Organic Chemistry Second Edition David Klein Chapter 24


- Slides: 33

Organic Chemistry Second Edition David Klein Chapter 24 Carbohydrates Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

24. 1 Introduction to Carbohydrates • Carbohydrates (sugars) are abundant in nature – High energy biomolecules – Provide structural rigidity for organisms (plants, crustaceans, etc. ) – The polymer backbone on which DNA and RNA are assembled – Expressed on cells so they can recognize one another • The term, carbohydrate, evolved to describe the formula for such molecules: Cx(H 2 O)x • Carbohydrates are NOT true hydrates. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -2 Klein, Organic Chemistry 2 e

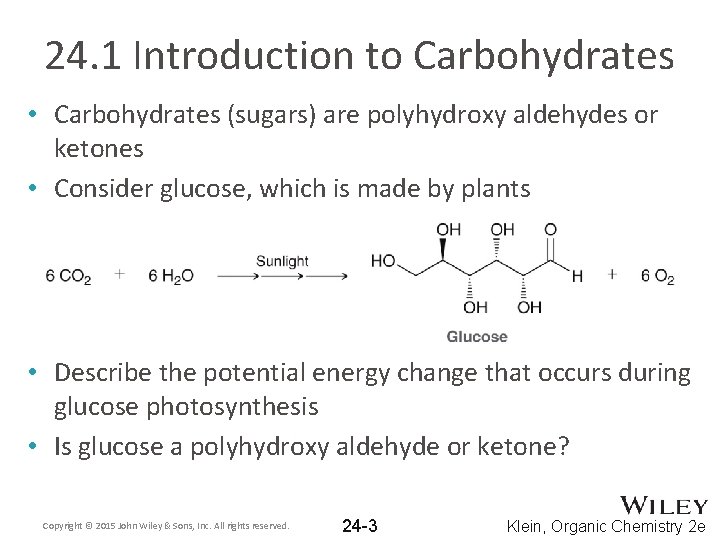
24. 1 Introduction to Carbohydrates • Carbohydrates (sugars) are polyhydroxy aldehydes or ketones • Consider glucose, which is made by plants • Describe the potential energy change that occurs during glucose photosynthesis • Is glucose a polyhydroxy aldehyde or ketone? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -3 Klein, Organic Chemistry 2 e

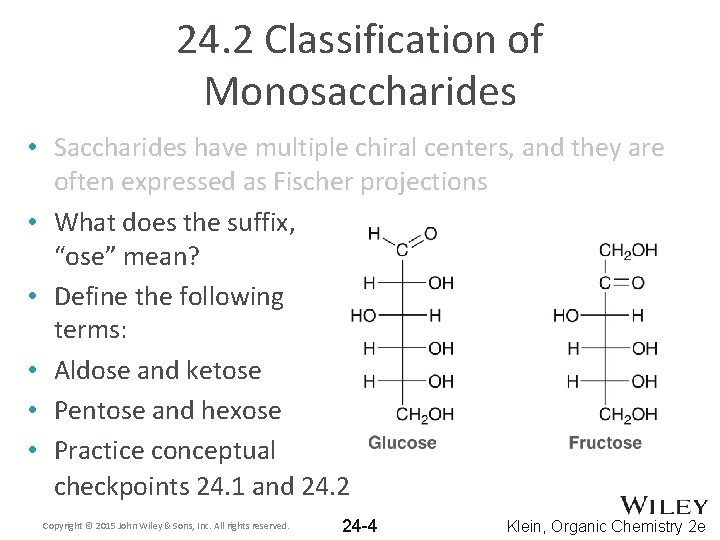
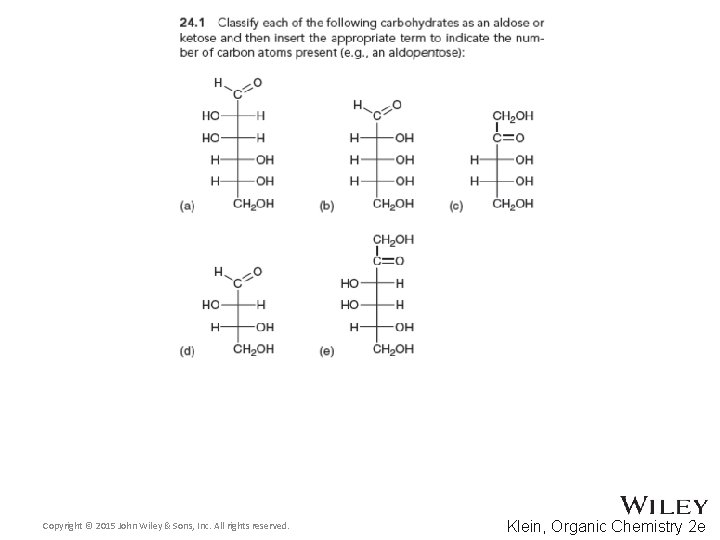
24. 2 Classification of Monosaccharides • Saccharides have multiple chiral centers, and they are often expressed as Fischer projections • What does the suffix, “ose” mean? • Define the following terms: • Aldose and ketose • Pentose and hexose • Practice conceptual checkpoints 24. 1 and 24. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -4 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

