Organic Chemistry Prductive Student What is Organic Chemistry






- Slides: 48

Organic Chemistry Prductive. Student

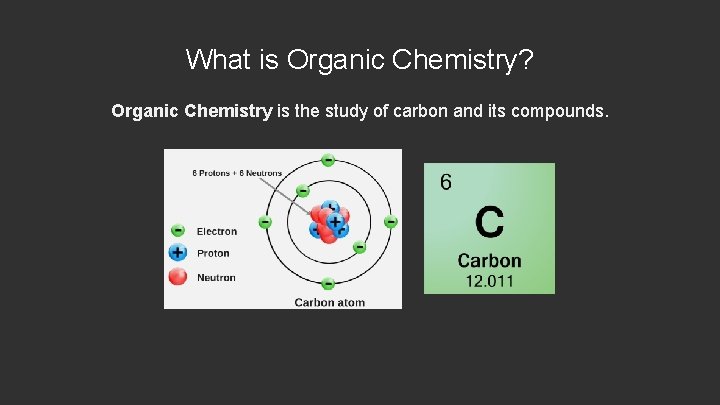
What is Organic Chemistry? Organic Chemistry is the study of carbon and its compounds.

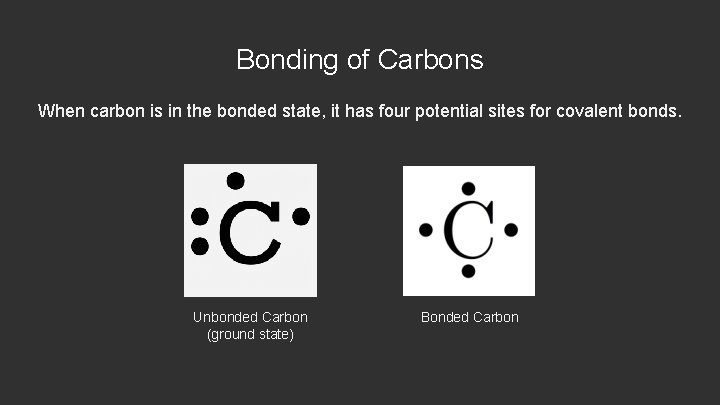
Bonding of Carbons When carbon is in the bonded state, it has four potential sites for covalent bonds. Unbonded Carbon (ground state) Bonded Carbon

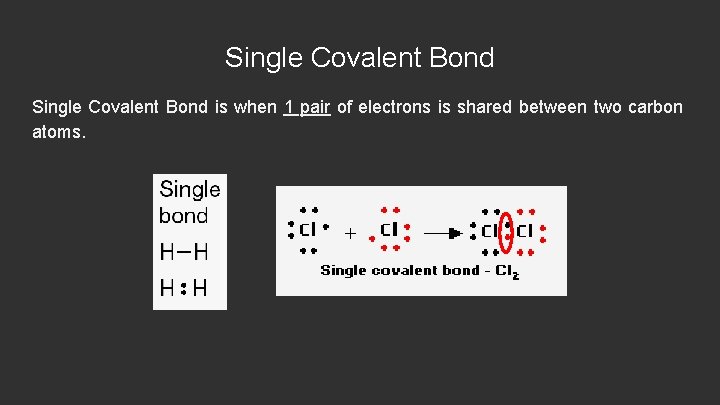
Single Covalent Bond is when 1 pair of electrons is shared between two carbon atoms.

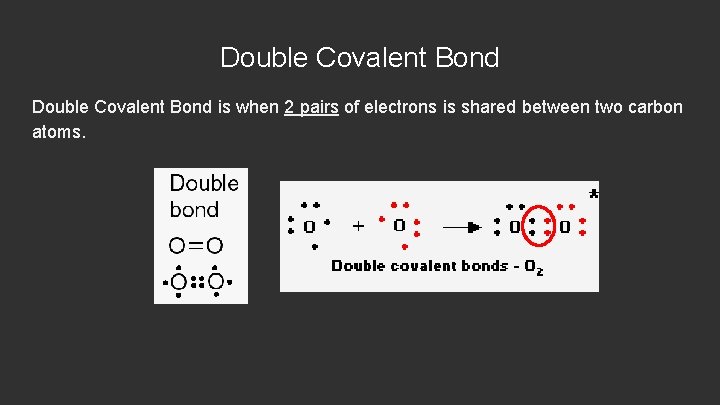
Double Covalent Bond is when 2 pairs of electrons is shared between two carbon atoms.

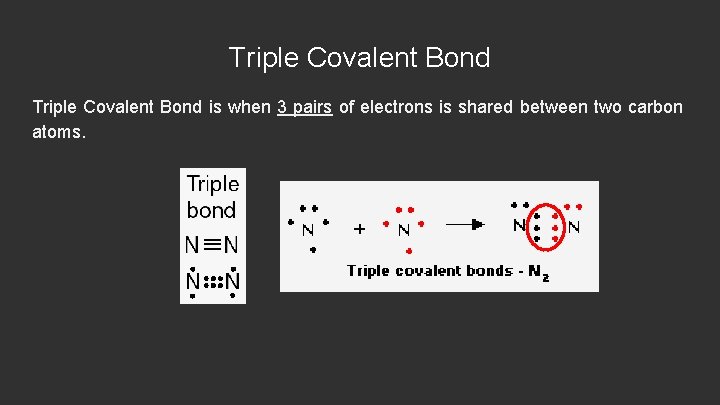
Triple Covalent Bond is when 3 pairs of electrons is shared between two carbon atoms.

