steps occur often in SN 2 reactions for the same reasons they occur in SN 1 reactions. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -48 Klein, Organic Chemistry 2 e

7. 7 SN 2 Complete Mechanisms • Proton transfer steps occur often in SN 2 reactions for the same reasons they occur in SN 1 reactions. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -49 Klein, Organic Chemistry 2 e

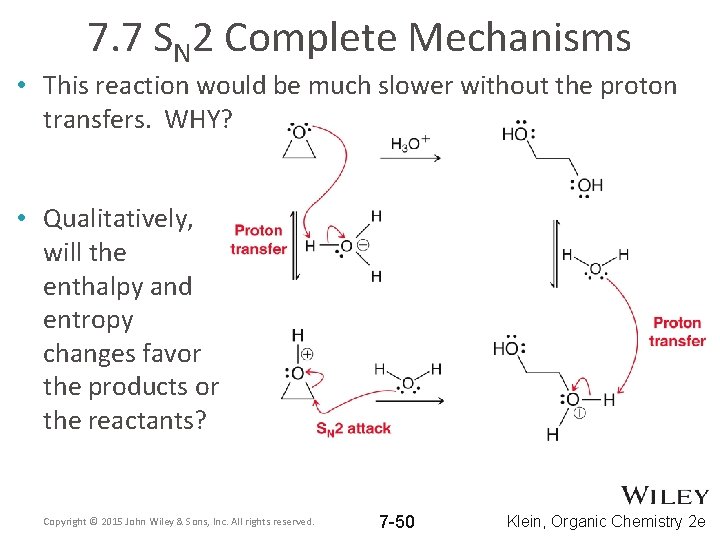
7. 7 SN 2 Complete Mechanisms • This reaction would be much slower without the proton transfers. WHY? • Qualitatively, will the enthalpy and entropy changes favor the products or the reactants? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -50 Klein, Organic Chemistry 2 e

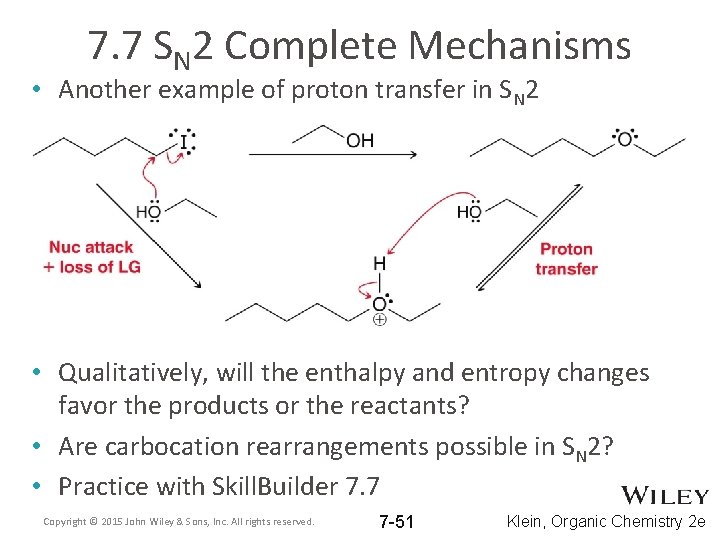
7. 7 SN 2 Complete Mechanisms • Another example of proton transfer in SN 2 • Qualitatively, will the enthalpy and entropy changes favor the products or the reactants? • Are carbocation rearrangements possible in SN 2? • Practice with Skill. Builder 7. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -51 Klein, Organic Chemistry 2 e

7. 8 SN 1 vs. SN 2 • There are four main factors that determine whether a substitution reaction is more likely to occur by SN 1 or S N 2 • Lets examine them in order of importance 1. The substrate (both sterics and the stability of the carbocation) 2. The quality of the leaving group 3. The strength of the nucleophile 4. The solvent Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -52 Klein, Organic Chemistry 2 e

7. 8 SN 1 vs. SN 2 • Before we can examine carbocation stability, let’s review some terminology ad learn some new 1. Vinyl 2. Allyl • Let’s learn some new terminology 1. Benzyl 2. Aryl Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -53 Klein, Organic Chemistry 2 e