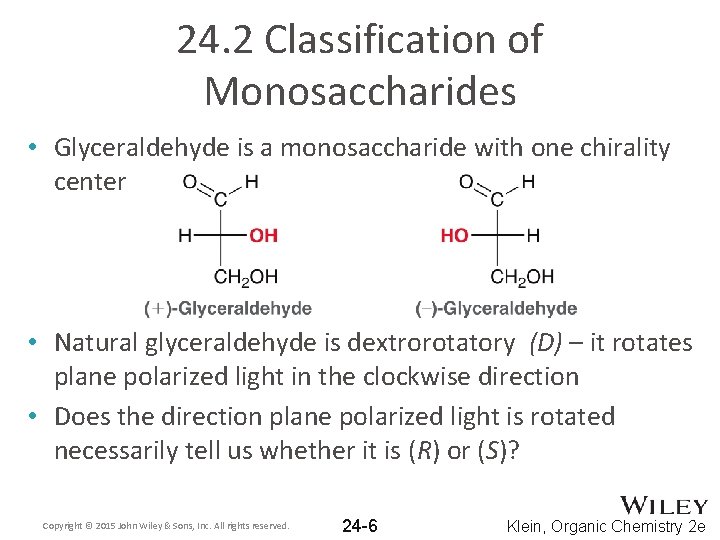
24. 2 Classification of Monosaccharides • Glyceraldehyde is a monosaccharide with one chirality center • Natural glyceraldehyde is dextrorotatory (D) – it rotates plane polarized light in the clockwise direction • Does the direction plane polarized light is rotated necessarily tell us whether it is (R) or (S)? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -6 Klein, Organic Chemistry 2 e

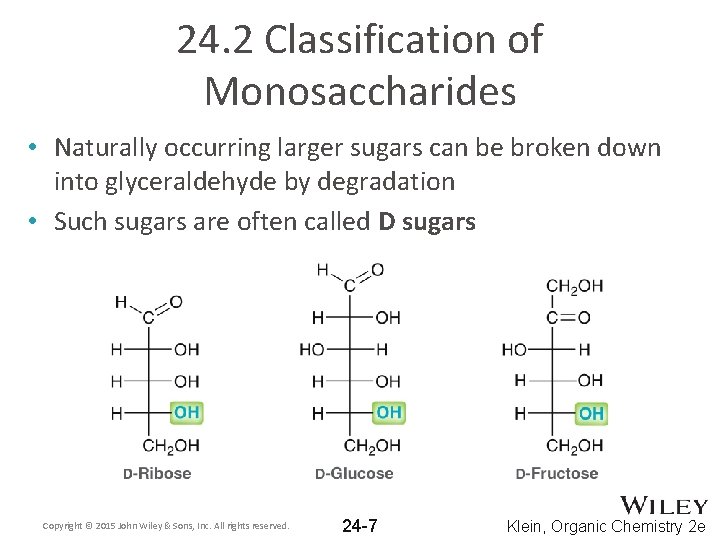
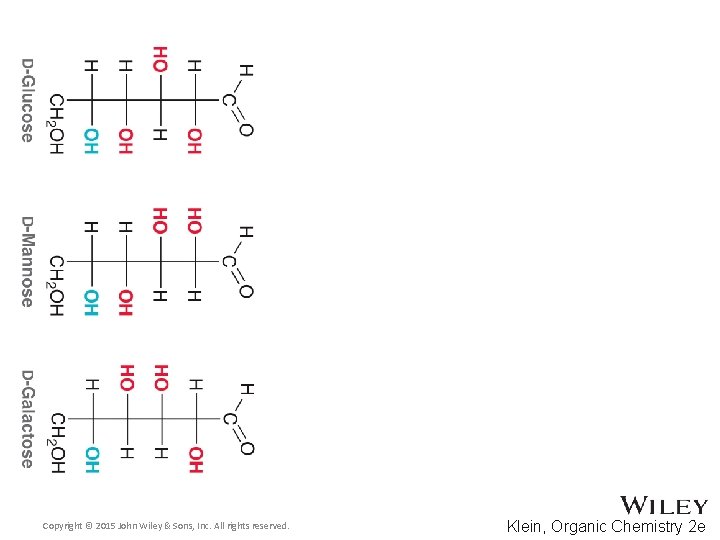
24. 2 Classification of Monosaccharides • Naturally occurring larger sugars can be broken down into glyceraldehyde by degradation • Such sugars are often called D sugars Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -7 Klein, Organic Chemistry 2 e

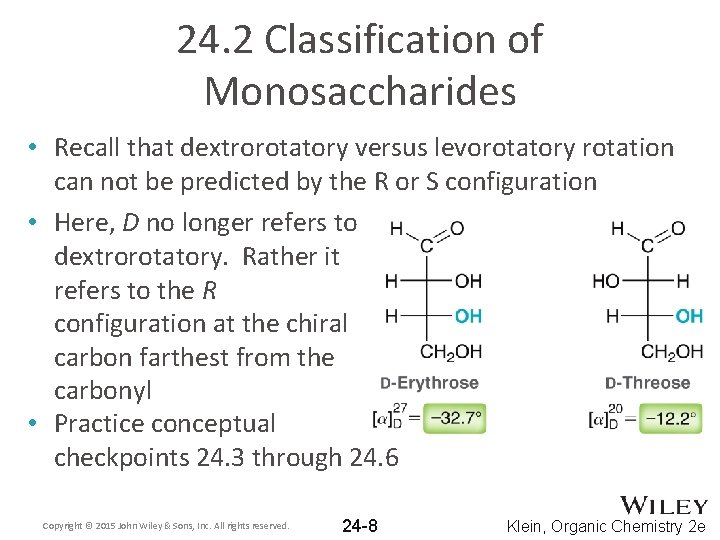
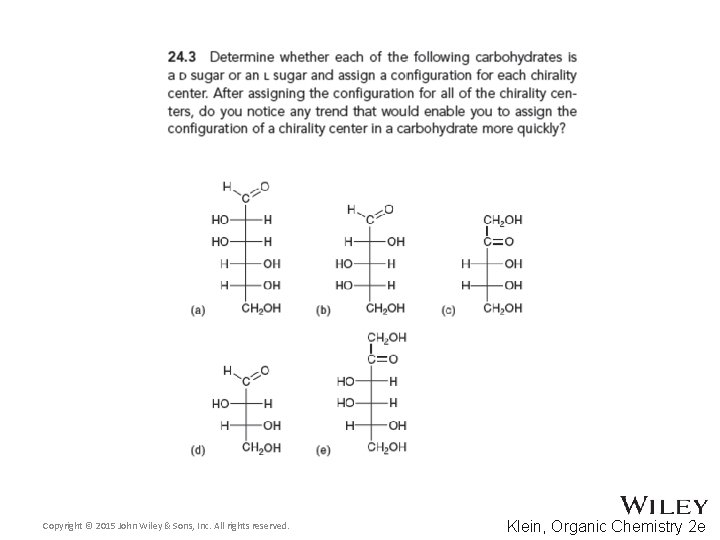
24. 2 Classification of Monosaccharides • Recall that dextrorotatory versus levorotatory rotation can not be predicted by the R or S configuration • Here, D no longer refers to dextrorotatory. Rather it refers to the R configuration at the chiral carbon farthest from the carbonyl • Practice conceptual checkpoints 24. 3 through 24. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -8 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