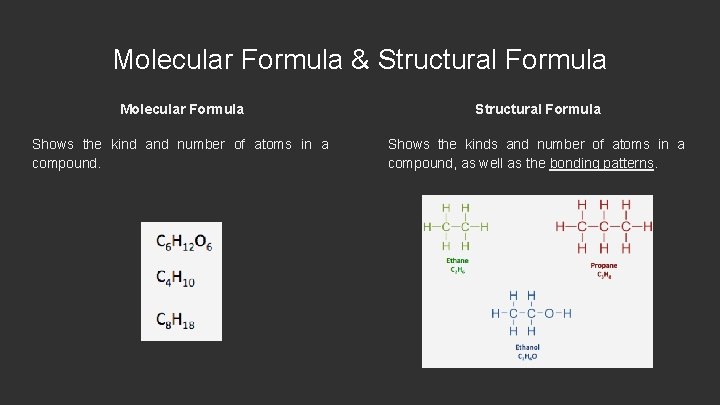
Molecular Formula & Structural Formula Molecular Formula Structural Formula Shows the kind and number of atoms in a compound. Shows the kinds and number of atoms in a compound, as well as the bonding patterns.

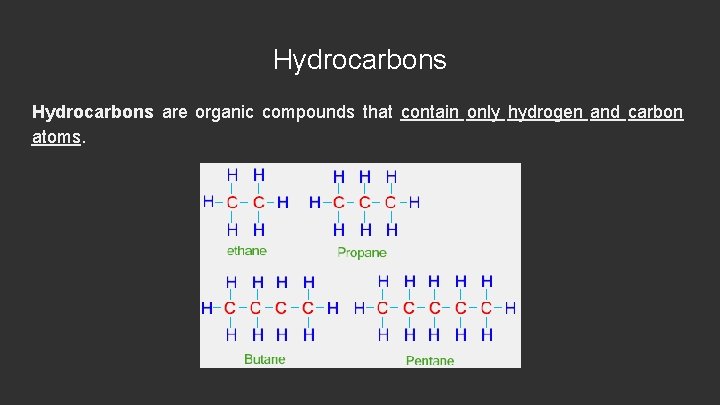
Hydrocarbons are organic compounds that contain only hydrogen and carbon atoms.

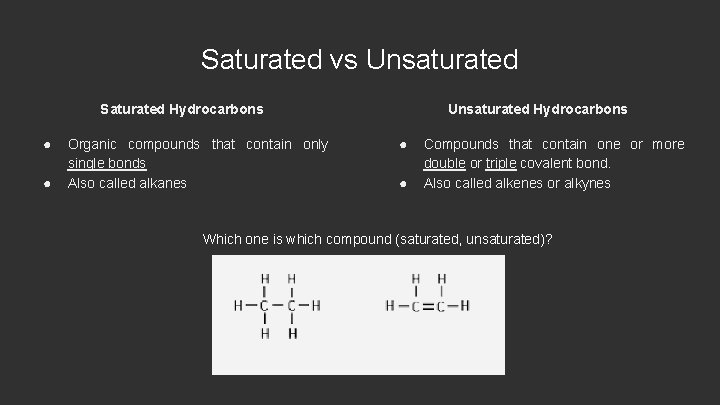
Saturated vs Unsaturated Saturated Hydrocarbons ● ● Organic compounds that contain only single bonds Also called alkanes Unsaturated Hydrocarbons ● ● Compounds that contain one or more double or triple covalent bond. Also called alkenes or alkynes Which one is which compound (saturated, unsaturated)?

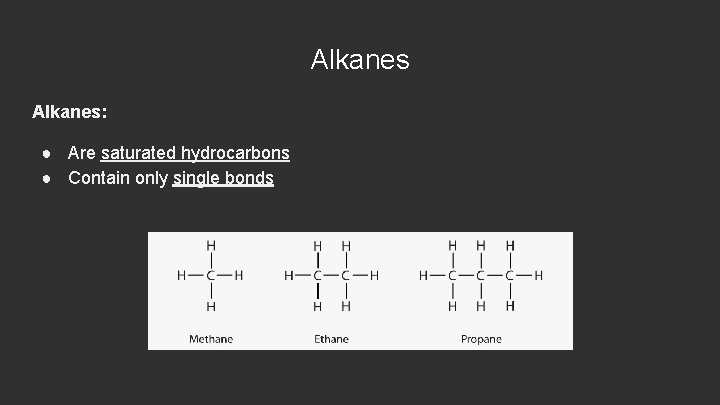
Alkanes: ● Are saturated hydrocarbons ● Contain only single bonds

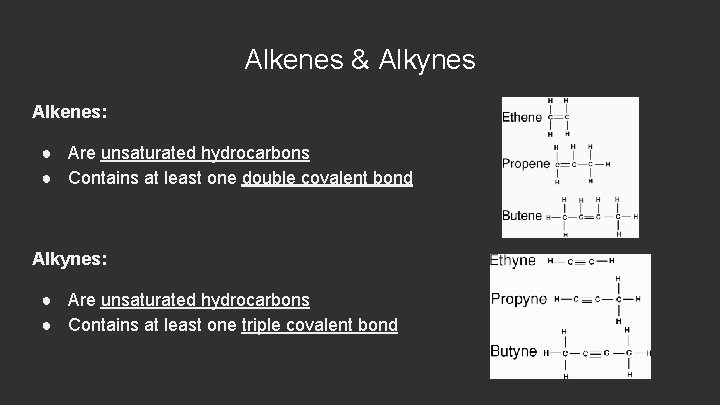
Alkenes & Alkynes Alkenes: ● Are unsaturated hydrocarbons ● Contains at least one double covalent bond Alkynes: ● Are unsaturated hydrocarbons ● Contains at least one triple covalent bond

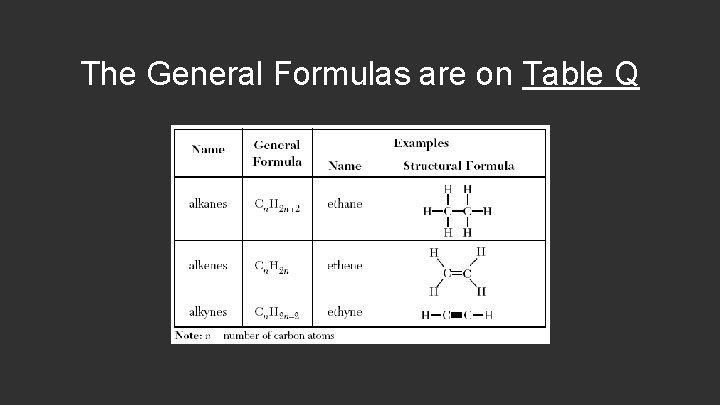
General Formula of Alkanes: If an alkane contains 5 carbons, then n=5. To find the number of hydrogen atoms, substitute the n value. C 5 H 2(5)+2 (2 x 5) + 2 = 12 12 Hydrogen Atoms