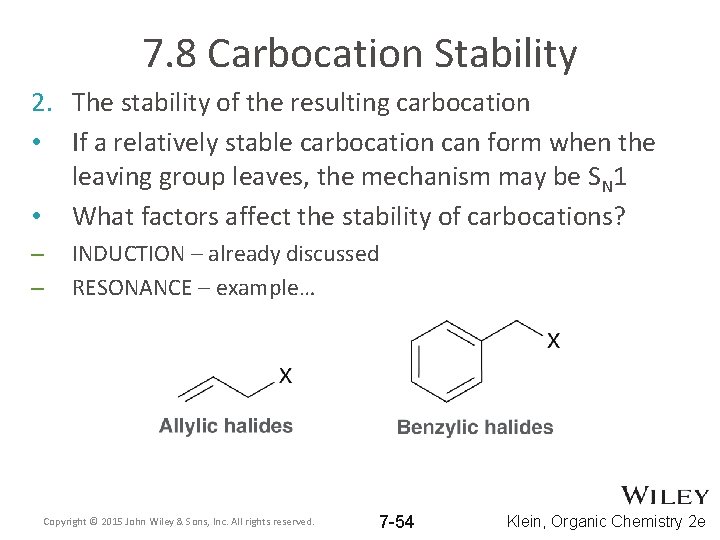
7. 8 Carbocation Stability 2. The stability of the resulting carbocation • If a relatively stable carbocation can form when the leaving group leaves, the mechanism may be SN 1 • What factors affect the stability of carbocations? – – INDUCTION – already discussed RESONANCE – example… Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -54 Klein, Organic Chemistry 2 e

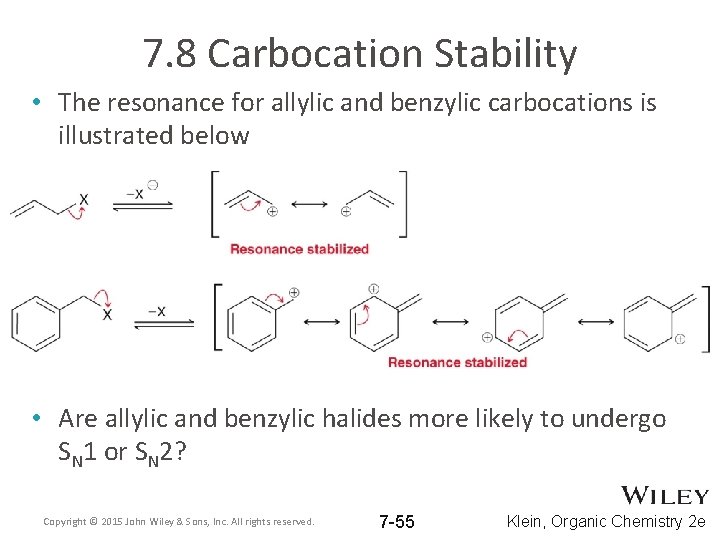
7. 8 Carbocation Stability • The resonance for allylic and benzylic carbocations is illustrated below • Are allylic and benzylic halides more likely to undergo SN 1 or SN 2? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -55 Klein, Organic Chemistry 2 e

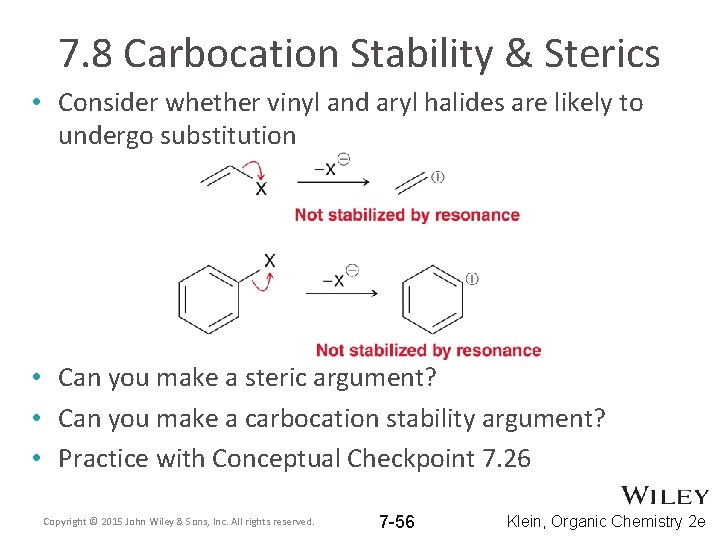
7. 8 Carbocation Stability & Sterics • Consider whether vinyl and aryl halides are likely to undergo substitution • Can you make a steric argument? • Can you make a carbocation stability argument? • Practice with Conceptual Checkpoint 7. 26 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -56 Klein, Organic Chemistry 2 e

7. 8 SN 1 vs. SN 2 (the nucleophile) 3. The quality of the nucleophile • What makes a nucleophile strong or weak? – Stability (induction, resonance, solvation) – Sterics • Give some examples of strong nucleophiles and some examples of weak ones • Will a strong nucleophile favor SN 1 or SN 2? WHY? • Practice with Conceptual Checkpoint 7. 27 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -57 Klein, Organic Chemistry 2 e