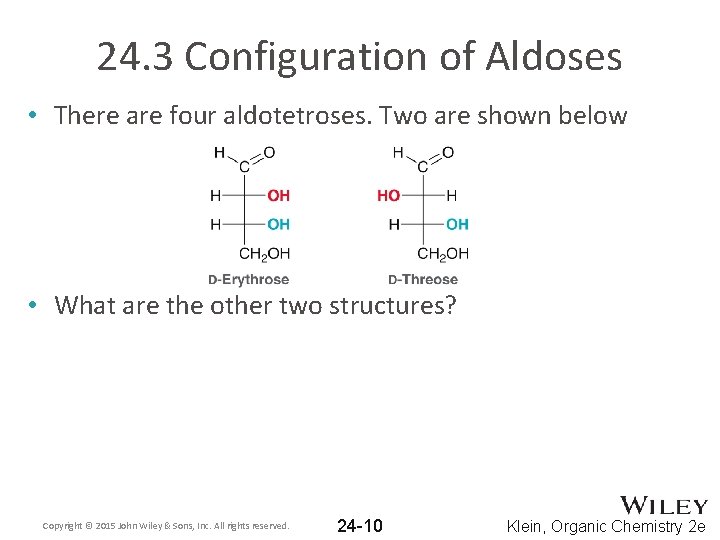
24. 3 Configuration of Aldoses • There are four aldotetroses. Two are shown below • What are the other two structures? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -10 Klein, Organic Chemistry 2 e

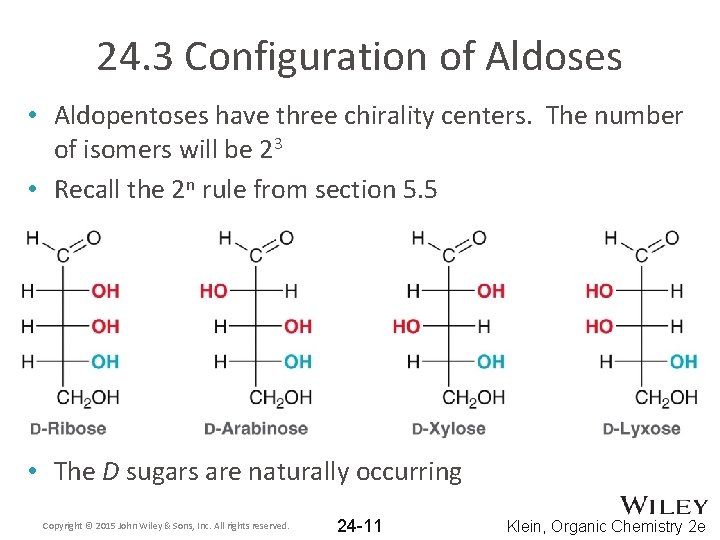
24. 3 Configuration of Aldoses • Aldopentoses have three chirality centers. The number of isomers will be 23 • Recall the 2 n rule from section 5. 5 • The D sugars are naturally occurring Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -11 Klein, Organic Chemistry 2 e

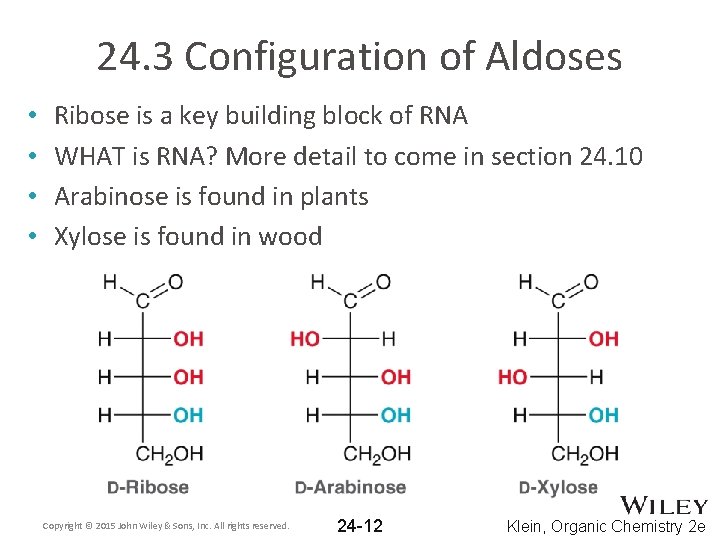
24. 3 Configuration of Aldoses • • Ribose is a key building block of RNA WHAT is RNA? More detail to come in section 24. 10 Arabinose is found in plants Xylose is found in wood Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -12 Klein, Organic Chemistry 2 e