General Formula of Alkenes: If an alkene contains 10 carbons, then n=10. To find the number of hydrogen atoms, substitute the n value. C 10 H 2(10) 2 x 10 = 20 20 Hydrogen Atoms

General Formula of Alkynes: If an alkene contains 2 carbons, then n=2. To find the number of hydrogen atoms, substitute the n value. C 2 H 2(2)-2 (2 x 2) - 2 = 2 2 Hydrogen Atoms

The General Formulas are on Table Q

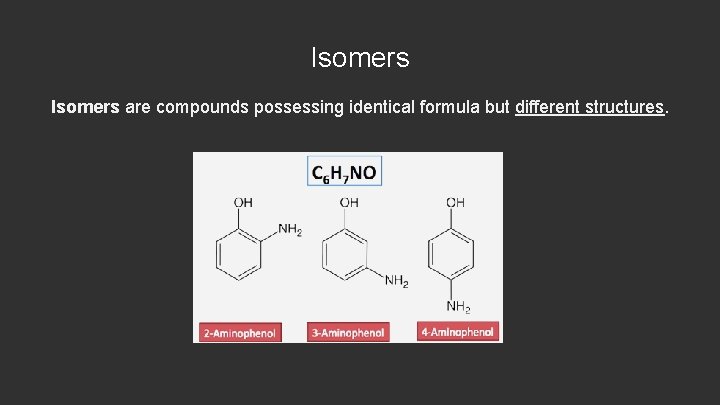
Isomers are compounds possessing identical formula but different structures.

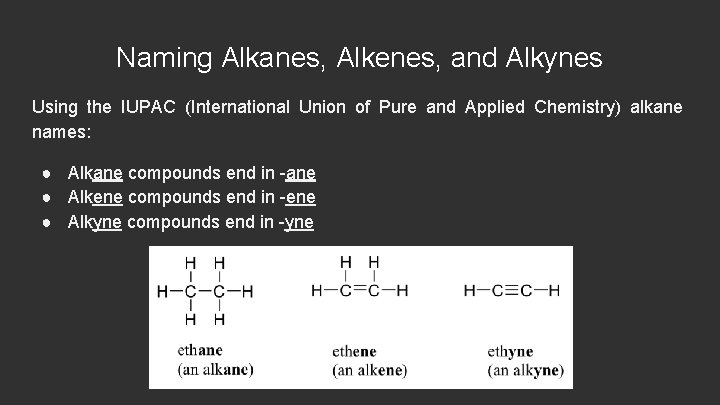
Naming Alkanes, Alkenes, and Alkynes Using the IUPAC (International Union of Pure and Applied Chemistry) alkane names: ● Alkane compounds end in -ane ● Alkene compounds end in -ene ● Alkyne compounds end in -yne

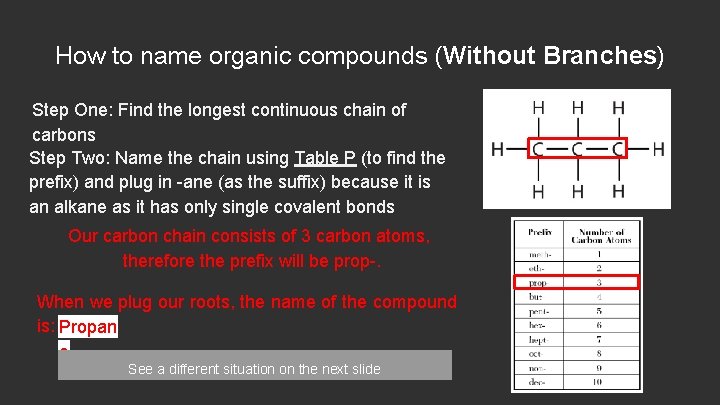
How to name organic compounds (Without Branches) Step One: Find the longest continuous chain of carbons Step Two: Name the chain using Table P (to find the prefix) and plug in -ane (as the suffix) because it is an alkane as it has only single covalent bonds Our carbon chain consists of 3 carbon atoms, therefore the prefix will be prop-. When we plug our roots, the name of the compound is: Propan e See a different situation on the next slide

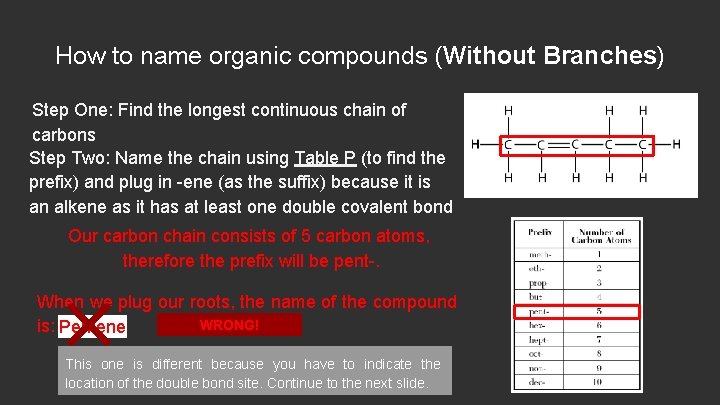
How to name organic compounds (Without Branches) Step One: Find the longest continuous chain of carbons Step Two: Name the chain using Table P (to find the prefix) and plug in -ene (as the suffix) because it is an alkene as it has at least one double covalent bond Our carbon chain consists of 5 carbon atoms, therefore the prefix will be pent-. When we plug our roots, the name of the compound WRONG! is: Pentene This one is different because you have to indicate the location of the double bond site. Continue to the next slide.