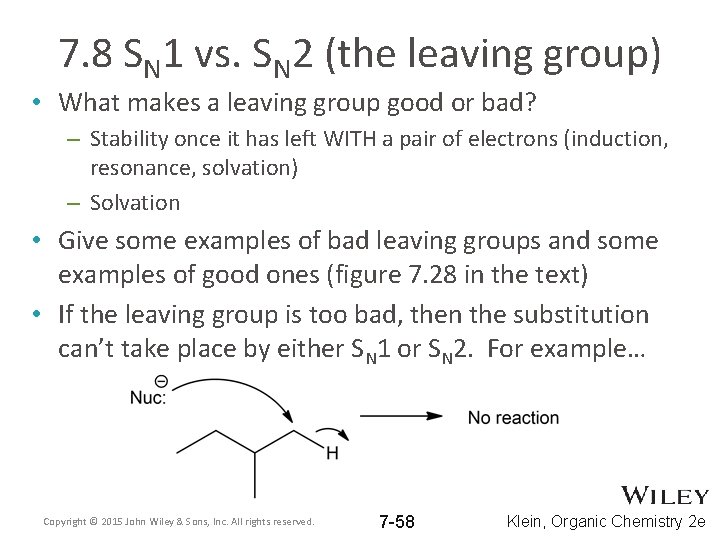
7. 8 SN 1 vs. SN 2 (the leaving group) • What makes a leaving group good or bad? – Stability once it has left WITH a pair of electrons (induction, resonance, solvation) – Solvation • Give some examples of bad leaving groups and some examples of good ones (figure 7. 28 in the text) • If the leaving group is too bad, then the substitution can’t take place by either SN 1 or SN 2. For example… Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -58 Klein, Organic Chemistry 2 e

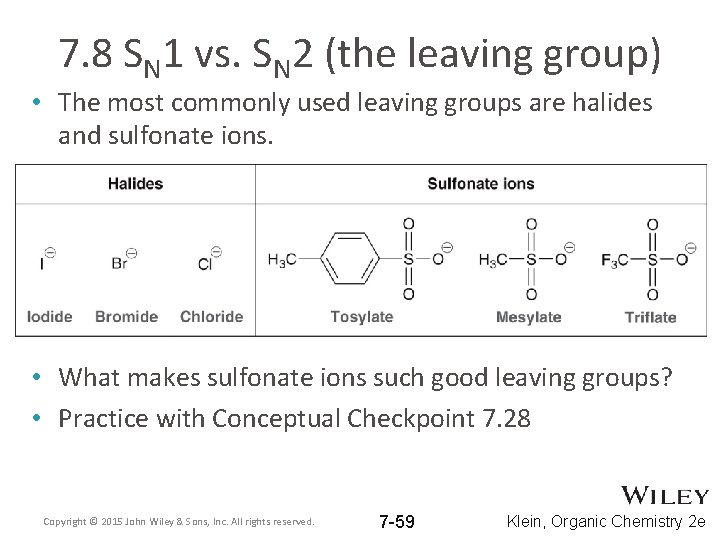
7. 8 SN 1 vs. SN 2 (the leaving group) • The most commonly used leaving groups are halides and sulfonate ions. • What makes sulfonate ions such good leaving groups? • Practice with Conceptual Checkpoint 7. 28 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -59 Klein, Organic Chemistry 2 e

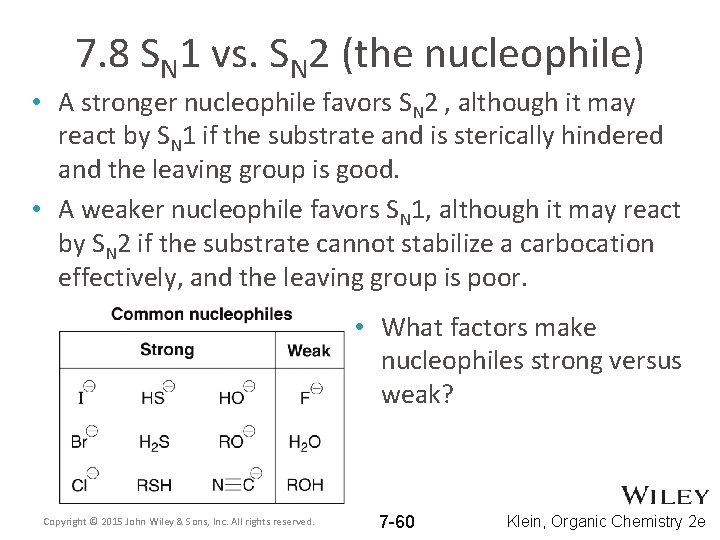
7. 8 SN 1 vs. SN 2 (the nucleophile) • A stronger nucleophile favors SN 2 , although it may react by SN 1 if the substrate and is sterically hindered and the leaving group is good. • A weaker nucleophile favors SN 1, although it may react by SN 2 if the substrate cannot stabilize a carbocation effectively, and the leaving group is poor. • What factors make nucleophiles strong versus weak? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -60 Klein, Organic Chemistry 2 e

7. 8 SN 1 vs. SN 2 (the solvent) 4. The solvent • The solvent surrounds each species in the mechanism including the transition state. How does that help to facilitate the reaction? See next slide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -61 Klein, Organic Chemistry 2 e