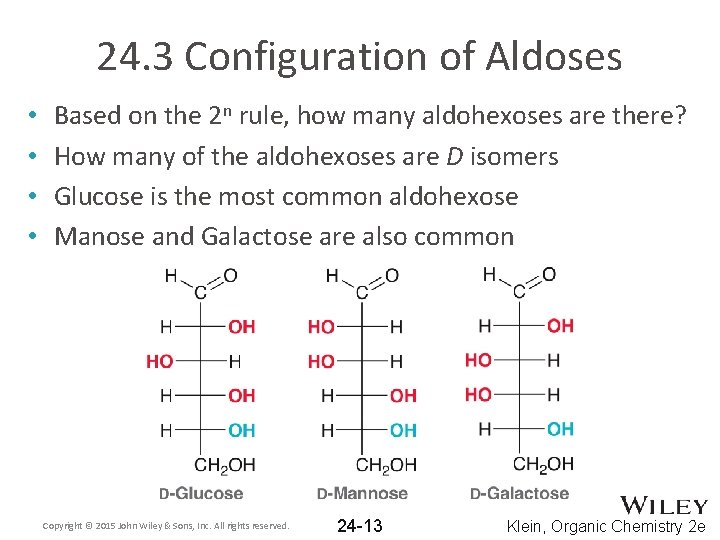
24. 3 Configuration of Aldoses • • Based on the 2 n rule, how many aldohexoses are there? How many of the aldohexoses are D isomers Glucose is the most common aldohexose Manose and Galactose are also common Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -13 Klein, Organic Chemistry 2 e

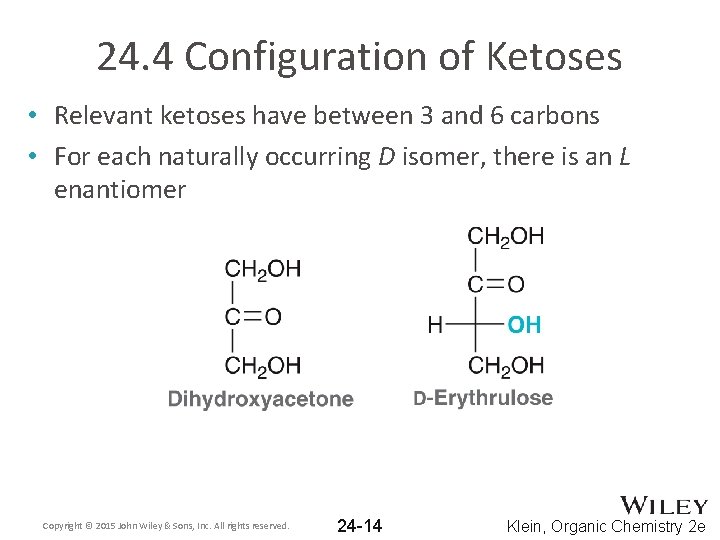
24. 4 Configuration of Ketoses • Relevant ketoses have between 3 and 6 carbons • For each naturally occurring D isomer, there is an L enantiomer Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -14 Klein, Organic Chemistry 2 e

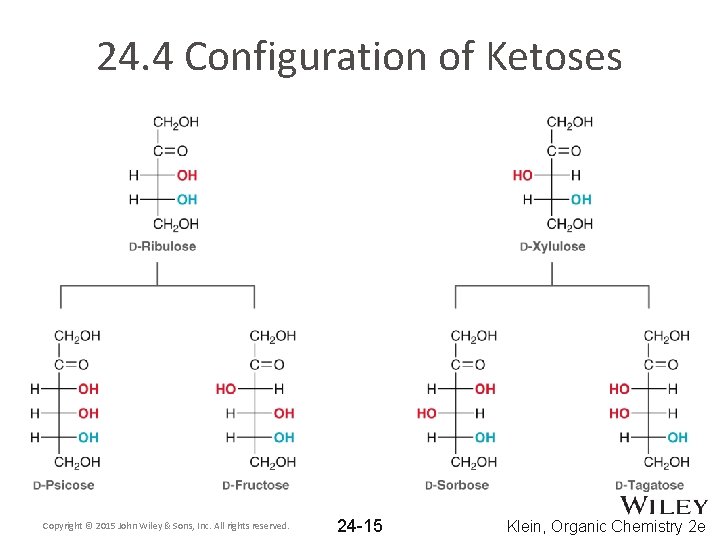
24. 4 Configuration of Ketoses Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -15 Klein, Organic Chemistry 2 e