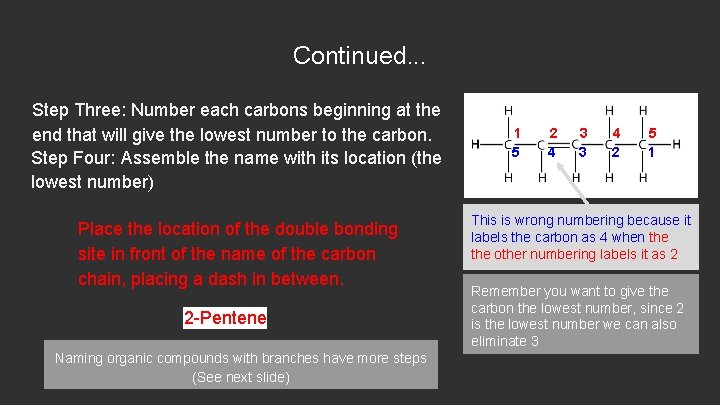
Continued. . . Step Three: Number each carbons beginning at the end that will give the lowest number to the carbon. Step Four: Assemble the name with its location (the lowest number) Place the location of the double bonding site in front of the name of the carbon chain, placing a dash in between. 2 -Pentene Naming organic compounds with branches have more steps (See next slide) 1 5 2 4 3 3 4 2 5 1 This is wrong numbering because it labels the carbon as 4 when the other numbering labels it as 2 Remember you want to give the carbon the lowest number, since 2 is the lowest number we can also eliminate 3

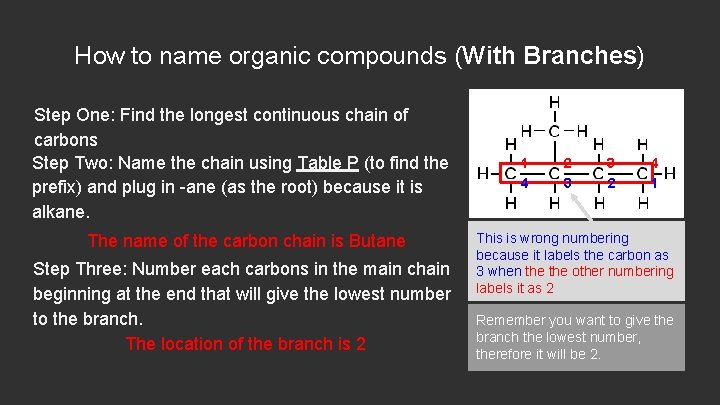
How to name organic compounds (With Branches) Step One: Find the longest continuous chain of carbons Step Two: Name the chain using Table P (to find the prefix) and plug in -ane (as the root) because it is alkane. The name of the carbon chain is Butane Step Three: Number each carbons in the main chain beginning at the end that will give the lowest number to the branch. The location of the branch is 2 1 4 2 3 3 2 4 1 This is wrong numbering because it labels the carbon as 3 when the other numbering labels it as 2 Remember you want to give the branch the lowest number, therefore it will be 2.

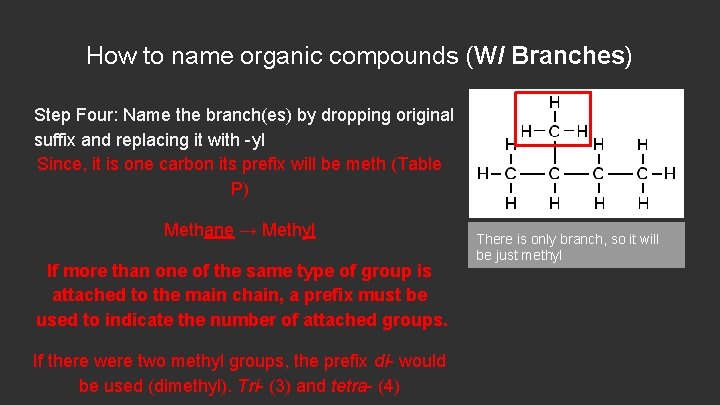
How to name organic compounds (W/ Branches) Step Four: Name the branch(es) by dropping original suffix and replacing it with -yl Since, it is one carbon its prefix will be meth (Table P) Methane → Methyl If more than one of the same type of group is attached to the main chain, a prefix must be used to indicate the number of attached groups. If there were two methyl groups, the prefix di- would be used (dimethyl). Tri- (3) and tetra- (4) There is only branch, so it will be just methyl

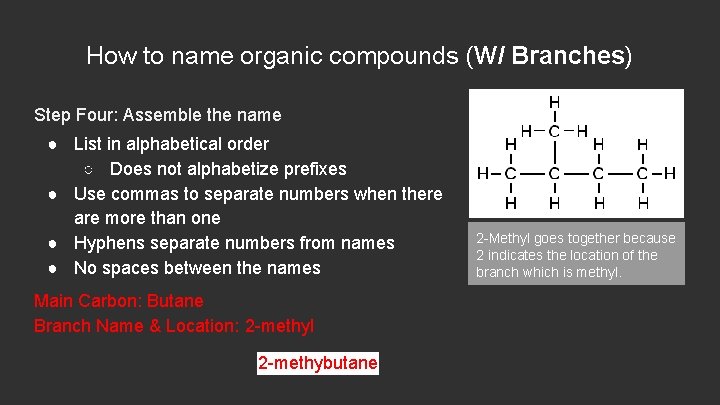
How to name organic compounds (W/ Branches) Step Four: Assemble the name ● List in alphabetical order ○ Does not alphabetize prefixes ● Use commas to separate numbers when there are more than one ● Hyphens separate numbers from names ● No spaces between the names Main Carbon: Butane Branch Name & Location: 2 -methylbutane 2 -Methyl goes together because 2 indicates the location of the branch which is methyl.