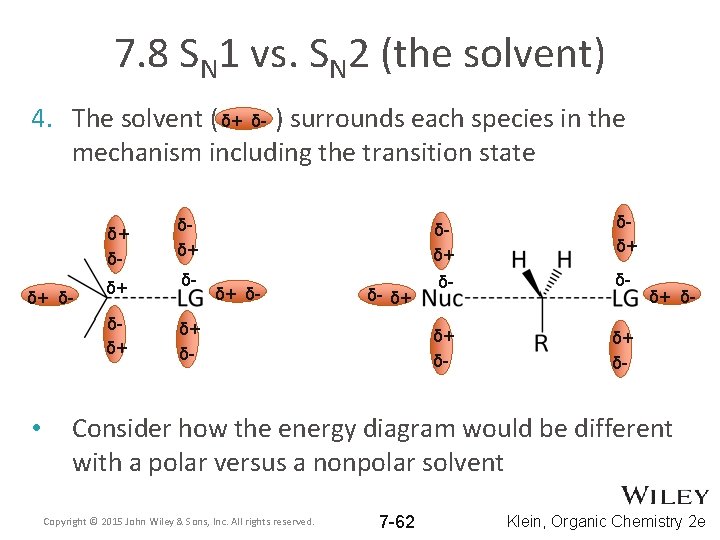
7. 8 SN 1 vs. SN 2 (the solvent) 4. The solvent ( δ+ δ- ) surrounds each species in the mechanism including the transition state δ+ δ- • δ+ δ- δδ+ δδ+ δ+ δ- δ- δ- δδ+ δ+ δ- δ- δ+ δ+ δ- Consider how the energy diagram would be different with a polar versus a nonpolar solvent Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -62 Klein, Organic Chemistry 2 e

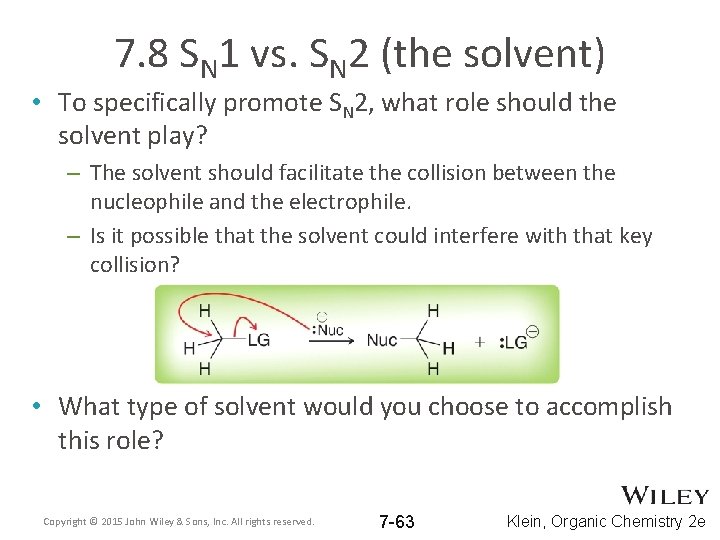
7. 8 SN 1 vs. SN 2 (the solvent) • To specifically promote SN 2, what role should the solvent play? – The solvent should facilitate the collision between the nucleophile and the electrophile. – Is it possible that the solvent could interfere with that key collision? • What type of solvent would you choose to accomplish this role? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -63 Klein, Organic Chemistry 2 e

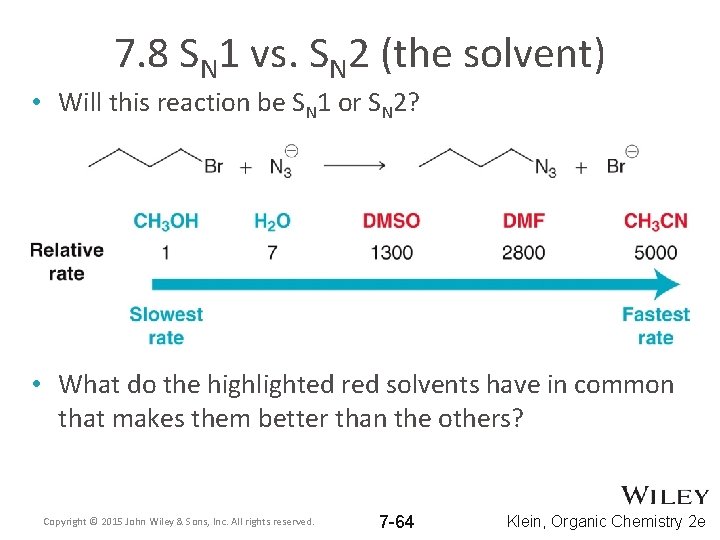
7. 8 SN 1 vs. SN 2 (the solvent) • Will this reaction be SN 1 or SN 2? • What do the highlighted red solvents have in common that makes them better than the others? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -64 Klein, Organic Chemistry 2 e