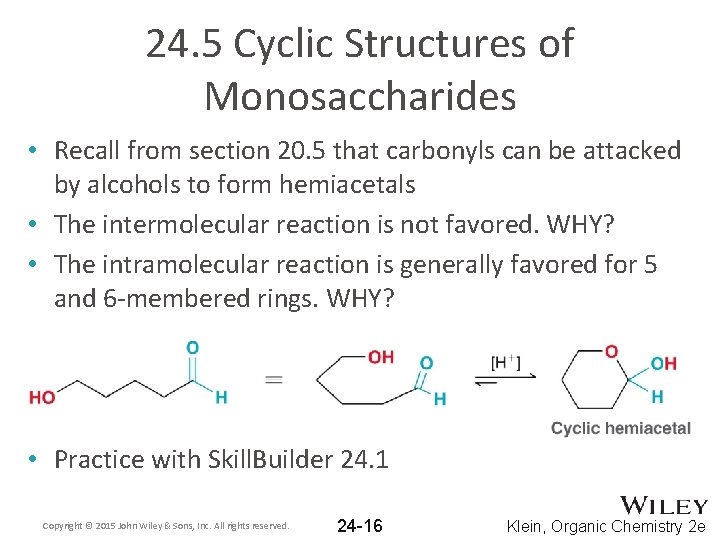
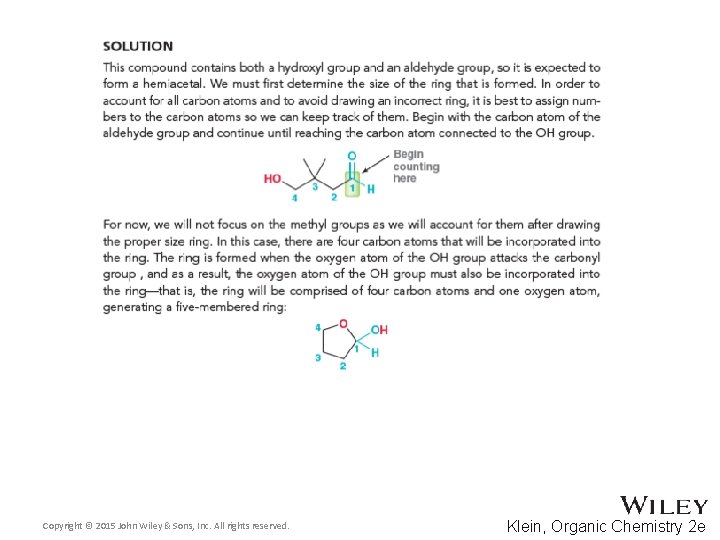
24. 5 Cyclic Structures of Monosaccharides • Recall from section 20. 5 that carbonyls can be attacked by alcohols to form hemiacetals • The intermolecular reaction is not favored. WHY? • The intramolecular reaction is generally favored for 5 and 6 -membered rings. WHY? • Practice with Skill. Builder 24. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -16 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

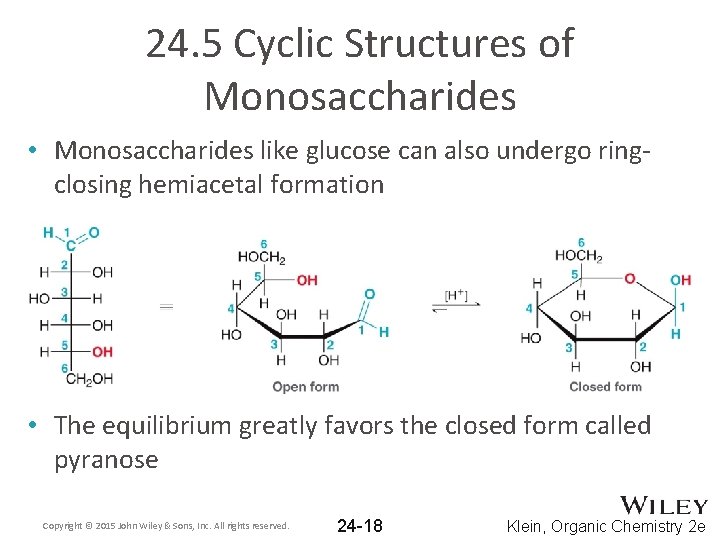
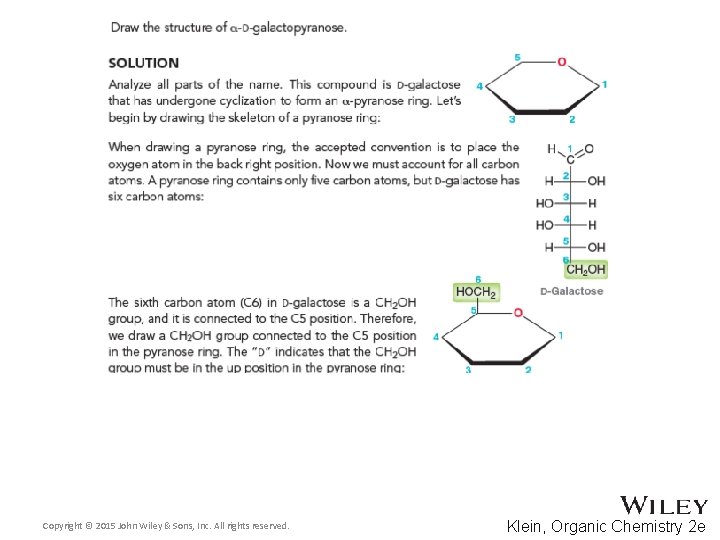
24. 5 Cyclic Structures of Monosaccharides • Monosaccharides like glucose can also undergo ringclosing hemiacetal formation • The equilibrium greatly favors the closed form called pyranose Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -18 Klein, Organic Chemistry 2 e