Functional Groups is the atom or atoms that replace a hydrogen atom in a hydrocarbon and give the compound distinctive chemical and physical properties. See next slide for the different types of functional groups

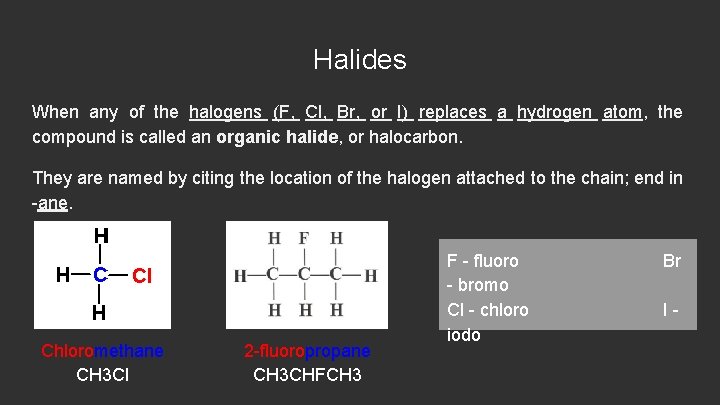
Halides When any of the halogens (F, Cl, Br, or I) replaces a hydrogen atom, the compound is called an organic halide, or halocarbon. They are named by citing the location of the halogen attached to the chain; end in -ane. Chloromethane CH 3 Cl 2 -fluoropropane CH 3 CHFCH 3 F - fluoro - bromo Cl - chloro iodo Br I-

Alcohols are organic compounds in which one or more hydrogen atoms of a hydrocarbon are replaced by an -OH group. The -OH group is called a hydroxyl group. Names end in -ol There are different types of alcohol. Each type is dependent on the number of hydroxyl groups and their position on the main carbon chain.

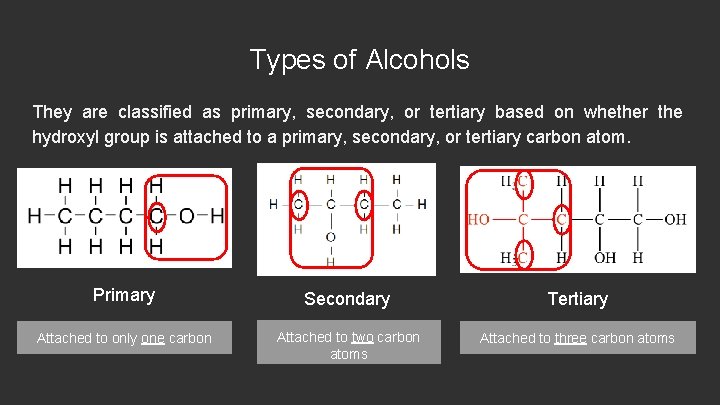
Types of Alcohols They are classified as primary, secondary, or tertiary based on whether the hydroxyl group is attached to a primary, secondary, or tertiary carbon atom. Primary Secondary Tertiary Attached to only one carbon Attached to two carbon atoms Attached to three carbon atoms

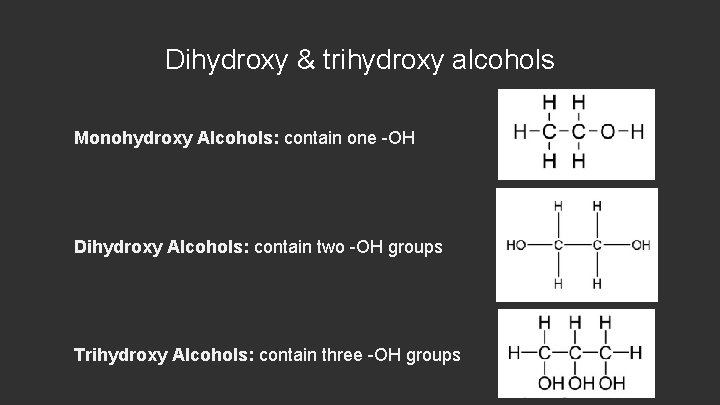
Dihydroxy & trihydroxy alcohols Monohydroxy Alcohols: contain one -OH Dihydroxy Alcohols: contain two -OH groups Trihydroxy Alcohols: contain three -OH groups

Carbonyl Group is a group consisting of a carbon atom double-bonded to oxygen. Both aldehydes and ketones contain the carbonyl group.

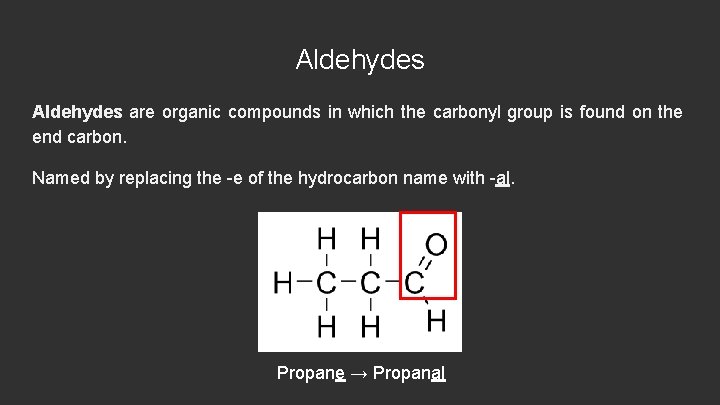
Aldehydes are organic compounds in which the carbonyl group is found on the end carbon. Named by replacing the -e of the hydrocarbon name with -al. Propane → Propanal