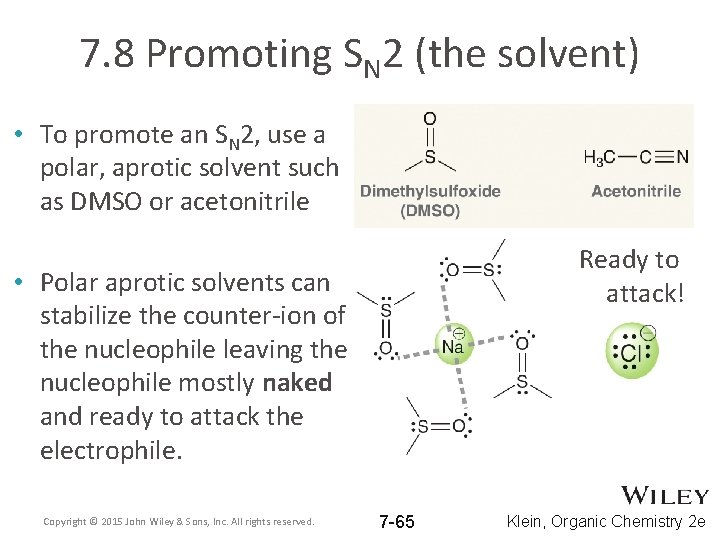
7. 8 Promoting SN 2 (the solvent) • To promote an SN 2, use a polar, aprotic solvent such as DMSO or acetonitrile Ready to attack! • Polar aprotic solvents can stabilize the counter-ion of the nucleophile leaving the nucleophile mostly naked and ready to attack the electrophile. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -65 Klein, Organic Chemistry 2 e

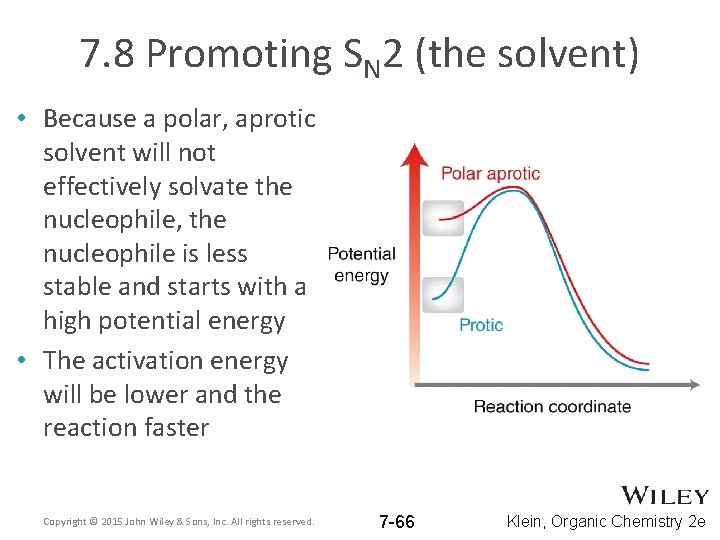
7. 8 Promoting SN 2 (the solvent) • Because a polar, aprotic solvent will not effectively solvate the nucleophile, the nucleophile is less stable and starts with a high potential energy • The activation energy will be lower and the reaction faster Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -66 Klein, Organic Chemistry 2 e

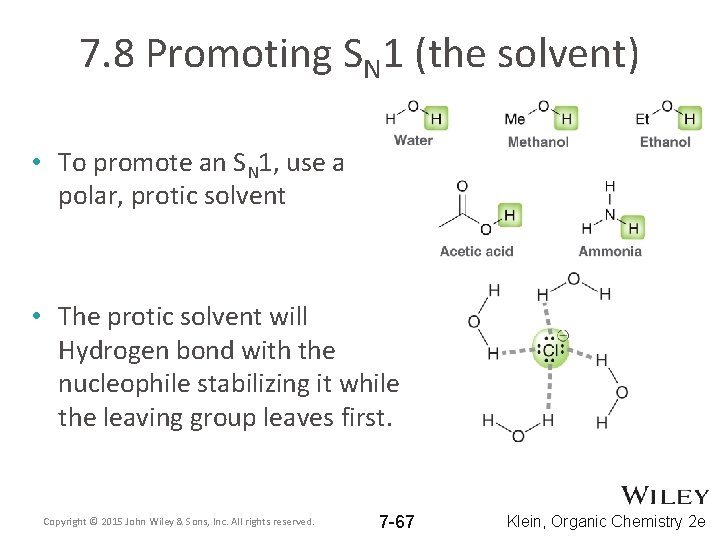
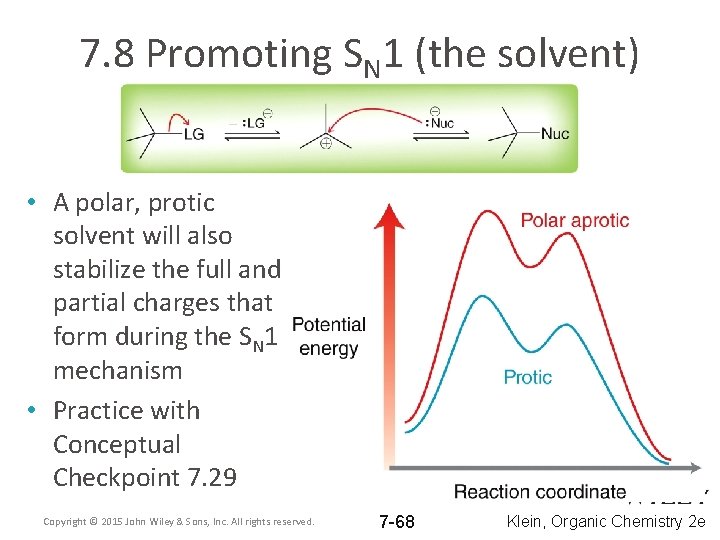
7. 8 Promoting SN 1 (the solvent) • To promote an SN 1, use a polar, protic solvent • The protic solvent will Hydrogen bond with the nucleophile stabilizing it while the leaving group leaves first. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -67 Klein, Organic Chemistry 2 e