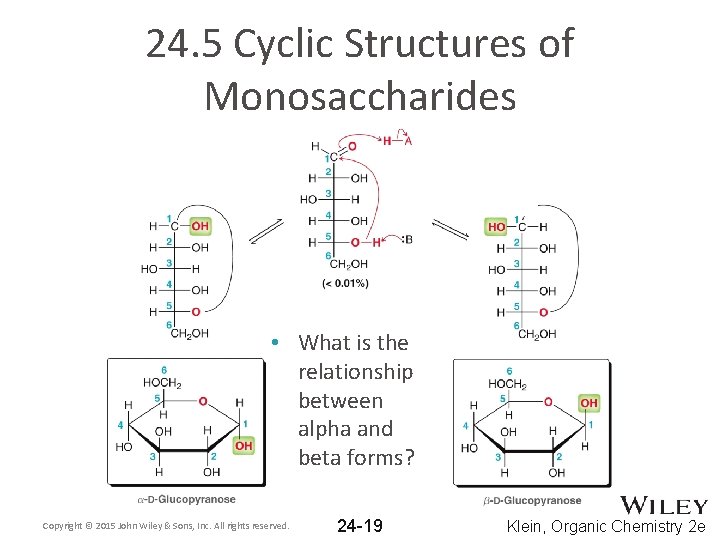
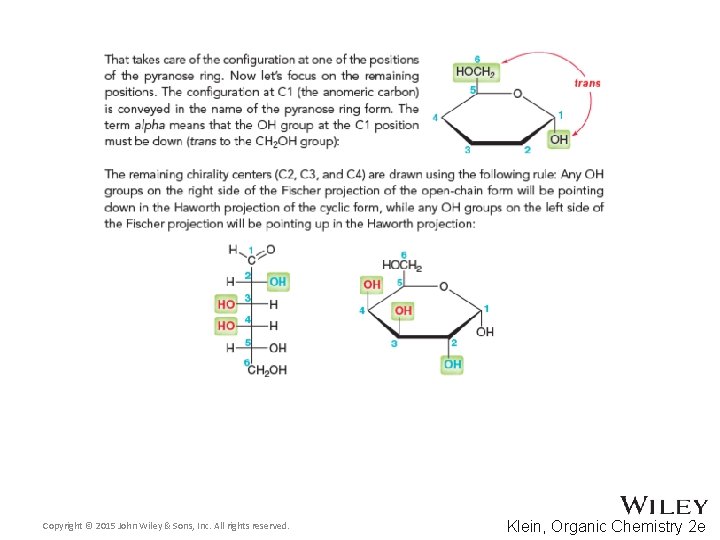
24. 5 Cyclic Structures of Monosaccharides • What is the relationship between alpha and beta forms? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -19 Klein, Organic Chemistry 2 e

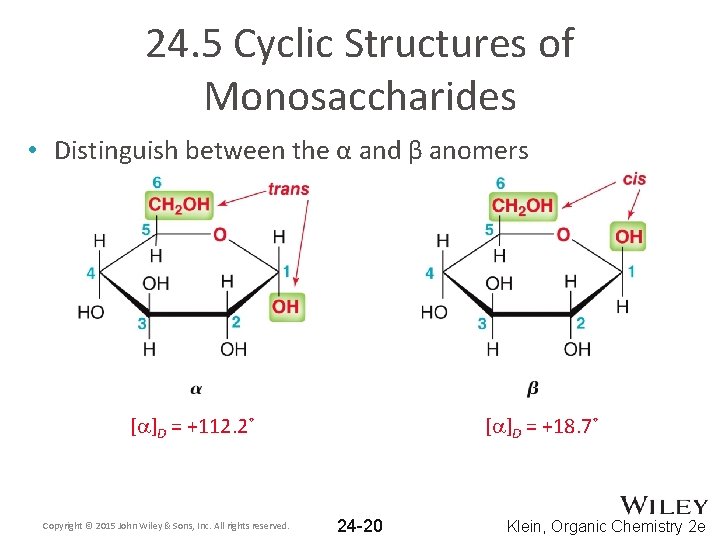
24. 5 Cyclic Structures of Monosaccharides • Distinguish between the α and β anomers [a]D = +112. 2˚ Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. [a]D = +18. 7˚ 24 -20 Klein, Organic Chemistry 2 e

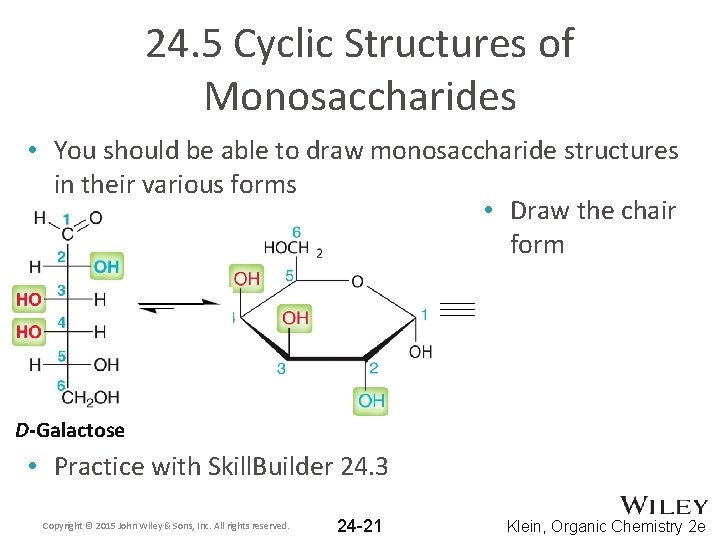
24. 5 Cyclic Structures of Monosaccharides • You should be able to draw monosaccharide structures in their various forms • Draw the chair form D-Galactose • Practice with Skill. Builder 24. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -21 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