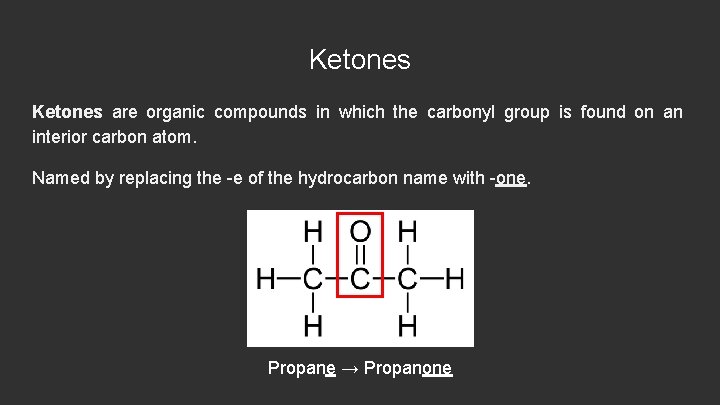
Ketones are organic compounds in which the carbonyl group is found on an interior carbon atom. Named by replacing the -e of the hydrocarbon name with -one. Propane → Propanone

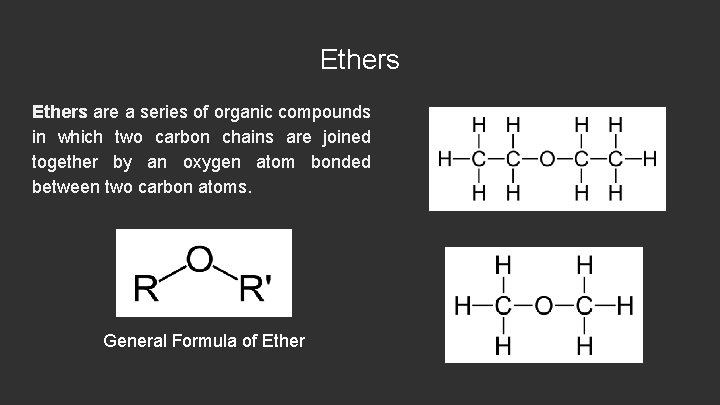
Ethers are a series of organic compounds in which two carbon chains are joined together by an oxygen atom bonded between two carbon atoms. General Formula of Ether

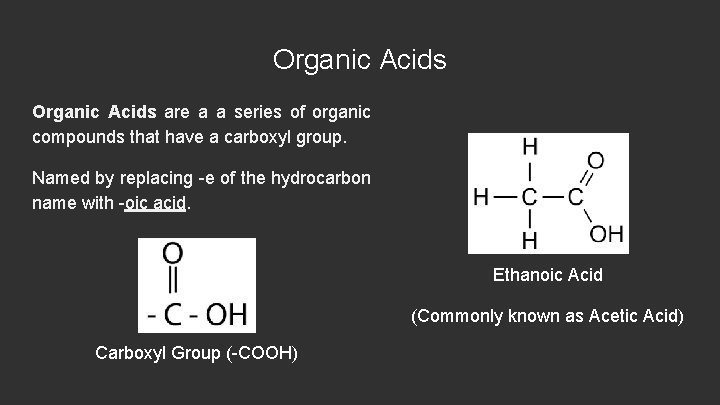
Organic Acids are a a series of organic compounds that have a carboxyl group. Named by replacing -e of the hydrocarbon name with -oic acid. Ethanoic Acid (Commonly known as Acetic Acid) Carboxyl Group (-COOH)

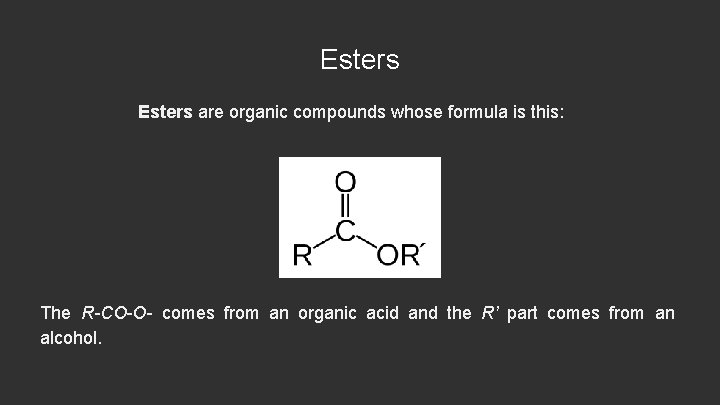
Esters are organic compounds whose formula is this: The R-CO-O- comes from an organic acid and the R’ part comes from an alcohol.

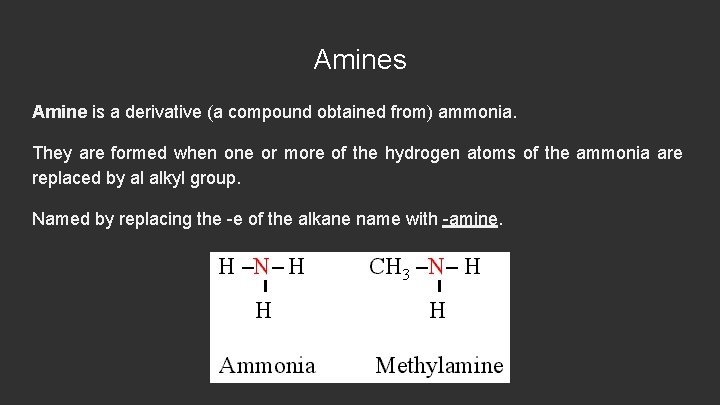
Amines Amine is a derivative (a compound obtained from) ammonia. They are formed when one or more of the hydrogen atoms of the ammonia are replaced by al alkyl group. Named by replacing the -e of the alkane name with -amine.

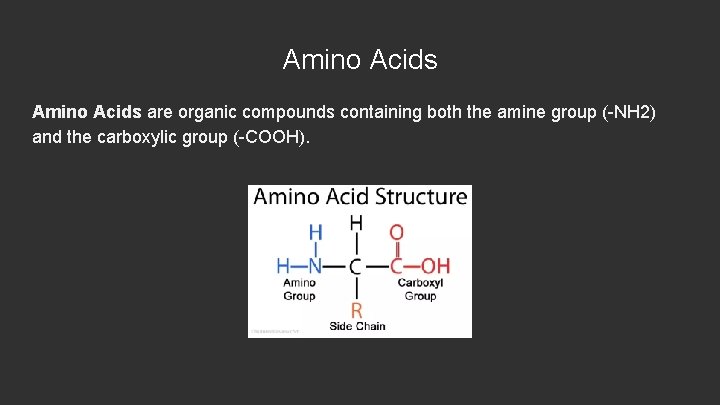
Amino Acids are organic compounds containing both the amine group (-NH 2) and the carboxylic group (-COOH).