7. 8 Promoting SN 1 (the solvent) • A polar, protic solvent will also stabilize the full and partial charges that form during the SN 1 mechanism • Practice with Conceptual Checkpoint 7. 29 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -68 Klein, Organic Chemistry 2 e

7. 8 Solvent Effect on Halide Nucleophiles • Consider the nucleophiles, F-, Cl-, Br-, and I • In a polar, protic solvent, which should be most reactive? WHY? • In a polar, aprotic solvent, which should be most reactive? WHY? • Why does the size of the halide affect its ability to attract a polar protic solvent? • Practice with Skill. Builder 7. 8 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -69 Klein, Organic Chemistry 2 e

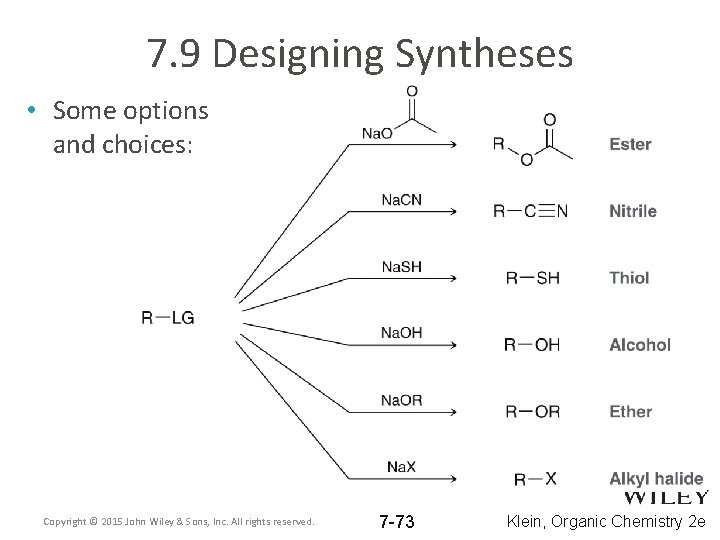
7. 9 Designing Syntheses • How do we use what we have learned to set up successful reactions? – We must choose appropriate substrate, nucleophile, leaving group, solvent, etc. • If you are working with a 1° substrate, the reaction will be SN 2, so what are the best conditions? – Nucleophile? – Leaving Group? – Solvent? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -70 Klein, Organic Chemistry 2 e

7. 9 Designing Syntheses • If you are working with a 3° substrate, the reaction will be SN 1, so what are the best conditions? – Nucleophile? – Leaving Group? – Solvent? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -71 Klein, Organic Chemistry 2 e

7. 9 Designing Syntheses • If you are working with a 2° substrate, the reaction could be SN 1 or SN 2, so what are the best conditions to get the stereochemistry you want, and WHY? – Nucleophile? – Leaving Group? – Solvent? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -72 Klein, Organic Chemistry 2 e

7. 9 Designing Syntheses • Some options and choices: Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -73 Klein, Organic Chemistry 2 e