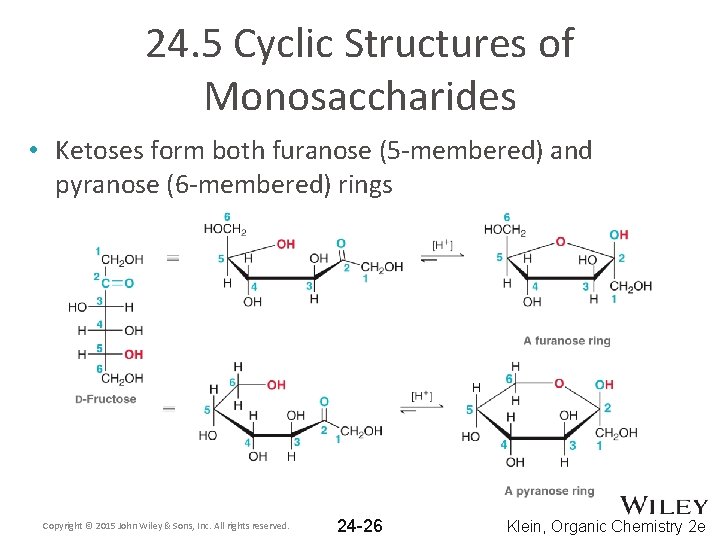
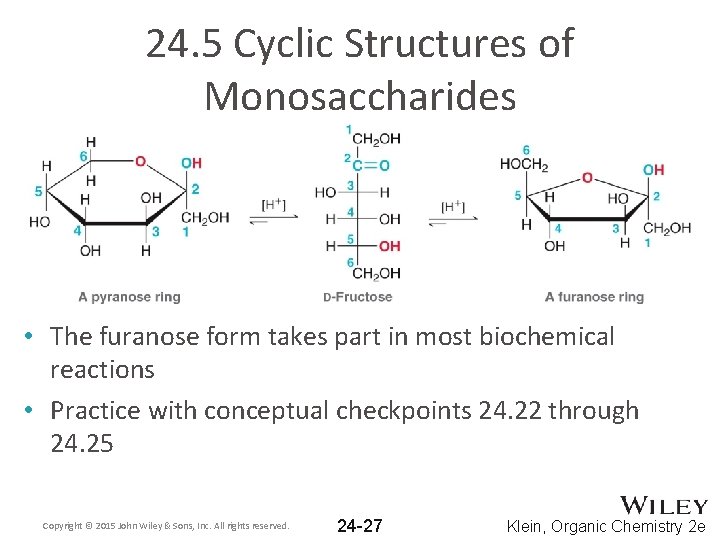
24. 5 Cyclic Structures of Monosaccharides • Ketoses form both furanose (5 -membered) and pyranose (6 -membered) rings Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -26 Klein, Organic Chemistry 2 e

24. 5 Cyclic Structures of Monosaccharides • The furanose form takes part in most biochemical reactions • Practice with conceptual checkpoints 24. 22 through 24. 25 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -27 Klein, Organic Chemistry 2 e

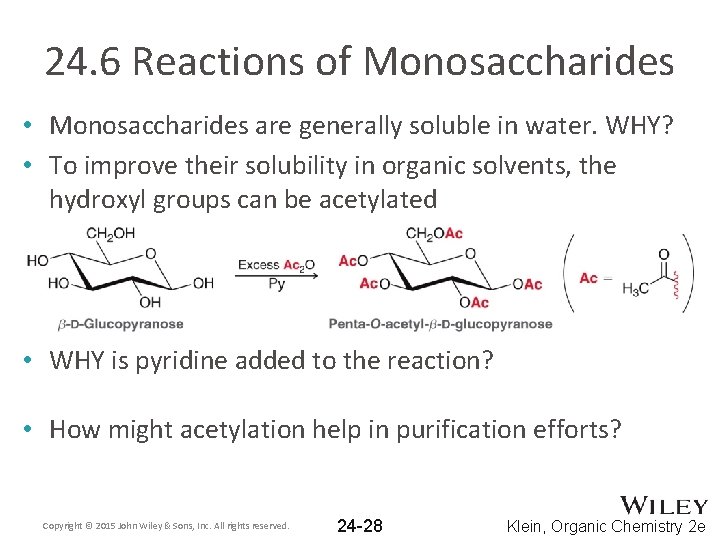
24. 6 Reactions of Monosaccharides • Monosaccharides are generally soluble in water. WHY? • To improve their solubility in organic solvents, the hydroxyl groups can be acetylated • WHY is pyridine added to the reaction? • How might acetylation help in purification efforts? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -28 Klein, Organic Chemistry 2 e

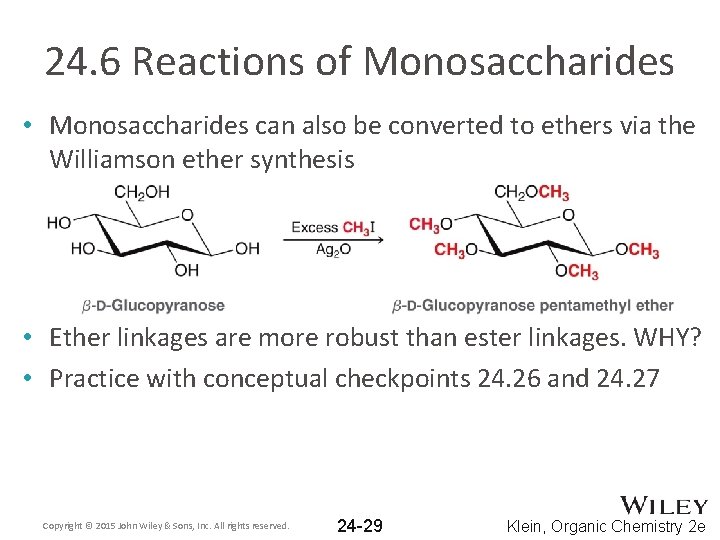
24. 6 Reactions of Monosaccharides • Monosaccharides can also be converted to ethers via the Williamson ether synthesis • Ether linkages are more robust than ester linkages. WHY? • Practice with conceptual checkpoints 24. 26 and 24. 27 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -29 Klein, Organic Chemistry 2 e