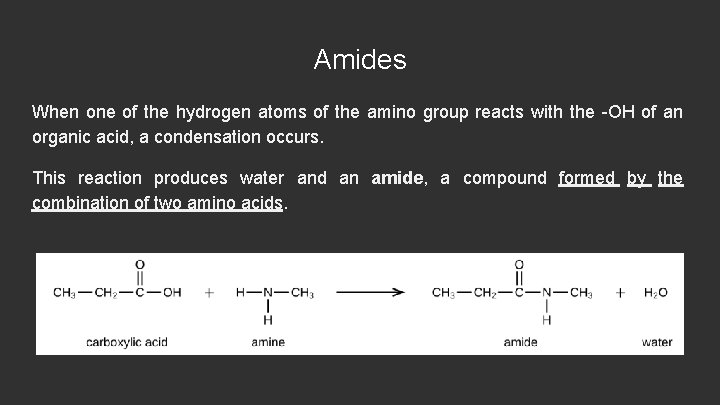
Amides When one of the hydrogen atoms of the amino group reacts with the -OH of an organic acid, a condensation occurs. This reaction produces water and an amide, a compound formed by the combination of two amino acids.

Organic Reactions

Organic Reactions occur more slowly than inorganic reactions due to strong bonds in the molecules. See next slide for the different types of organic reactions

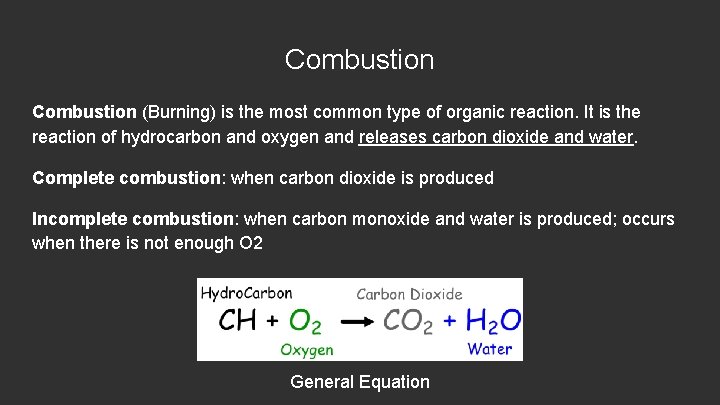
Combustion (Burning) is the most common type of organic reaction. It is the reaction of hydrocarbon and oxygen and releases carbon dioxide and water. Complete combustion: when carbon dioxide is produced Incomplete combustion: when carbon monoxide and water is produced; occurs when there is not enough O 2 General Equation

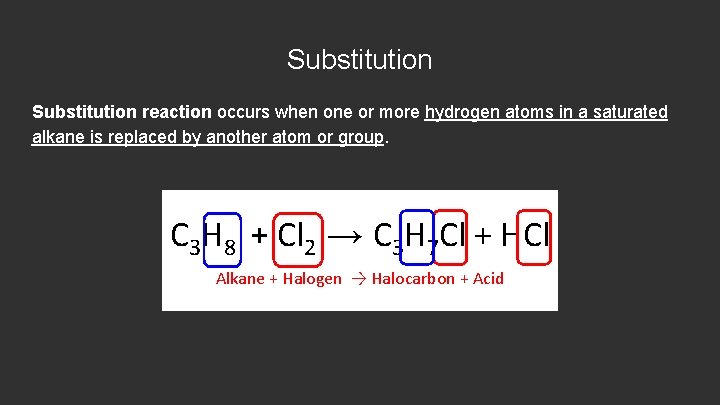
Substitution reaction occurs when one or more hydrogen atoms in a saturated alkane is replaced by another atom or group. C 3 H 8 + Cl 2 → C 3 H 7 Cl + HCl Alkane + Halogen → Halocarbon + Acid

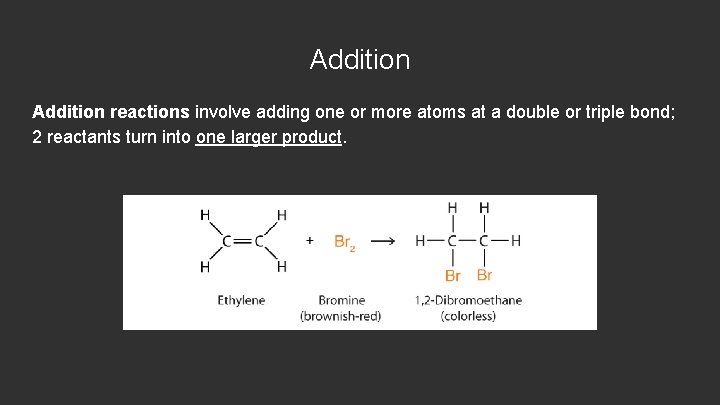
Addition reactions involve adding one or more atoms at a double or triple bond; 2 reactants turn into one larger product.