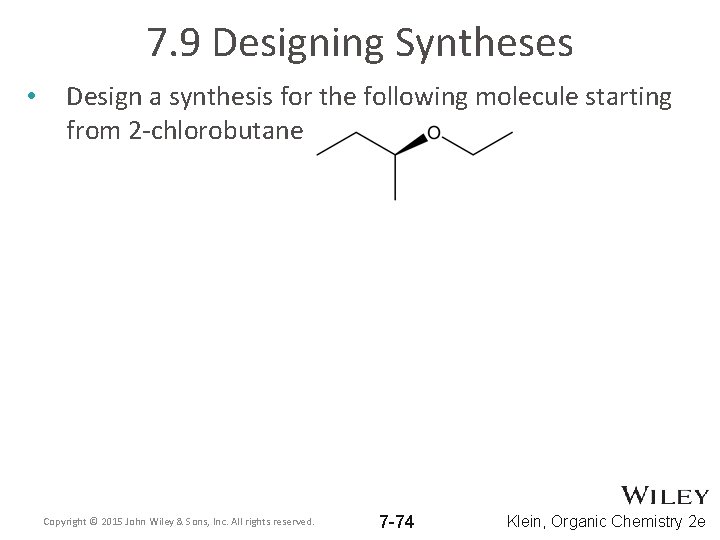
7. 9 Designing Syntheses • Design a synthesis for the following molecule starting from 2 -chlorobutane Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -74 Klein, Organic Chemistry 2 e

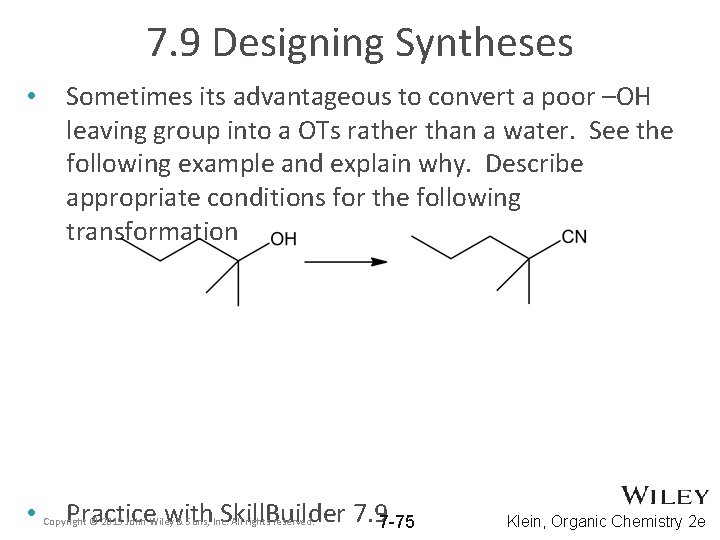
7. 9 Designing Syntheses • • Sometimes its advantageous to convert a poor –OH leaving group into a OTs rather than a water. See the following example and explain why. Describe appropriate conditions for the following transformation Practice with Skill. Builder 7. 97 -75 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

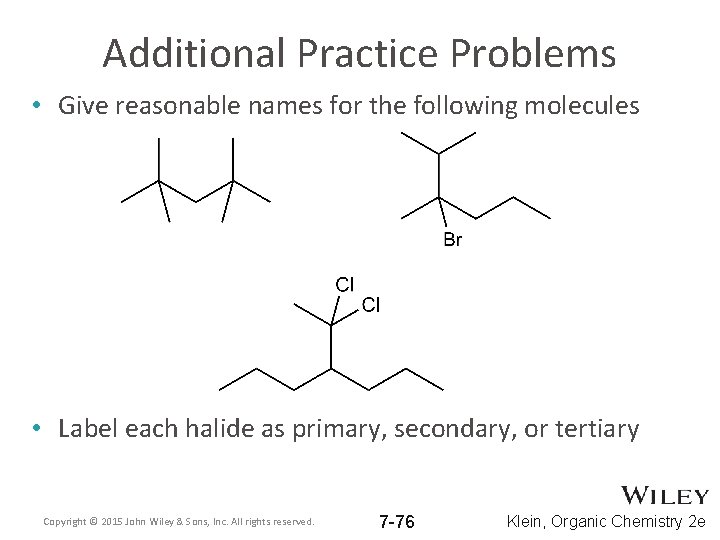
Additional Practice Problems • Give reasonable names for the following molecules • Label each halide as primary, secondary, or tertiary Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -76 Klein, Organic Chemistry 2 e

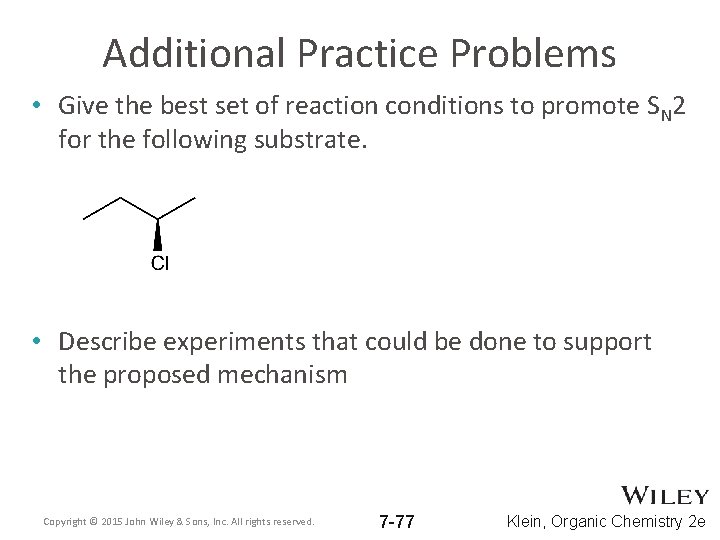
Additional Practice Problems • Give the best set of reaction conditions to promote SN 2 for the following substrate. • Describe experiments that could be done to support the proposed mechanism Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -77 Klein, Organic Chemistry 2 e