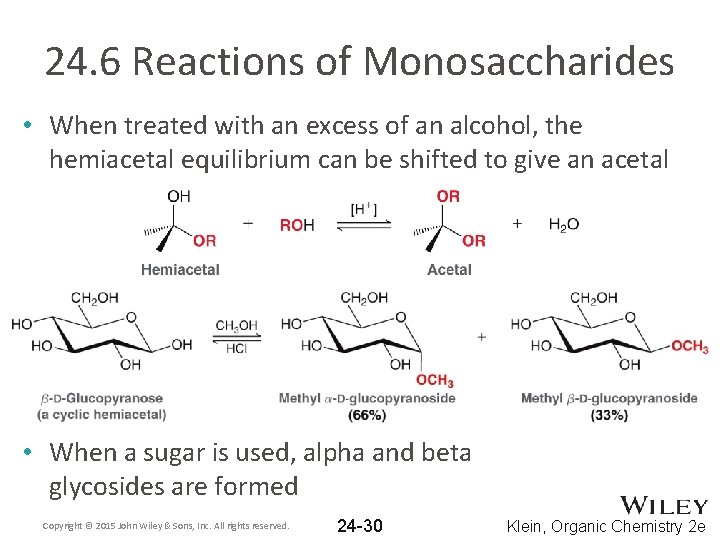
24. 6 Reactions of Monosaccharides • When treated with an excess of an alcohol, the hemiacetal equilibrium can be shifted to give an acetal • When a sugar is used, alpha and beta glycosides are formed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -30 Klein, Organic Chemistry 2 e

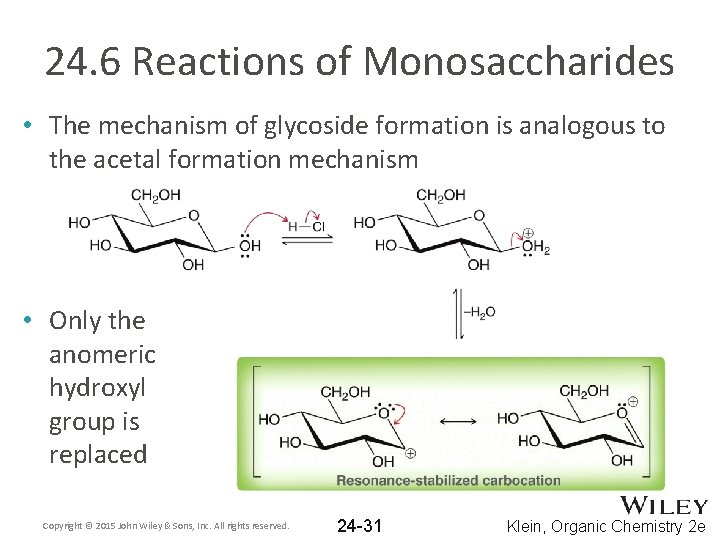
24. 6 Reactions of Monosaccharides • The mechanism of glycoside formation is analogous to the acetal formation mechanism • Only the anomeric hydroxyl group is replaced Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -31 Klein, Organic Chemistry 2 e

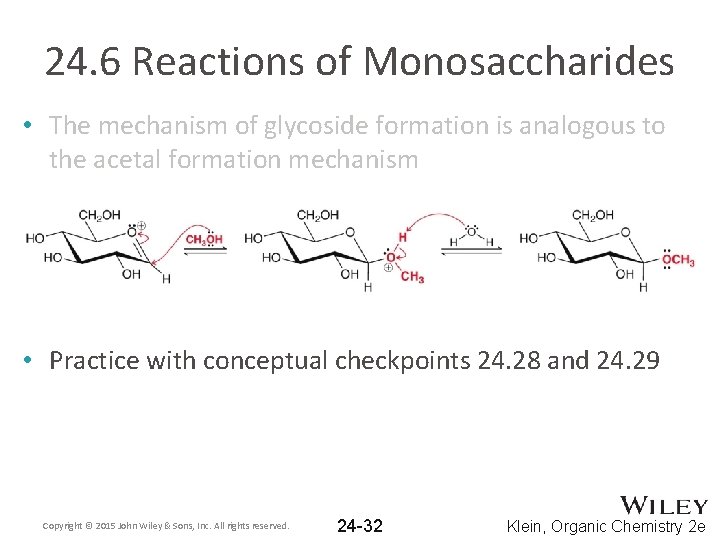
24. 6 Reactions of Monosaccharides • The mechanism of glycoside formation is analogous to the acetal formation mechanism • Practice with conceptual checkpoints 24. 28 and 24. 29 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 24 -32 Klein, Organic Chemistry 2 e

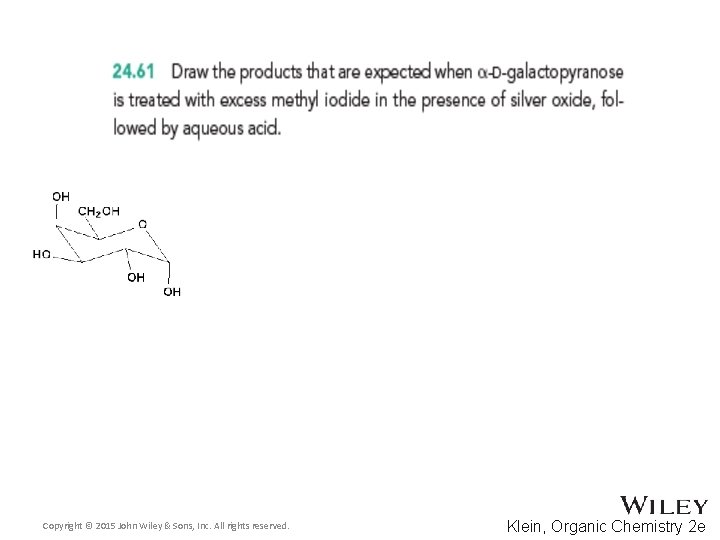
Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e