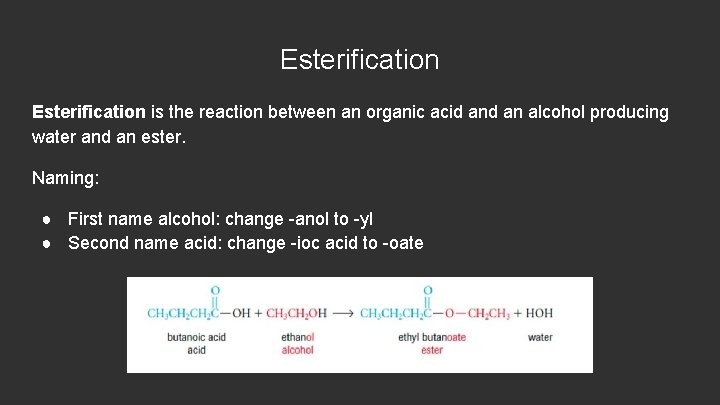
Esterification is the reaction between an organic acid an alcohol producing water and an ester. Naming: ● First name alcohol: change -anol to -yl ● Second name acid: change -ioc acid to -oate

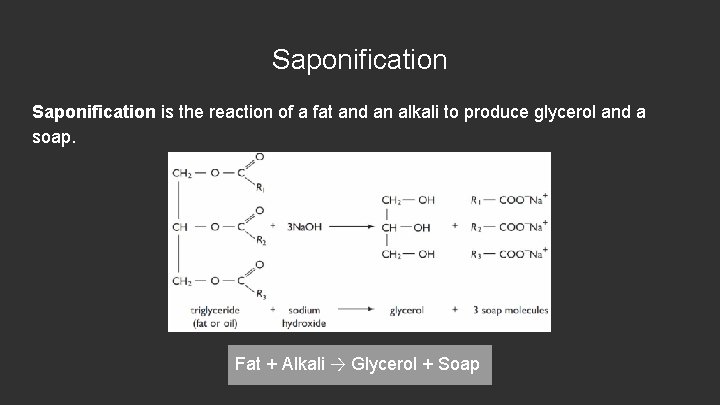
Saponification is the reaction of a fat and an alkali to produce glycerol and a soap. Fat + Alkali → Glycerol + Soap

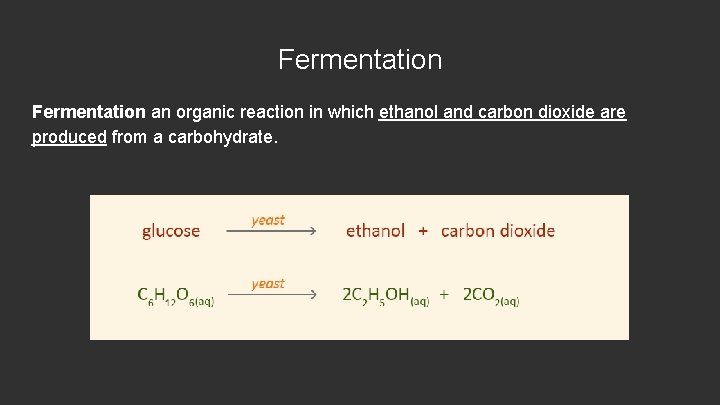
Fermentation an organic reaction in which ethanol and carbon dioxide are produced from a carbohydrate.

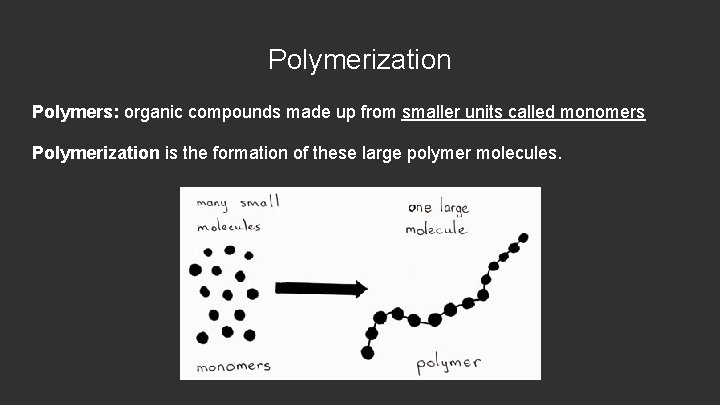
Polymerization Polymers: organic compounds made up from smaller units called monomers Polymerization is the formation of these large polymer molecules.

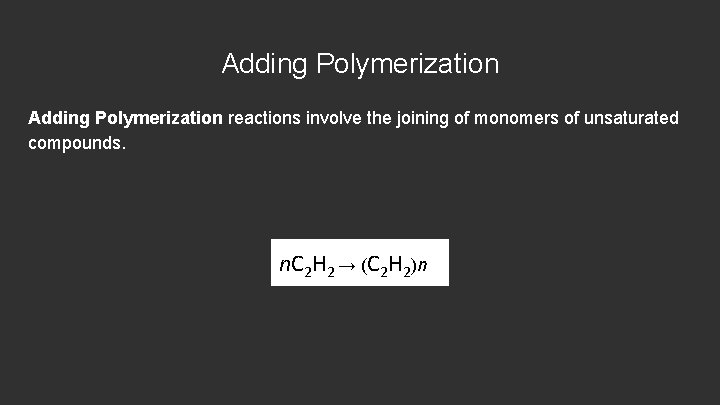
Adding Polymerization reactions involve the joining of monomers of unsaturated compounds. n. C 2 H 2 → (C 2 H 2)n

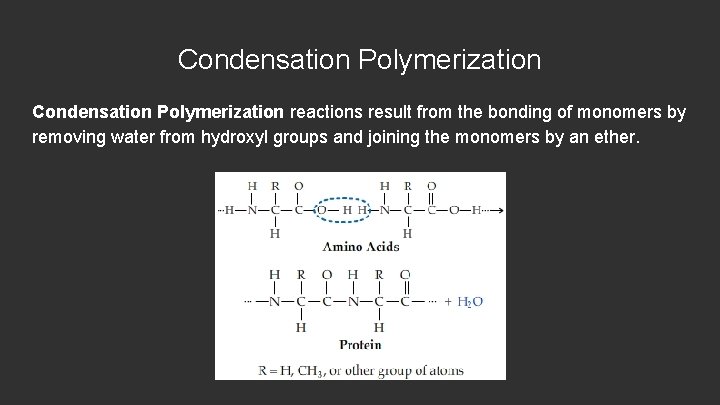
Condensation Polymerization reactions result from the bonding of monomers by removing water from hydroxyl groups and joining the monomers by an ether.