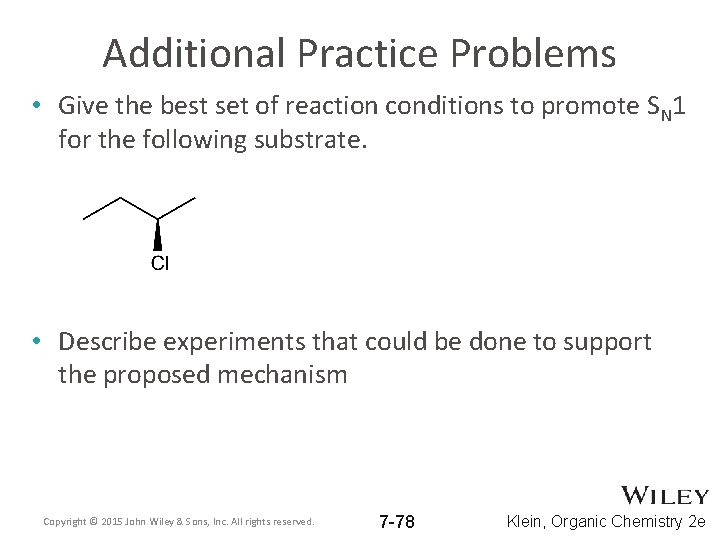
Additional Practice Problems • Give the best set of reaction conditions to promote SN 1 for the following substrate. • Describe experiments that could be done to support the proposed mechanism Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -78 Klein, Organic Chemistry 2 e

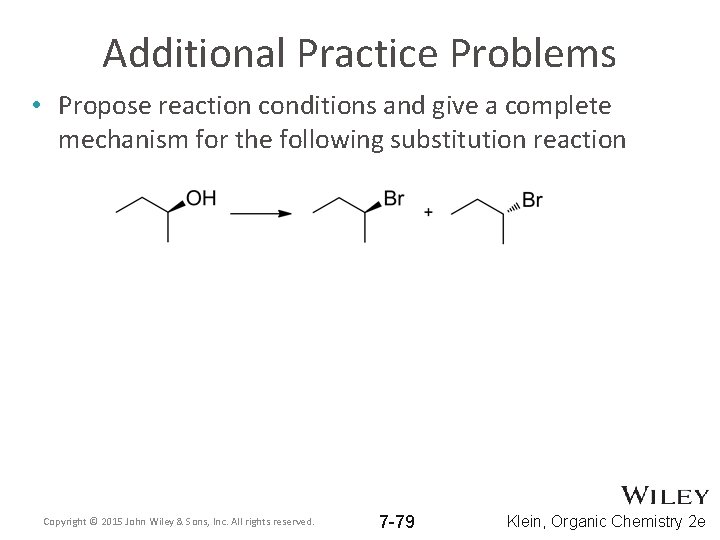
Additional Practice Problems • Propose reaction conditions and give a complete mechanism for the following substitution reaction Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -79 Klein, Organic Chemistry 2 e

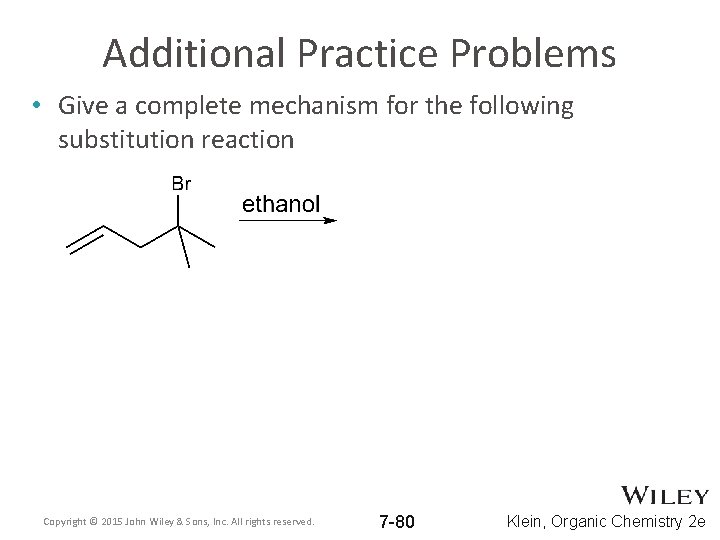
Additional Practice Problems • Give a complete mechanism for the following substitution reaction Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 7 -80 Klein, Organic Chemistry 2 e